Introduction
Diffuse large B-cell lymphoma (DLBCL) is the major
histological subtype of non-Hodgkin lymphoma and accounts for
30–40% of all new diagnoses (1,2). It is an
aggressive but potentially curable lymphoma. R-CHOP (rituximab plus
cyclophosphamide, doxorubicin, vincristine and prednisone)
chemotherapy regimens have been the primary treatment methods over
the past decade (3). However, only
~60% of patients with DLBCL achieve durable remission following
chemotherapy (4,5). Previous studies have demonstrated that
the categorization of DLBCL phenotypes, particularly germinal
centre B-cell-like (GCB) and non-GCB, may be used to determine
patient prognosis (6,7). However, the results are controversial;
it has been demonstrated that patients with the GCB phenotype have
a better survival rate than those in the non-GCB group (8), however this has not been identified in
other studies (9,10). Therefore, a reliable and reproducible
prediction method is crucial to optimize patient care.
The standard International Prognostic Index (S-IPI)
(4) is the most widely used and
accepted prognostic tool for patients with aggressive lymphomas.
Five individual risk factors, patient age, lactate dehydrogenase
(LDH) concentration, Eastern Cooperative Oncology Group (ECOG)
performance status (11), involvement
of extra-nodal sites and Ann Arbor stage (12) are considered. S-IPI divides patients
into four prognostic subgroups based on the number of risk factors
present: A low risk group (0–1 risk factors), a low intermediate
risk group (2 risk factors), a high intermediate risk group (3 risk
factors) and a high-risk group (4–5 risk factors) (13). However, the addition of rituximab to
CHOP-like regimens for patients with DLBCL resulted in a major
improvement of patient outcome. Therefore, an updated version of
IPI, the revised-IPI (R-IPI), was established by Sehn et al
(14). Furthermore, an enhanced IPI
(NCCN-IPI) was proposed by Zhou et al (15) which incorporates the same five
variables as the S-IPI, but assigns different weights to age
[>40–60, 1 point (pt); >60–75, 2 pts; >75, 3 pts] and
elevated LDH [>1–3 (upper limit of normal; ULN), 1 pt; ≥3 ULN, 2
pts] and identifies the presence of extranodal involvement in the
bone marrow, central nervous system (CNS), liver, gastrointestinal
tract or lung as a positive parameter. These methods of predicting
patient prognosis (S-IPI, R-IPI and NCCN-IPI) are based solely on
the clinical features of patients prior to chemotherapy. Owing to
the clinical and biological heterogeneity of DLBCL (7,16), it
would be beneficial to add the assessment of responses to
treatment.
F-18 fluorodeoxyglucose positive emission
tomography/computed tomography (18F-FDGPET/CT) may be a
powerful tool for monitoring the response to therapy in aggressive
lymphomas (17,18). It was demonstrated that PET/CT could
be used to evaluate the response in FDG-avid tissues using the
5-point scale (5-PS) (19) at the
11th International Conference, which was held in Lugano,
Switzerland. Studies have suggested that the use of FDG PET/CT to
monitor early responses may guide therapeutic strategies for
patients with DLBCL (20–22). Therefore, interim 18F-FDG
PET/CT following the 4th cycle of R-CHOP-like regimens was used to
monitor the response of patients with DLBCL to treatment in the
current study.
The present study aimed to explore the value of
S-IPI, R-IPI and NCCN-IPI in predicting the prognosis of patients
with DLBCL and to determine whether the prognostic value may be
improved by interim 18F-FDG PET/CT response.
Patients and methods
Patients
Between January 2004 and January 2014, a total of
185 patients with newly diagnosed DLBCL were enrolled from Nanfang
Hospital (Guangzhou, China) in the retrospective study, which was
approved by the institutional ethics review board of Nanfang
Hospital. Informed consent was obtained from all individual
participants included in the study. The patients' clinical and
biological characteristics are summarized in Table I.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | n (%) |
|---|
| Male/female
ratio | 116/69 |
| Median age, years
(range) | 49 (16–82) |
|
≤40 | 54 (29.2) |
|
40–60 | 87 (47.0) |
|
60–75 | 38 (20.6) |
|
>75 | 6 (3.2) |
| Ann Arbor
stage |
|
| I | 23 (12.4) |
| II | 38 (20.6) |
|
III | 27 (14.6) |
| IV | 97(52.4) |
| LDH,
normalized |
|
| ≤1 | 108 (58.4) |
|
1–3 | 57 (30.8) |
|
>3 | 20 (10.8) |
| ECOG performance
status |
|
|
0–1 | 142 (76.8) |
| ≥2 | 43 (23.2) |
| Extranodal
disease |
|
| Bone
marrow | 18 (9.7) |
|
CNS | 11 (5.9) |
|
Liver | 17 (9.2) |
| GI
tract | 41 (22.2) |
|
Lung | 14 (7.6) |
|
Others | 103 (55.7) |
| Standard IPI
score |
|
|
0–1 | 74 (40.0) |
| 2 | 40 (21.6) |
| 3 | 40 (21.6) |
|
4–5 | 31 (16.8) |
| Revised IPI
score |
|
| 0 | 35 (18.9) |
|
1–2 | 79 (42.7) |
|
3–5 | 71 (38.4) |
| NCCN-IPI score |
|
|
0–1 | 38 (20.6) |
|
2–3 | 90 (48.6) |
|
4–5 | 45 (24.3) |
|
6–8 | 12 (6.5) |
Inclusion criteria were: i) ≥16 years of age at
diagnosis, ii) histologically proven DLBCL, iii) treatment with
R-CHOP-like regimens with curative intent and iv) an interim FDG
PET/CT scan following the 4th cycle of chemotherapy. Patients were
excluded if they were treated with surgery or exhibited evidence of
a secondary malignant tumor. All patients were reviewed on
pathological diagnosis by two hematopathologists.
PET/CT scanning protocol
All patients underwent whole body 18F-FDG
PET/CT scan using a Discovery LS PET/CT scanner (GE Healthcare,
Chicago, IL, USA). Following 6 h fasting, 185–370 MBq
18F-FDG (5.18 MBq/kg) was administered intravenously to
each patient. Blood glucose levels were monitored prior to the scan
to ensure that blood glucose levels were normal (<7 mmol/l).
Approximately 60 min after the injection of FDG, whole-body PET/CT
(from the vertex of the skull to the mid-thigh) was performed
following the guidelines for tumor imaging with PET/CT (23). A spiral CT scan was performed with a
scout view using an 0.8 sec rotation time, 80 mA, 140 kVp, 5-mm
slice thickness and a 4.25-mm interval in high-speed mode. A
whole-body PET/CT scan was acquired in the 2-dimensional
acquisition mode with 3 min per bed position. Acquired PET and CT
images were sent to the Xeleris workstation (version 2.1; GE
Healthcare) for registration and fusion.
PET/CT analysis
The interim PET scan was performed following the 4th
cycle of chemotherapy, with a median interval of 16 days after the
first day of the second or third cycle (range, 14–21 days). All
PET/CT images were interpreted by two experienced nuclear
physicians in consensus using the Xeleris (version 2.1; GE
Healthcare) workstation. A visual interpretation was performed
using the Deauville five-point scale (24): 1, no residual uptake; 2, uptake
≤mediastinum; 3, uptake >mediastinum but ≤liver; 4, uptake
moderately >liver; and 5, uptake markedly increased compared
with the liver and/or progression of new lesions (25). Interim PET/CT images were reclassified
into negative and positive groups; scores of 1–3 were considered
negative and scores of 4–5 were considered positive (24). These criteria were used to determine
extranodal disease and Ann Arbor stage.
S-IPI, R-IPI and NCCN-IPI
S-IPI, R-IPI and NCCN-IPI were examined in this
cohort of 18F-FDG PET/CT staged patients with DLBCL. The
risk factors identified by S-IPI were: Age >60 years, ECOG
performance score ≥2, elevated LDH (>ULN), involvement of >1
extranodal site and Ann Arbor stage III/IV. S-IPI divided the
patients into four prognostic subgroups, based on the number of
risk factors present: A low risk group (0–1 risk factors), a low
intermediate risk group (2 risk factors), a high intermediate risk
group (3 risk factors) and a high-risk group (4–5 risk factors)
(13). The R-IPI involves the same
individual factors, but with only three risk groups: Very good (0
risk factors), good (1–2 risk factors) and poor (>2 risk
factors) (14). NCCN-IPI also uses
the same five risk factors as the IPI but further refines the
categorization of age and normalized LDH and specific sites of
involvement (bone marrow, CNS, liver, gastrointestinal tract or
lung). Four risk groups were formed: A low risk group (0–1), a low
intermediate group (2–3), a high intermediate group (4–5) and a
high-risk group (6–8) (15). All
risk factors of patients were available in the present study.
Statistical analysis
Descriptive statistics of clinical characteristics
were generated as proportions. Fisher's exact test was analyzed to
compare the differences between groups of categorical values. End
points were 2-year progression free survival (PFS; defined as time
from diagnosis to progression, relapse or mortality from any cause)
and 2-year overall survival (OS; defined as time from diagnosis to
mortality from any cause). PFS and OS were determined by
Kaplan-Meier analysis and differences across groups were analyzed
using a log-rank test. Prognostic factors were tested using a Cox
proportional hazard model. All tests were considered significant
when P<0.05 and were not adjusted for multiple comparisons.
Statistical analyses were performed by GraphPad Prism version 5.0
(GraphPad software, Inc., La Jolla, CA, USA).
Results
Patient outcomes
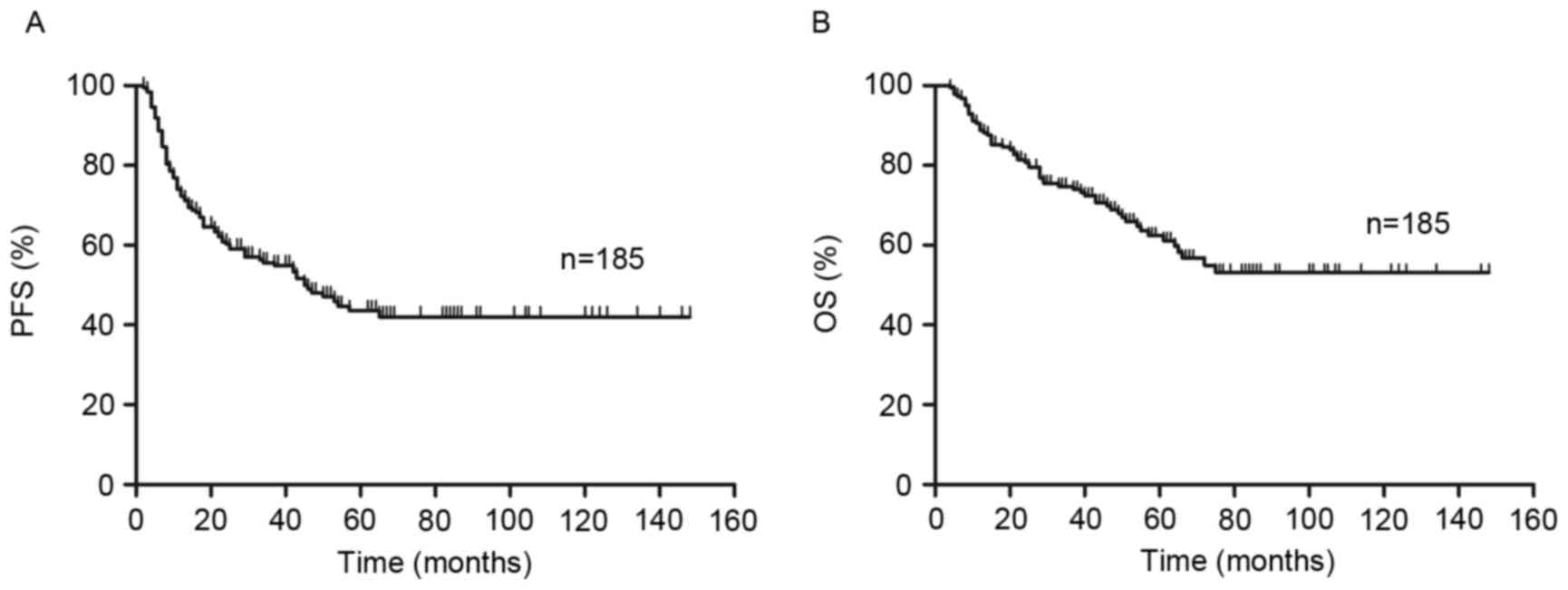
The median follow-up time of patients was 44 months
(range 4–148 months). Of all 185 patients, 88 patients exhibited no
progression (PFS 47.6%) and the median time prior to relapse was 34
months (range 2–148 months). By the end of follow-up (148 months
was the longest follow-up time for one patient), OS was 67%
(124/185 patients).
Outcomes according to S-IPI and
interim PET/CT
PFS and OS curves present the outcome of patients
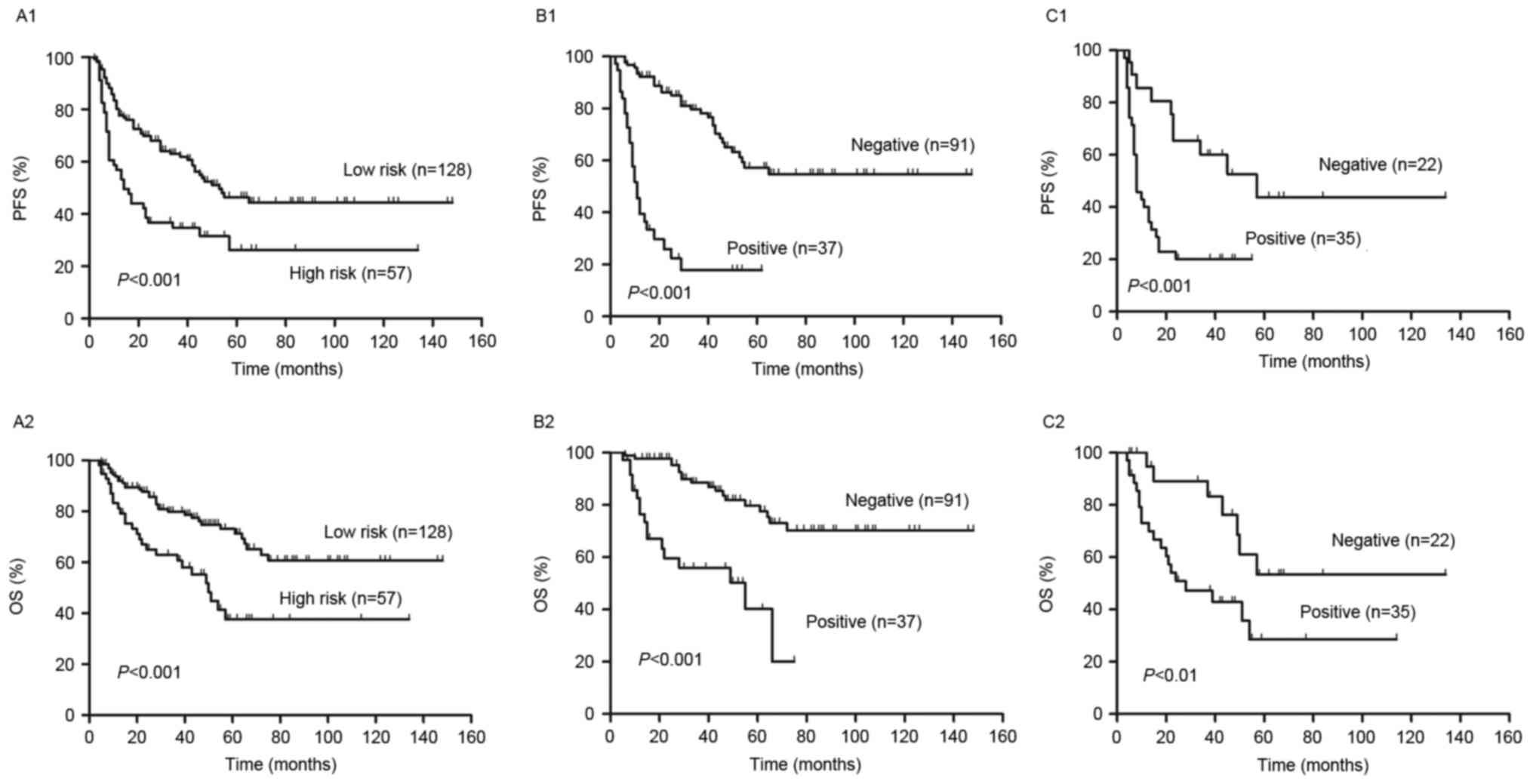
treated with R-CHOP like regimens (Fig.
1). The 2-year PFS and OS of all risk subgroups are presented
in Table II. Of the entire cohort,
2-year PFS and OS were 60% [95% confidence interval (CI), 53–67%]
and 81% (95% CI, 74–86%), respectively (Fig. 1). As demonstrated in Fig. 2, analysis of S-IPI results identified
statistically significant differences in PFS and OS between
patients in the lower and higher risk groups (P<0.001; Fig. 2A). The results also identified
statistically significant differences in the lower risk group
between score 0–1 and score 2 in PFS (P=0.01) and OS (P<0.01).
However, in the higher risk group, it exhibited no statistically
significant difference in PFS (P=0.47) and OS (P=0.16) between
score 3 and score 4–5.
 | Table II.Comparison of S-IPI, R-IPI to
NCCN-IPI for risk stratification and outcomes of 2-year PFS and OS
in these risk groups redistributed by interim PET/CT into PET
negative (−) and positive (+) groups. |
Table II.
Comparison of S-IPI, R-IPI to
NCCN-IPI for risk stratification and outcomes of 2-year PFS and OS
in these risk groups redistributed by interim PET/CT into PET
negative (−) and positive (+) groups.
|
| S-IPI | R-IPI | NCCN-IPI |
|---|
|
|
|
|
|
|---|
| Variable | Low risk (0–2) | High risk
(3–5) | Very good (0) | Good (1–2) | Poor (3–5) | Low risk (0–3) | High risk
(4–8) |
|---|
| 2-year | 74 (64–81) | 37 (26–48) | 97 (80–100) | 63 (51–73) | 37 (25–48) | 70 (61–77) | 37 (24–49) |
| PFS | PET(−) | PET(+) | PET(−) | PET(+) | PET(−) | PET(+) | PET(−) | PET(+) | PET(−) | PET(+) | PET(−) | PET(+) | PET(−) | PET(+) |
|
| 88 (78–94) | 31 (15–49) | 66 (44–80) | 18 (8–31) | 100 | 97 (80–100) | 82 (67–92) | 26 (11–44) | 66 (44–81) | 18 (8–31) | 85 (76–91) | 22 (10–38) | 65 (41–82) | 20 (9–34) |
| 2-year | 90 (82–94) | 66 (53–76) | 97 (80–100) | 85 (75–91) | 60 (47–71) | 88 (80–93) | 65 (51–76) |
| OS | PET(−) | PET(+) | PET(−) | PET(+) | PET(−) | PET(+) | PET(−) | PET(+) | PET(−) | PET(+) | PET(−) | PET(+) | PET(−) | PET(+) |
|
| 98 (90–99) | 65 (43–80) | 82 (59–93) | 45 (29–60) | 100 | 97 (80–100) | 94 (82–98) | 63 (40–79) | 92 (71–98) | 48 (32–63) | 95 (88–98) | 60 (41–74) | 89 (63–97) | 51 (33–66) |
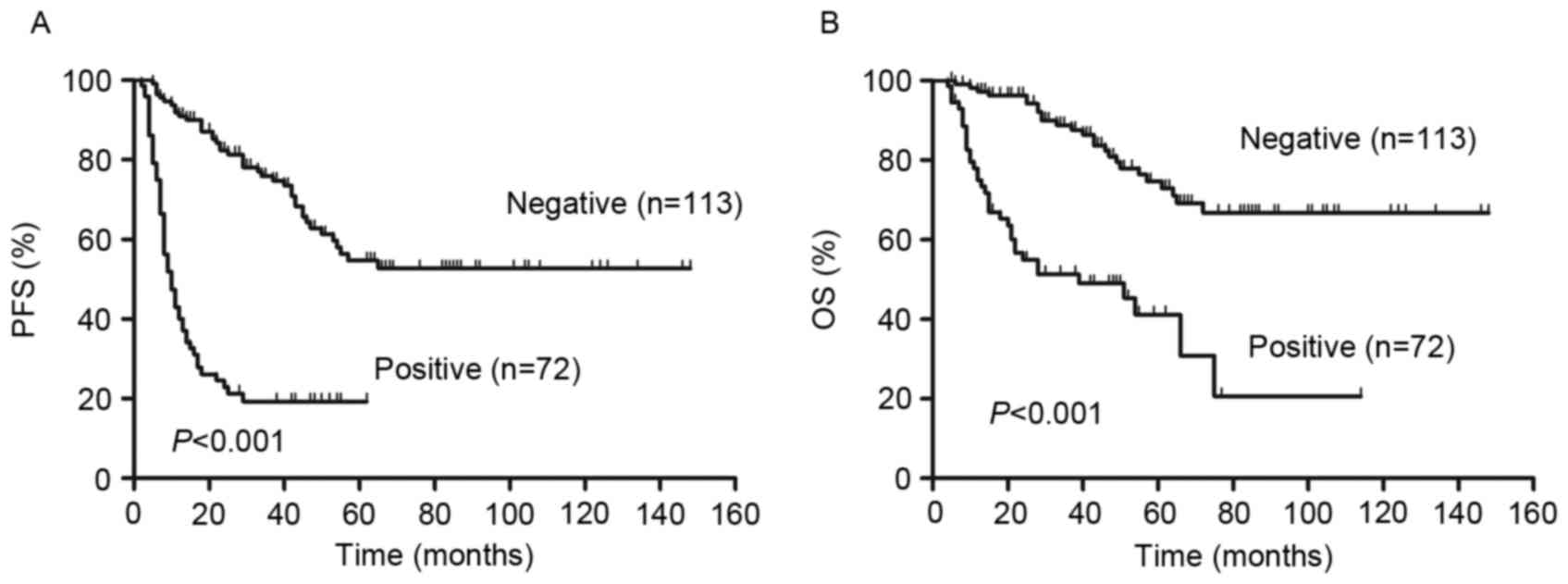
Analysis of visual 5-PS results demonstrated that
there were significant differences in the PFS and OS of patients
with a positive interim PET scan compared with patients that had a
negative interim PET scan (P<0.01; Fig. 3). The 2-year PFS and OS were 82% (95%
CI, 76–88%) and 96% (95% CI, 90–99%), respectively, in patients
with negative PET results. By contrast, in patients with positive
PET results, 2-year PFS and OS were 23% (95% CI 14–34%) and 55%
(95% CI 42–66%), respectively.
In the higher risk group, 2-year PFS and OS were 37%
(95% CI, 26–48%) and 66% (95% CI, 53–76%), respectively (Table II). Patients in the low risk group
were reclassified into PET negative and positive groups by interim
PET/CT and it was demonstrated that patients in the PET positive
group had a significantly lower PFS and OS than those in the PET
negative group (P<0.001; Fig. 2B).
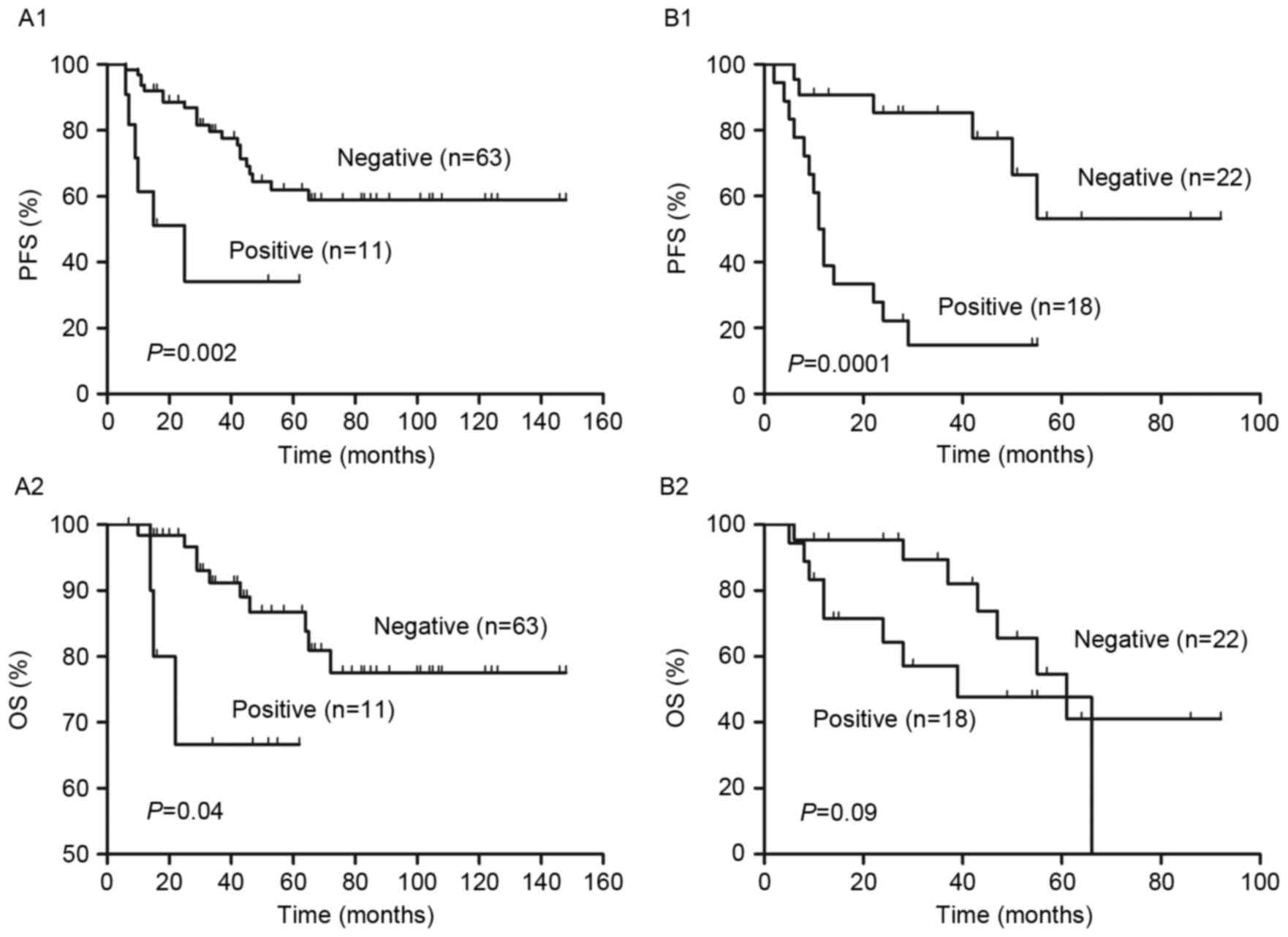
Furthermore, patients in the low and low intermediate risk groups
were reclassified into PET negative and positive groups using
interim PET/CT (Fig. 4). It was
demonstrated that the differences in PFS and OS between the PET
negative and positive groups were significant in the low risk group
(both P<0.05; Fig. 4A). However,
in the low intermediate risk group, the difference in PFS between
PET negative and positive patients was significant (P=0.0001);
however, the difference in OS was not (Fig. 4B).
Outcomes according to R-IPI and
interim PET/CT
R-IPI is a valid predictor of outcomes of patients
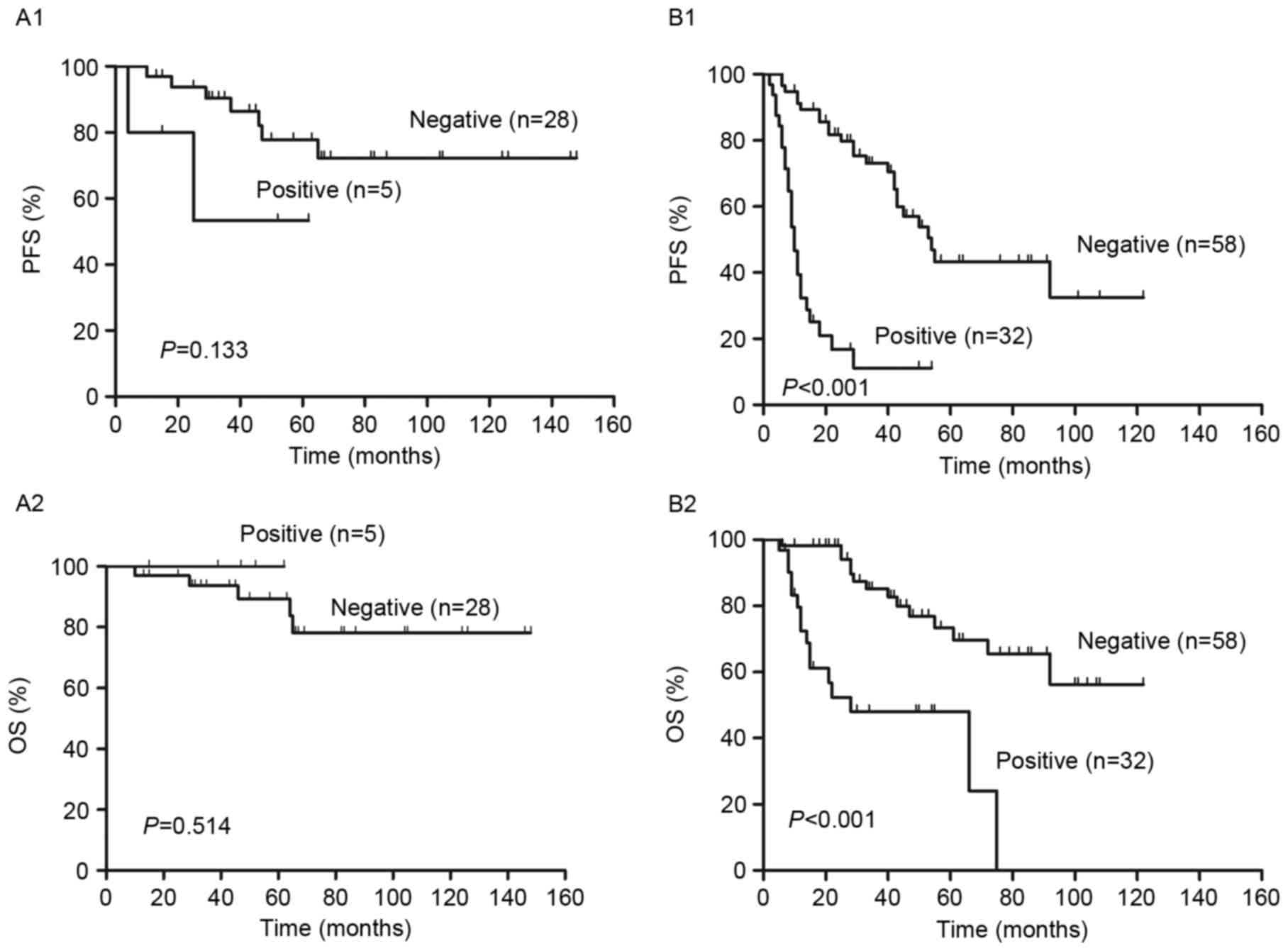
with DLBCL treated with R-CHOP like regimens. Fig. 5 demonstrates that R-IPI identified 3
distinct prognostic groups with a very good, good and poor outcome,
respectively, with significant differences between all groups in
PFS and OS (P<0.001; Fig. 5A). The
2-year PFS and OS of all risk subgroups are presented in Table II. Analysis of PET results identified
significant differences in the PFS and OS between PET positive and
negative patients in the good and poor risk groups (P<0.01;
Fig. 5C and D). However, no
significant differences in PFS (P=0.60) or OS (P=0.07) between PET
negative and positive patients were identified in the very good
risk group (Fig. 5B). In all groups,
2-year PFS and OS were decreased in PET positive patients compared
with PET negative patients (P<0.01; Fig. 5 and Table
II).
Outcomes according to NCCN-IPI and
interim PET/CT
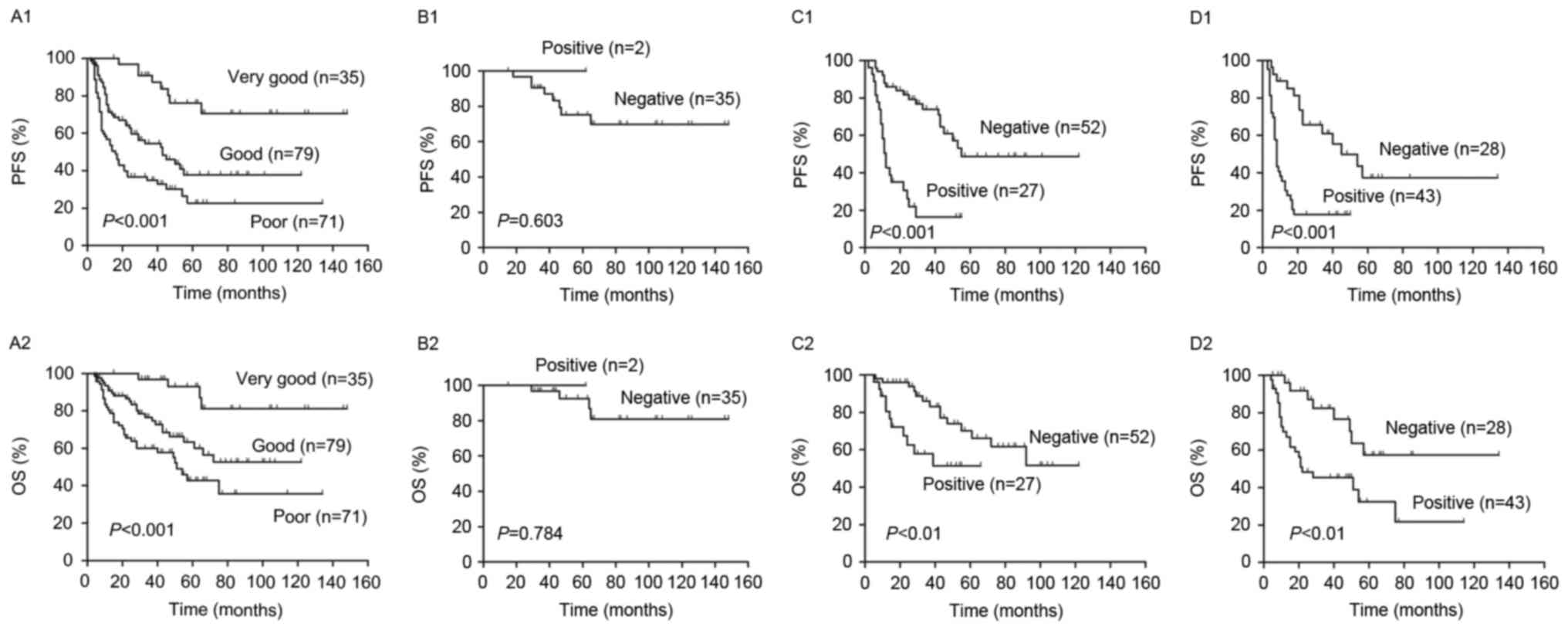
Patients were classified into high and low risk
groups, according to NCCN-IPI and it was determined that the PFS
and OS were significantly lower in high risk patients compared with
low risk patients (P<0.001, Fig.
6A). The 2-year PFS and OS of the risk subgroups are presented
in Table II. In the higher risk
group, there was no significant difference in PFS (P=0.84) and OS
(P=0.51) between scores 4–5 and 6–8. However, there were
significant differences in the PFS (P<0.01) and OS (P<0.01)
of patients with scores 0–1 and 2–3.
The interim PET results divided patients in the high
and low risk groups into PET negative and positive groups (Fig. 6). The 2-year PFS and OS were
significantly higher in the PET negative group compared with the
PET positive group in the low and high risk groups (P<0.01,
Fig. 6B and C). Patients in the low
risk were further subdivided into a low risk group and a low
intermediate risk group. In the low intermediate risk group, PFS
and OS were significantly lower in PET negative patients compared
with PET positive patients (P<0.001; Fig. 7B). However, in the low risk group,
there were no significant differences in PFS and OS between the PET
negative and positive groups (P>0.05, Fig. 7A).
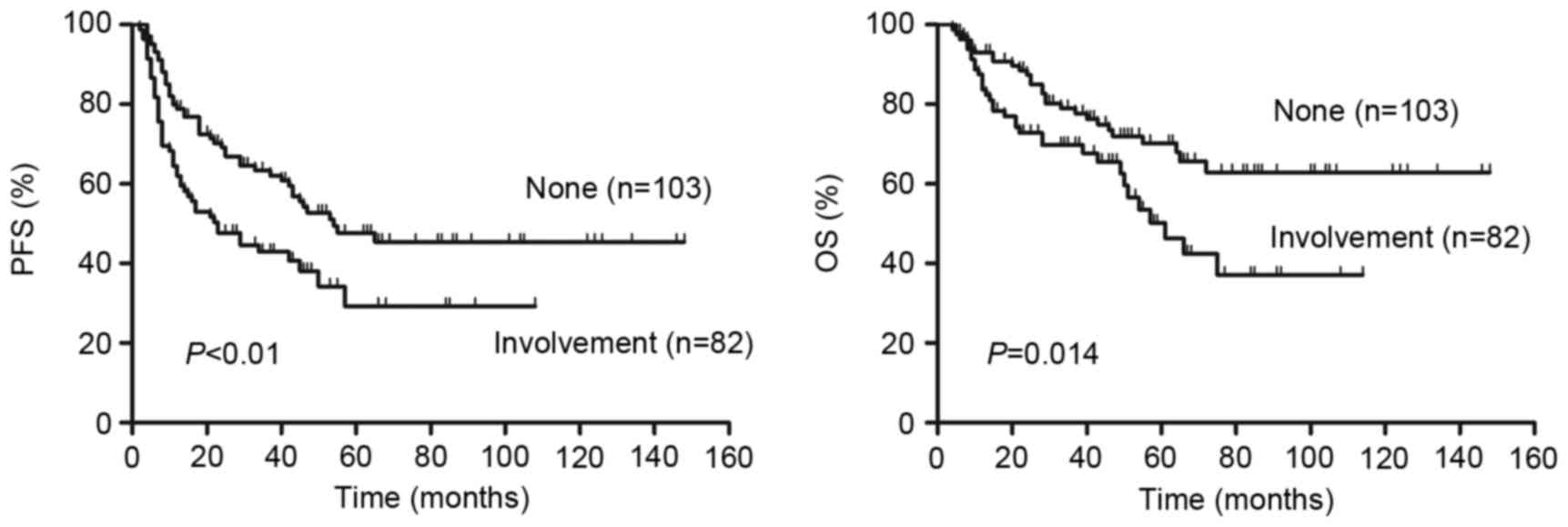
Patients that had DLBCL with involvement of bone
marrow, CNS, liver, gastrointestinal tract or lung exhibited a
significantly lower PFS (P=0.005, Fig.
8A) and OS (P=0.014, Fig. 8B)
than patients without involvement of these organs. No significant
difference in survival was observed between these involved organs
for patients with DLBCL.
Discussion
In the present study, the prognostic value of S-IPI,
R-IPI and NCCN-IPI in patients with DLBCL treated with R-CHOP-like
regimens was assessed. Interim PET following 4 cycles induction
chemotherapy was used to predict the 2-year PFS and OS of patients
with DLBCL. The results of the current study demonstrated that
S-IPI, R-IPI and NCCN-IPI are three clinically useful prognostic
indexes that may guide the treatment planning of patients with
DLBCL. Furthermore, interim PET/CT improves the prognostic value of
S-IPI, R-IPI and NCCN-IPI in predicting the 2-year PFS and OS of
patients with DLBCL, particularly in patients with high IPI
scores.
The use of R-CHOP-like regimens has resulted in a
major improvement of survival in patients with DLBCL across all
risk groups (3,26). Consequently, the usefulness of S-IPI
in discriminating between patients in different risk groups has
declined, particularly in higher risk groups (4,14). In the
current study, the S-IPI revealed no significant differences in PFS
and OS between patients in the high intermediate (score 3) and high
risk groups (score 4–5). NCCN-IPI is more powerful than S-IPI at
predicting the survival of low- and high-risk patients (15). However, the results of the current
study demonstrated that differences in PFS and OS between the high
intermediate (score 4–5) and high-risk groups (score 6–8) of the
NCCN-IPI were not significant. R-IPI identified 3 distinct
prognostic groups with very good (score 0), good (score 1–2) and
poor (score 3–5) outcomes (P<0.001). From this, the R-IPI
integrated the high intermediate risk group (score 3) and the high
risk group (score 4–5) in S-IPI into one group: The poor risk group
(score 3–5). However, R-IPI was not able to differentiate between
the high intermediate and the high risk groups. Therefore, the
ability of S-IPI, NCCN-IPI and R-IPI to differentiate between the
high and high intermediate risk groups is limited.
Alizadeh et al (16) demonstrated that DLBCL is a
heterogeneous group of malignancies rather than a single clinical
or pathological entity. The World Health Organization acknowledged
the biological heterogeneity of DLBCL in the version of lymphoid
malignancies in 2008 (27). To date,
the Hans algorithm has been the most widely used method of
reclassifying DLBCL patients into GCB and non-GCB groups, based on
the presence of three immunohistochemical markers [cluster of
differentiation 10, B-Cell lymphoma 6 and melanoma associated
antigen (mutated) 1] (28). However,
the results of previous studies with regard to the prognostic value
of GCB and non-GCB phenotypes for patients with DLBCL are
conflicting (8–10). S-IPI, R-IPI and NCCN-IPI are all based
solely on the clinical features of patients prior to chemotherapy.
Considering the clinical and biological heterogeneity of DLBCL,
improving the prediction of patient responses to treatment based on
the IPI score is imperative.
18F-FDG PET/CT is a powerful method of
evaluating the response to chemotherapy in patients with DLBCL
(29). It has been demonstrated that
the use of interim PET/CT to assess the response to treatment may
induce chemosensitivity and may help to guide therapeutic
strategies for patients with DLBCL (21,30). Itti
et al (31) reported that the
accuracy at four cycles was better than at two cycles when visual
interpretation was used. Patients who were PET2-positive (PET
imaging following two cycles of chemotherapy) became PET4-negative
(PET imaging following four cycles of chemotherapy), of whom only a
small number experienced an event, whereas patients who were
PET2-positve remained PET4-positive, of whom most rapidly had an
event. By comparison, all PET2-negative patients who underwent
PET-4 remained negative and few experienced an event (31). Therefore, in the current study,
interim PET following four cycles of chemotherapy was used to
monitor the treatment response of patients with DLBCL.
The results of the current study confirmed that the
redistribution of high risk patients into PET negative and positive
groups provided a more clinically relevant prediction of patient
outcome. Compared with the PET negative group, PET positive
patients had a significantly lower PFS and OS. Therefore, an
investigational approach involving clinical trials on PET positive
patients in high or poor risk groups should be considered to ensure
potential curative therapy. Although S-IPI and NCCN-IPI were able
to discriminate between low and low intermediate risk groups in
predicting PFS and OS, the results indicated that PFS and OS were
higher in patients in the lower risk group that were PET negative
than those that were evaluated using IPI scores alone. However, no
significant differences were observed between PET negative and PET
positive groups in patients determined to be in the low
intermediate risk group by S-IPI. In the low risk NCCN-IPI group,
there were also no significant differences in 2-year PFS and OS
between PET negative and positive patients. This may have been due
to the fact that the number of patients in these groups was too
small. Furthermore, the number of patients that were PET positive
in the very good risk group of R-IPI was too small to obtain a
valid statistical interpretation.
Zhou et al (15) suggested that the involvement of the
bone marrow, CNS, liver, gastrointestinal tract or lung in lymphoma
may be a better predictor of survival in patients with DLBCL than
simply the number of extranodal sites involved. The results of the
current study demonstrated that patients with involvement of these
organs had a significantly lower 2-year PFS and OS than those
without involvement. No significant difference of survival was
observed among these involved organs for patients with DLBCL. This
may be due to the fact that the involvement of the organs is small
and often overlapping, thus it is difficult to clearly identify the
effects of organ involvement alone on PFS and OS.
The present study used three different meaningful
prognostic tools, S-IPI, R-IPI and NCCN-IPI, to evaluate the
prognosis of patients with DLBCL. All of them identified
significant differences in the PFS and OS between low and high risk
groups. The results of the current study demonstrated that low and
high risk groups can be further reclassified into PET positive and
negative groups using interim PET/CT at the 4th cycle of treatment
with an R-CHOP like regimen. Patients that were PET negative in the
low or high risk groups had a higher PFS and OS than those that
were PET positive. However, the current study was a retrospective
analysis and there were low numbers of patients in the low
intermediate risk group in S-IPI, the low risk group in NCCN-IPI
and in the very good risk group in R-IPI that were PET positive.
Therefore, the results should be validated prospectively in a
larger population of patients with DLBCL.
In conclusion, the present study demonstrated that
the S-IPI, R-IPI and NCCN-IPI are three clinically useful
prognostic indexes that may guide the treatment planning of
patients with DLBCL. The results suggest that interim PET/CT
improves the risk stratifications of S-IPI, R-IPI and NCCN-IPI in
predicting 2-year PFS and OS, particularly in patients with high
IPI scores.
Acknowledgements
The study was funded by the National Natural Science
Foundation of China (grant no. 81271641) and the projects of
medical and health technology program in Zhejiang province (grant
no. 2018KY676).
References
|
1
|
No authors listed: A clinical evaluation
of the International Lymphoma Study Group classification of
non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification
Project. Blood. 89:3909–3918. 1997.PubMed/NCBI
|
|
2
|
Coiffier B: Diffuse large cell lymphoma.
Curr Opin Oncol. 13:325–334. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coiffier B, Lepage E, Briere J, Herbrecht
R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G,
Gaulard P, et al: CHOP chemotherapy plus rituximab compared with
CHOP alone in elderly patients with diffuse large-B-cell lymphoma.
N Engl J Med. 346:235–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ziepert M, Hasenclever D, Kuhnt E, Glass
B, Schmitz N, Pfreundschuh M and Loeffler M: Standard international
prognostic index remains a valid predictor of outcome for patients
with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin
Oncol. 28:2373–2380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pfreundschuh M, Schubert J, Ziepert M,
Schmits R, Mohren M, Lengfelder E, Reiser M, Nickenig C, Clemens M,
Peter N, et al: Six versus eight cycles of bi-weekly CHOP-14 with
or without rituximab in elderly patients with aggressive CD20+
B-cell lymphomas: A randomised controlled trial (RICOVER-60).
Lancet Oncol. 9:105–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Campo E, Swerdlow SH, Harris NL, Pileri S,
Stein H and Jaffe ES: The 2008 WHO classification of lymphoid
neoplasms and beyond: Evolving concepts and practical applications.
Blood. 117:5019–5032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dybkær K, Bøgsted M, Falgreen S, Bødker
JS, Kjeldsen MK, Schmitz A, Bilgrau AE, Xu-Monette ZY, Li L,
Bergkvist KS, et al: Diffuse large B-cell lymphoma classification
system that associates normal B-cell subset phenotypes with
prognosis. J Clin Oncol. 33:1379–1388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barrans SL, Carter I, Owen RG, Davies FE,
Patmore RD, Haynes AP, Morgan GJ and Jack AS: Germinal center
phenotype and bcl-2 expression combined with the International
Prognostic Index improves patient risk stratification in diffuse
large B-cell lymphoma. Blood. 99:1136–1143. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Colomo L, López-Guillermo A, Perales M,
Rives S, Martínez A, Bosch F, Colomer D, Falini B, Montserrat E and
Campo E: Clinical impact of the differentiation profile assessed by
immunophenotyping in patients with diffuse large B-cell lymphoma.
Blood. 101:78–84. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Linderoth J, Jerkeman M, Cavallin-Ståhl E,
Kvaløy S and Torlakovic E: Nordic Lymphoma Group Study:
Immunohistochemical expression of CD23 and CD40 may identify
prognostically favorable subgroups of diffuse large B-cell
lymphoma: A nordic lymphoma group study. Clin Cancer Res.
9:722–728. 2003.PubMed/NCBI
|
|
11
|
Young J, Badgery-Parker T, Dobbins T,
Jorgensen M, Gibbs P, Faragher I, Jones I and Currow D: Comparison
of ECOG/WHO performance status and ASA score as a measure of
functional status. J Pain Symptom Manage. 49:258–264. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Greene FL: The American Joint Committee on
Cancer: Updating the strategies in cancer staging. Bull Am Coll
Surg. 87:13–15. 2002.PubMed/NCBI
|
|
13
|
International Non-Hodgkin's Lymphoma
Prognostic Factors Project: A predictive model for aggressive
non-Hodgkin's lymphoma. N Engl J Med. 329:987–994. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sehn LH, Berry B, Chhanabhai M, Fitzgerald
C, Gill K, Hoskins P, Klasa R, Savage KJ, Shenkier T, Sutherland J,
et al: The revised International Prognostic Index (R-IPI) is a
better predictor of outcome than the standard IPI for patients with
diffuse large B-cell lymphoma treated with R-CHOP. Blood.
109:1857–1861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou Z, Sehn LH, Rademaker AW, Gordon LI,
Lacasce AS, Crosby-Thompson A, Vanderplas A, Zelenetz AD, Abel GA,
Rodriguez MA, et al: An enhanced International Prognostic Index
(NCCN-IPI) for patients with diffuse large B-cell lymphoma treated
in the rituximab era. Blood. 123:837–842. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alizadeh AA, Eisen MB, Davis RE, Ma C,
Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, et al:
Distinct types of diffuse large B-cell lymphoma identified by gene
expression profiling. Nature. 403:503–511. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Terasawa T, Nihashi T, Hotta T and Nagai
H: 18F-FDG PET for posttherapy assessment of Hodgkin's disease and
aggressive Non-Hodgkin's lymphoma: A systematic review. J Nucl Med.
49:13–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Terasawa T, Lau J, Bardet S, Couturier O,
Hotta T, Hutchings M, Nihashi T and Nagai H:
Fluorine-18-fluorodeoxyglucose positron emission tomography for
interim response assessment of advanced-stage Hodgkin's lymphoma
and diffuse large B-cell lymphoma: A systematic review. J Clin
Oncol. 27:1906–1914. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheson BD, Fisher RI, Barrington SF,
Cavalli F, Schwartz LH, Zucca E and Lister TA: Alliance,
Australasian Leukaemia and Lymphoma Group; Eastern Cooperative
Oncology Group; European Mantle Cell Lymphoma Consortium:
Recommendations for initial evaluation, staging, and response
assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano
classification. J Clin Oncol. 32:3059–3068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Casasnovas RO, Meignan M,
Berriolo-Riedinger A, Bardet S, Julian A, Thieblemont C, Vera P,
Bologna S, Brière J, Jais JP, et al: SUVmax reduction improves
early prognosis value of interim positron emission tomography scans
in diffuse large B-cell lymphoma. Blood. 118:37–43. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fuertes S, Setoain X, Lopez-Guillermo A,
Carrasco JL, Rodríguez S, Rovira J and Pons F: Interim FDG PET/CT
as a prognostic factor in diffuse large B-cell lymphoma. Eur J Nucl
Med Mol Imaging. 40:496–504. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nols N, Mounier N, Bouazza S, Lhommel R,
Costantini S, Vander Borght T, Vekemans MC, Sonet A, Bosly A,
Michaux L, et al: Quantitative and qualitative analysis of
metabolic response at interim positron emission tomography scan
combined with International Prognostic Index is highly predictive
of outcome in diffuse large B-cell lymphoma. Leuk Lymphoma.
55:773–780. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Delbeke D, Coleman RE, Guiberteau MJ,
Brown ML, Royal HD, Siegel BA, Townsend DW, Berland LL, Parker JA,
Hubner K, et al: Procedure guideline for tumor imaging with 18F-FDG
PET/CT 1.0. J Nucl Med. 47:885–895. 2006.PubMed/NCBI
|
|
24
|
Meignan M, Gallamini A, Haioun C and
Polliack A: Report on the second international workshop on interim
positron emission tomography in lymphoma held in Menton, France,
8–9 April 2010. Leuk Lymphoma. 51:2171–2180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meignan M, Gallamini A, Meignan M,
Gallamini A and Haioun C: Report on the first international
workshop on interim-PET-scan in lymphoma. Leuk Lymphoma.
50:1257–1260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Habermann TM, Weller EA, Morrison VA,
Gascoyne RD, Cassileth PA, Cohn JB, Dakhil SR, Woda B, Fisher RI,
Peterson BA and Horning SJ: Rituximab-CHOP versus CHOP alone or
with maintenance rituximab in older patients with diffuse large
B-cell lymphoma. J Clin Oncol. 24:3121–3127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vardiman JW, Thiele J, Arber DA, Brunning
RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM,
Hellström-Lindberg E, Tefferi A and Bloomfield CD: The 2008
revision of the World Health Organization (WHO) classification of
myeloid neoplasms and acute leukemia: Rationale and important
changes. Blood. 114:937–951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hans CP, Weisenburger DD, Greiner TC,
Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E,
Braziel RM, Jaffe ES, et al: Confirmation of the molecular
classification of diffuse large B-cell lymphoma by
immunohistochemistry using a tissue microarray. Blood. 103:275–282.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kwon SH, Kang DR, Kim J, Yoon JK, Lee SJ,
Jeong SH, Lee HW and An YS: Prognostic value of negative interim
2-[18F]-fluoro-2-deoxy-d-glucose PET/CT in diffuse large
B-cell lymphoma. Clin Radiol. 71:280–286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang DH, Min JJ, Song HC, Jeong YY, Chung
WK, Bae SY, Ahn JS, Kim YK, Bom HS, Chung IJ, et al: Prognostic
significance of interim 18F-FDG PET/CT after three or
four cycles of R-CHOP chemotherapy in the treatment of diffuse
large B-cell lymphoma. Eur J Cancer. 47:1312–1318. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Itti E, Lin C, Dupuis J, Paone G,
Capacchione D, Rahmouni A, Haioun C and Meignan M: Prognostic value
of interim 18F-FDG PET in patients with diffuse large B-Cell
lymphoma: SUV-based assessment at 4 cycles of chemotherapy. J Nucl
Med. 50:527–533. 2009. View Article : Google Scholar : PubMed/NCBI
|