Introduction
Colorectal cancer (CRC) is the third most frequent
type of cancer in males and the second in females, and the fourth
most common cause of oncological mortality worldwide (1). Surgical resection is the major treatment
for patients with CRC. However, ~75% of patients with resectable
metastatic CRC undergo postoperative recurrence within 18 months of
surgery (2). CRC is difficult to
treat due to unlimited cell proliferation and resistance to cell
apoptosis. Therefore, chemotherapy which executes an antitumor
effect through decreasing cell proliferation or inducing cell
apoptosis, is administered to alleviate symptoms and prolong
survival (3). However, these
chemotherapeutic agents exhibit significant side effects on
off-target cells, including normal cells. Drug resistance, drug
toxicity and conditions including anemia, leucopenia,
thrombocytopenia and peripheral neuropathy limit the effectiveness
of these treatments, increasing the requirement for the development
of novel therapeutic approaches (4).
Traditional Chinese medicine (TCM) has been used to
treat cancer for thousands of years in China. TCM combined with
modern treatments may improve symptoms, enhance quality of life,
prevent recurrence and metastasis, and prolong patient survival.
Additionally, TCM has potential advantages in patients who are not
suitable candidates for radiotherapy and chemotherapy (5). TCM involves numerous chemical compounds,
and is therefore considered to be multi-component and multi-target
exerting therapeutic functions in a more holistic way. Discovering
naturally occurring agents is a promising approach for the
treatment of cancer (6).
Hedyotis diffusa Willd (HDW) is a well-known herbal
medicine, which exhibits a variety of bioactivities, including
anti-inflammatory, antioxidative, immune-modulating and anticancer
properties (7–9). Belonging to the Rubiaceae family of
plants, HDW is widely distributed in Northeast Asia. It is used in
TCM to clear away heat and toxic material (7,8), to
promote blood circulation and has long been used clinically to
treat various types of cancer, including breast (10), colorectal (11) and liver (12) cancer. Our previous studies
demonstrated that HDW inhibited the growth of CRC, possibly by
inducing cancer cell apoptosis and inhibiting cell proliferation
and tumor angiogenesis (13–17). However, HDW has a complicated chemical
composition and the effectiveness of its various polar fractions
remains largely unclear. In the present study, the effect of polar
extracts of HDW on four human colorectal cancer cell lines, SW620,
HT-29, HCT116 and HCT-8, were compared, and the potential
underlying molecular mechanisms were investigated.
Materials and methods
Materials and reagents
Dulbecco's modified Eagle's medium (DMEM), RPMI-1640
medium, fetal bovine serum (FBS), penicillin-streptomycin, 0.25%
trypsin-EDTA, DreamTaq Green PCR Master Mix and Pierce
Bicinchoninic Acid (BCA) Protein Assay kit were purchased from
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). MTT was
purchased from Beijing Solarbio Science and Technology Co., Ltd.
(Beijing, China). Carboxyfluorescein diacetate succinimidyl ester
(CFDA-SE) Cell Proliferation and Tracking kit and Annexin
V-Fluorescein Isothiocyante (FITC) Apoptosis Detection kit were
purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China).
RNAiso Plus and PrimeScript RT Reagent kit with gDNA Eraser
(Perfect Real Time) was purchased from Takara Biotechnology Co.,
Ltd. (Dalian, China). Radio immunoprecipitation assay (RIPA) lysis
buffer and blocking buffer were purchased from Beyotime Institute
of Biotechnology (Haimen, China). Rabbit polyclonal antibodies
against β-actin (20536–1-AP), Survivin (0508-1-AP), protein kinase
B (AKT; 10176-2-AP) and extracellular-signal-regulated kinase (ERK;
16443-1-AP) were purchased from Proteintech Wuhan Sanying
Biotechnology (Wuhan, China). Rabbit polyclonal antibodies against
Bcl-2-associated X-protein (Bax; D220073), B-cell lymphoma 2
(Bcl-2; D160117), cyclin-dependent kinase 4 (CDK4; D220396) and
proliferating cell nuclear antigen (PCNA; D120014) were purchased
from Sangon Biotech Co., Ltd. (Shanghai, China). Rabbit polyclonal
antibodies against Cyclin D1 (sc-753), phospho-AKT (p-AKT;
sc-135650) and phospho-ERK (p-ERK; sc-16982-R) were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Horseradish
peroxidase (HRP)-conjugated goat anti-rabbit (E030120-01) was
purchased from Earthox LLC (Millbrae, CA, USA). Culture flasks and
plates were purchased from NEST Biotechnology Co., Ltd. (Wuxi,
Jiangsu, China). All other chemicals used, unless otherwise stated,
were purchased from Sigma Aldrich; Merck KGaA (Darmstadt,
Germany).
Preparation of the HDW extract
HDW was purchased from the Guo Yi Tang Chinese
Herbal Medicine Store (Fujian, China). Using a procedure described
previously (18), 500 g HDW was
extracted. A series of solvents were applied including chloroform,
petroleum ether, n-butanol and ethyl acetate resulting in the
chloroform extract of HDW (CEHDW), the petroleum ether extract of
HDW (PEEHDW), the n-butanol extract of HDW (NBEHDW) and the ethyl
acetate extract of HDW (EAEHDW), respectively. All extracts were
separately evaporated on a rotary evaporator. Subsequently, powders
of the extracts were dissolved in 100% dimethyl sulfoxide (DMSO) to
a stock concentration of 100 mg/ml and stored at −20°C. The final
concentration of DMSO in the medium for all experiments was
<0.5%.
Cell culture
The human colorectal cancer cell lines SW620, HT-29,
HCT116 and HCT-8 were purchased from the Cell Bank of the Chinese
Academy of Sciences (Shanghai, China). SW620 and HT-29 cells were
cultured in DMEM supplemented with 10% (v/v) FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin. HCT116 and HCT-8 cells were
cultured in RPMI-1640 medium supplemented with 10% (v/v) FBS, 100
U/ml penicillin and 100 µg/ml streptomycin. All of the cell lines
were cultured at 37°C in a humidified incubator containing 5%
CO2.
Cell viability evaluation
An MTT assay was used to assess the cell viability.
SW620, HT-29, HCT116 and HCT-8 cells were incubated in 96-well
plates at a density of 1×105 cells/ml in 100 µl culture
medium for 12 h and treated with various concentrations (0, 150,
300 and 500 µg/ml) of HDW extract for 24 h at 37°C. In addition,
SW620 cells were treated with various concentrations (0, 12.5, 25,
50, 75 and 100 µg/ml) of CEHDW for 24 h at 37°C in an additional
MTT assay. Subsequently, the medium was replaced with 100 µl MTT
(0.5 mg/ml in PBS) and cells were incubated at 37°C. After 4 h, the
MTT solution was removed and 100 µl DMSO was added to solubilize
the purple-blue formazan precipitate. The resulting absorbance was
measured at 570 nm using an ELISA reader (model ELX800; BioTek
Instruments, Inc., Winooski, VT, USA).
Colony formation assay
SW620 cells were seeded into 6-well plates at a
density of 3×105 cells/ml in 2 ml culture medium.
Following treatment with various concentrations (0, 50, 75 and 100
µg/ml) of CEHDW for 24 h at 37°C, cells were harvested and diluted
with fresh medium without CEHDW, and subsequently reseeded in
6-well plates at a density of 1,000 cells/well in 2 ml. The medium
was replaced with fresh medium every 4 days. After 10 days,
colonies were fixed with 10% formaldehyde for 15 min at room
temperature, stained with 0.01% crystal violet for 10 min at room
temperature and photographed with digital camera.
CFDA-SE cell proliferation assay
The CFDA-SE probe was used to determine the cell
proliferation (19). Briefly, SW620
cells were stained with 5 µM CFDA-SE for 30 min at 37°C in the dark
then seeded in 12-well plates at a density of 2.5×105
cells/ml in 1 ml medium according to the manufacturer's protocol.
Subsequently, cells were exposed to a series of concentrations of
CEHDW for 24 h. CFDA-SE fluorescence was detected using a
FACSCaliber instrument (BD Biosciences, San Jose, CA, USA).
Analysis of apoptosis using annexin V
and propidium iodide (PI) double staining
SW620 cells were treated with various concentrations
(0, 50, 75 and 100 µg/ml) of CEHDW for 24 h at 37°C. Cell apoptosis
was determined using flow cytometry. Annexin V/PI staining was
performed prior to analysis using a FACSCaliber instrument,
according to the manufacturer's protocol. In this assay, the
annexin V/PI double-negative population indicates viable cells, and
the annexin V-positive/PI-negative or annexin V/PI double-positive
population represents cells undergoing early or late apoptosis,
respectively.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
SW620 cells were treated with various concentrations
(0, 50, 75 and 100 µg/ml) of CEHDW for 24 h at 37°C. RNA from cell
samples was isolated with RNAiso Plus. Oligo-dT-primed RNA (1 µg)
was reverse-transcribed using PrimeScript RT Reagent kit with gDNA
Eraser (Perfect Real Time), according to the manufacturer's
protocol. The resultant cDNA was used to determine the amount of
Survivin, PCNA, Cyclin D1, CDK4, Bcl-2 and Bax mRNA using PCR with
DreamTaq Green PCR Master Mix. PCR was performed using the 3-step
method, with a denaturation stage at 95°C for 30 sec, an annealing
stage at an appropriate temperature (55°C for Survivin, CDK4, Bcl-2
and Bax, and 58°C for PCNA, Cyclin D1 and GAPDH) for 30 sec and an
extension stage at 72°C for 30 sec for 30 cycles. GAPDH was used as
an internal control. The primers were synthesized by Invitrogen;
Thermo Fisher Scientific, Inc., and the sequences are listed in
Table I.
 | Table I.Primer sequences for reverse
transcription-polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-polymerase chain reaction.
| Gene | Primer sequence
(5′-3′) | Product size, bp |
|---|
| Survivin | Forward,
5′-CTGGGCTATGGGTGAGGTTC-3′ | 686 |
|
| Reverse,
5′-CCCTAGAATCAGACAGCCGAC-3′ |
|
| PCNA | Forward,
5′-GCTGACATGGGACACTTA-3′ | 165 |
|
| Reverse,
5′-CTCAGGTACAAACTTGGTG-3′ |
|
| Cyclin D1 | Forward,
5′-TGGATGCTGGAGGTCTGCGAGGAA-3′ | 573 |
|
| Reverse,
5′-GGCTTCGATCTGCTCCTGGCAGGC-3′ |
|
| CDK4 | Forward,
5′-GGTCAAAGATTTTGCCCAAC-3′ | 138 |
|
| Reverse,
5′-CCGAAGTTCTTCTGCAGTCC-3′ |
|
| Bcl-2 | Forward,
5′-CAGCTGCACCTGACGCCCTT-3′ | 231 |
|
| Reverse,
5′-GCCTCCGTTATCCTGGATCC-3′ |
|
| Bax | Forward,
5′-TGCTTCAGGGTTTCATCCAGG-3′ | 276 |
|
| Reverse,
5′-TGGCAAAGTAGAAAAGGGCGA-3′ |
|
| GAPDH | Forward,
5′-CGACCACTTTGTCAAGCTCA-3′ | 228 |
|
| Reverse,
5′-AGGGGTCTACATGGCAACTG-3′ |
|
Western blot analysis
SW620 cells were treated with various concentrations
(0, 50, 75 and 100 µg/ml) of CEHDW for 24 h at 37°C. Cells were
washed with PBS three times and lysed with RIPA lysis buffer
containing EASYpack protease inhibitor cocktail (Roche Diagnostics,
Basel, Switzerland) and PhosSTOP (Roche Diagnostics). The
concentrations of resultant protein were quantified using the BCA
Protein Assay kit. Proteins (50 µg) were separated by SDS-PAGE and
transferred onto nitrocellulose membranes (EMD Millipore
Corporation, Darmstadt, Germany). The membranes were blocked with
blocking buffer for 2 h at room temperature and incubated with
antibodies against Survivin, PCNA, Cyclin D1, CDK4, Bcl-2, Bax,
AKT, ERK, p-AKT, p-ERK or β-actin (all 1:1,000 dilution) for 16 h
at 4°C, and then washed three times with Tris-buffered saline with
Tween-20 (TBST), respectively. Subsequently, the membranes were
incubated with HRP-conjugated goat anti-rabbit secondary antibodies
for 1 h at room temperature. Cells were washed again in TBST and
membranes were visualized using a BeyoECL Plus instrument. Image
Lab™ software (version 3.0; Beyotime Institute of Biotechnology)
was used for densitometric analysis and quantification of western
blots.
Statistical analysis
Data were analyzed using the SPSS package for
Windows (version 17.0; SPSS Inc., Chicago, IL, USA) using one-way
analysis of variance. Fisher's least significant difference and
Dunnett's test were used as post-hoc tests. P<0.05 was
considered to indicate a statistically significant difference.
Results
CEHDW exhibits the most potent
inhibitory effect on the viability of colorectal cancer cells
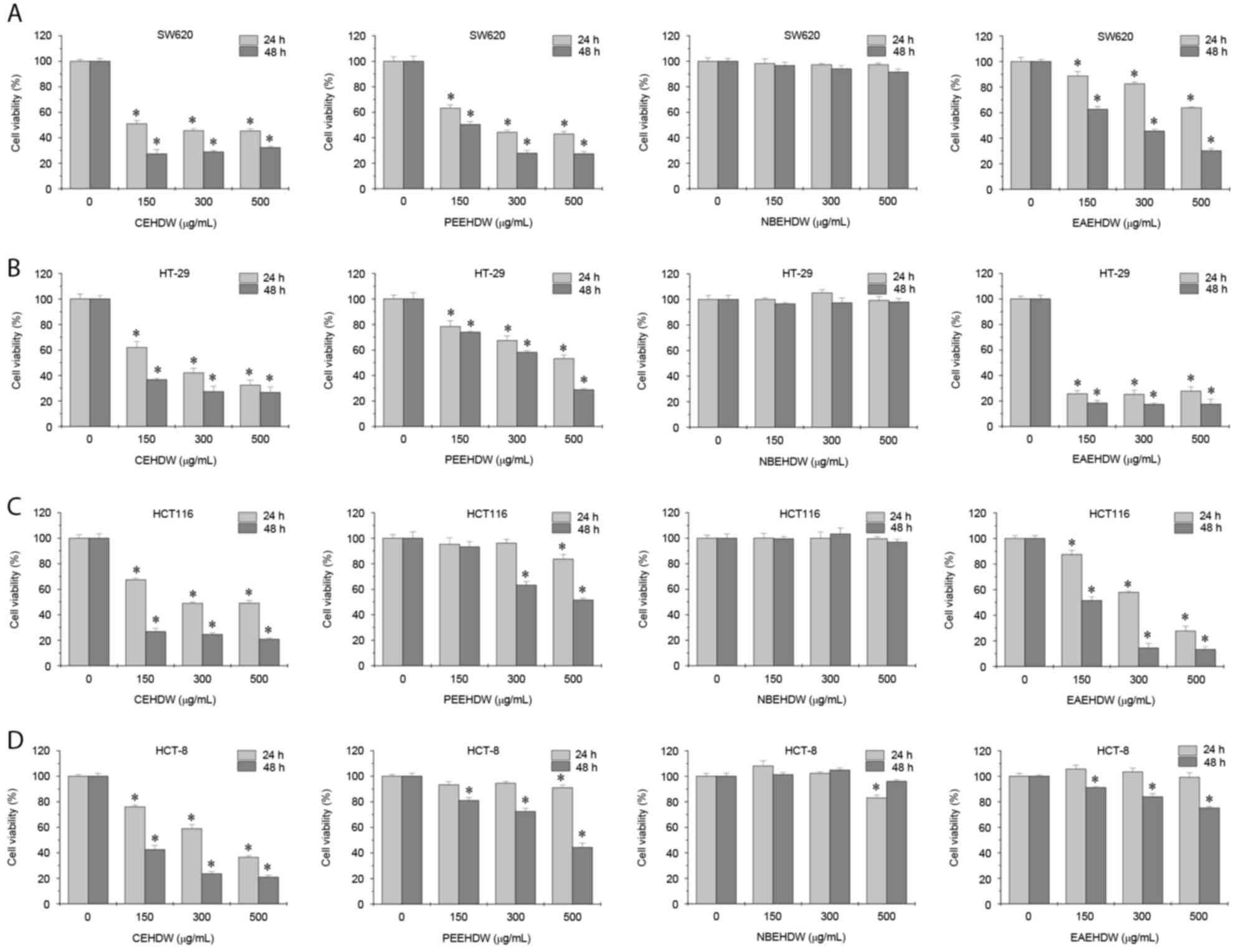
The inhibitory effects of CEHDW, PEEHDW, NBEHDW and
EAEHDW on the viability of the CRC cell lines were determined using
an MTT assay. As presented in Fig.
1A-D, the four cell lines were exposed to various
concentrations of the four extracts for 24 or 48 h. CEHDW exhibited
the most antitumor activity in all cell lines. NBEHDW exhibited no
significant inhibitory effects. PEEHDW decreased the cell viability
of SW620 and HT-29 cells, but exhibited a limited effect on HCT116
and HCT-8 cells. Similarly, EAEHDW significantly inhibited the
viability of SW620, HT-29 and HCT116 cells, but not HCT-8
cells.
CEHDW inhibits the viability of SW620
cells
Following CEHDW treatment, SW620 demonstrated the
most drug sensitivity as presented in Fig. 1A. Therefore, the SW620 cell line was
selected for further study. In order to evaluate the effect of
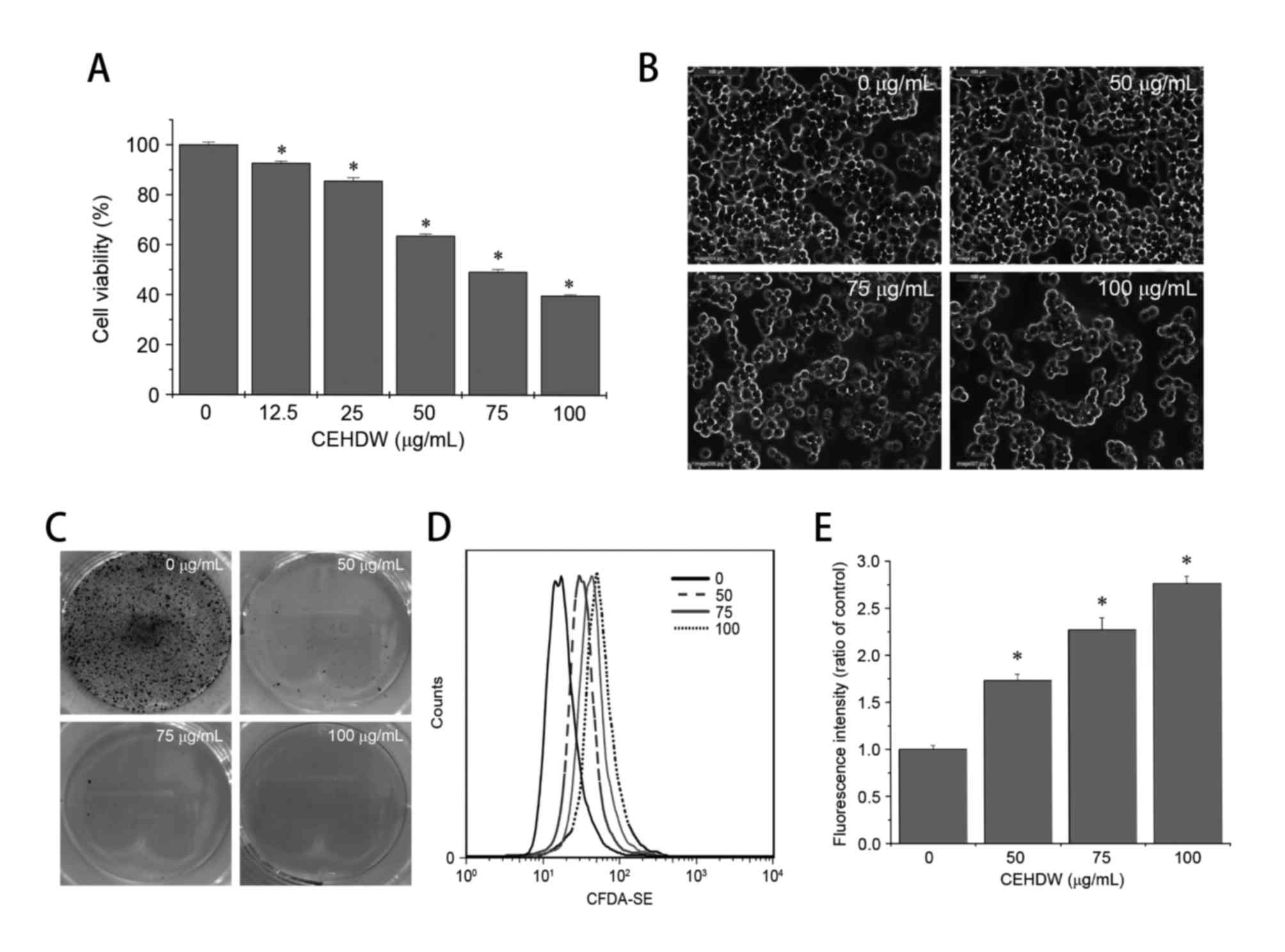
CEHDW on SW620 cells, viability was determined using an MTT assay.
Treatment with between 12.5 and 100 µg/ml CEHDW for 24 h decreased
cell viability by between 7.3 and 60.23%, when compared with
untreated cells (Fig. 2A). To observe
the effects of CEHDW on cell morphology, the appearance of the
treated and untreated SW620 monolayers were compared using
phase-contrast microscopy. Untreated SW620 cells appeared as a
crowded and disorganized monolayer after 24 h (Fig. 2B). The cell density was decreased in
the confluent monolayers that had been treated with CEHDW, with the
attached cells exhibiting a round appearance (Fig. 2B). To estimate the effect of CEHDW on
cell survival, SW620 cells were examined by performing the colony
formation assay. The number of colonies was decreased following
CEHDW treatment, and almost no colonies were observed following
treatment with CEHDW at concentrations of 50, 75 and 100 µg/ml for
24 h (Fig. 2C). To determine the
effect of CEHDW on cell proliferation, a CFDA-SE assay was
performed. As presented in Fig. 2D and
E, the ratio of fluorescence intensity of cells significantly
increased following treatment with CEHDW (50, 75 and 100 µg/ml) for
24 h was 1.73, 2.27 and 2.77, respectively, compared with that of
the control group. These results suggest that CEHDW is potent in
suppressing the proliferation of SW620 cells.
CEHDW promotes apoptosis of SW620
cells
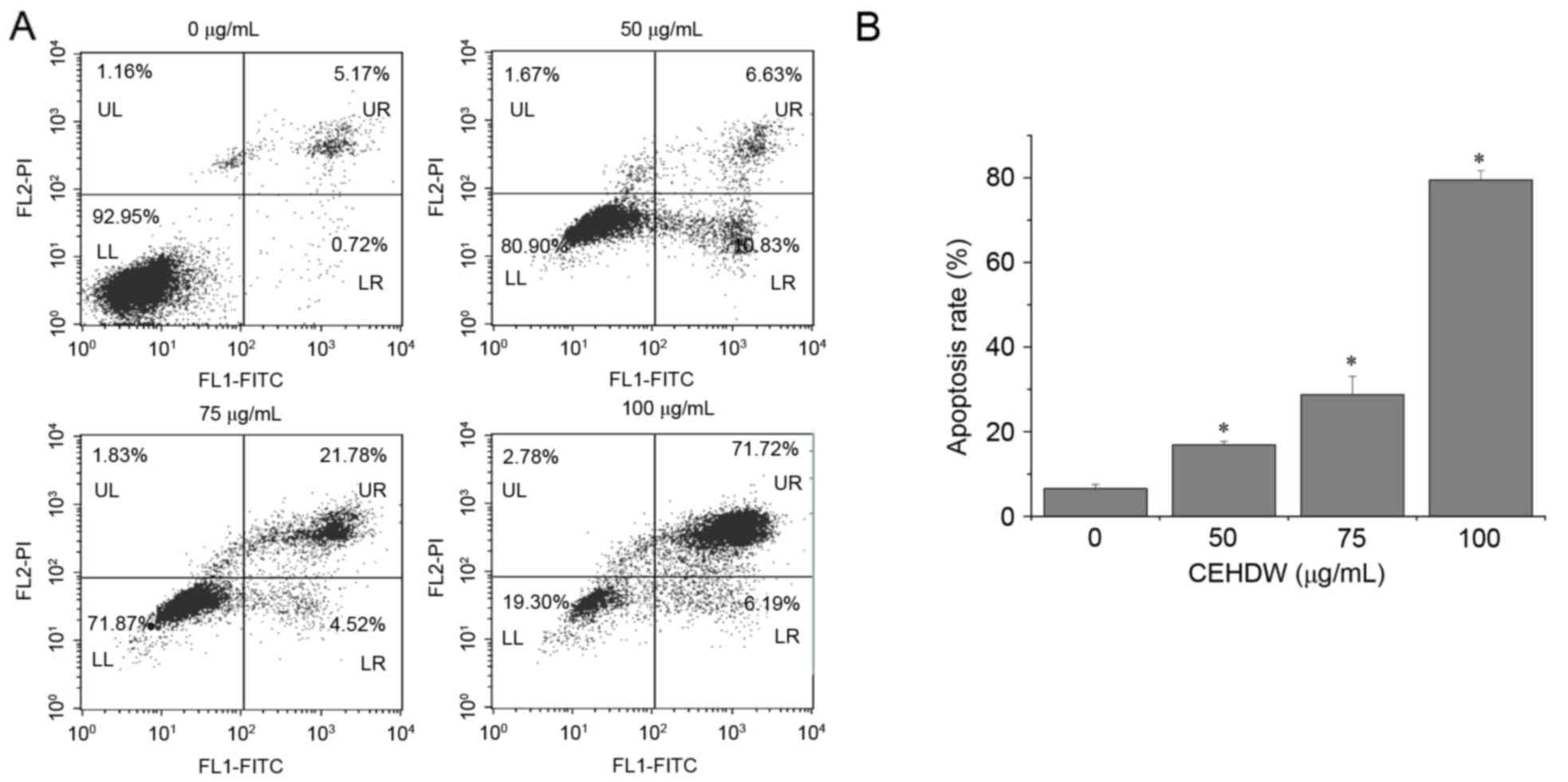
In order to investigate the underlying molecular
mechanism of the proliferation suppressing activity of CEHDW, its
effect on apoptosis in SW620 cells was assessed using annexin V/PI
staining followed by FACS analysis. As presented in Fig. 3A and B, the proportion of cells
undergoing either early apoptosis or late apoptosis following
treatment with 0, 50, 75 and 100 µg/ml CEHDW was 6.58, 16.86, 28.75
and 79.47%, respectively, suggesting that CEHDW treatment induces
apoptosis in SW620 cells in a dose-dependent manner.
CEHDW regulates the expression of
Survivin, PCNA, Cyclin D1, CDK4, Bcl-2 and Bax in SW620 cells
To further explore the underlying molecular
mechanism of the proliferation inhibition effect of CEHDW, RT-PCR
and western blot analysis were performed to determine the
expression of Survivin, PCNA, Cyclin D1, CDK4, Bcl-2 and Bax at the
mRNA and protein levels. RT-PCR results revealed that CEHDW
treatment decreased mRNA expression of the pro-proliferative PCNA,
Cyclin D1 and CDK4 and anti-apoptotic Bcl-2 and Survivin, while
also increasing expression of the pro-apoptotic Bax (Fig. 4A and B). The protein expression
patterns of Survivin, PCNA, Cyclin D1, CDK4, Bcl-2 and Bax were
similar to that observed for the respective mRNA (Fig. 4C and D).
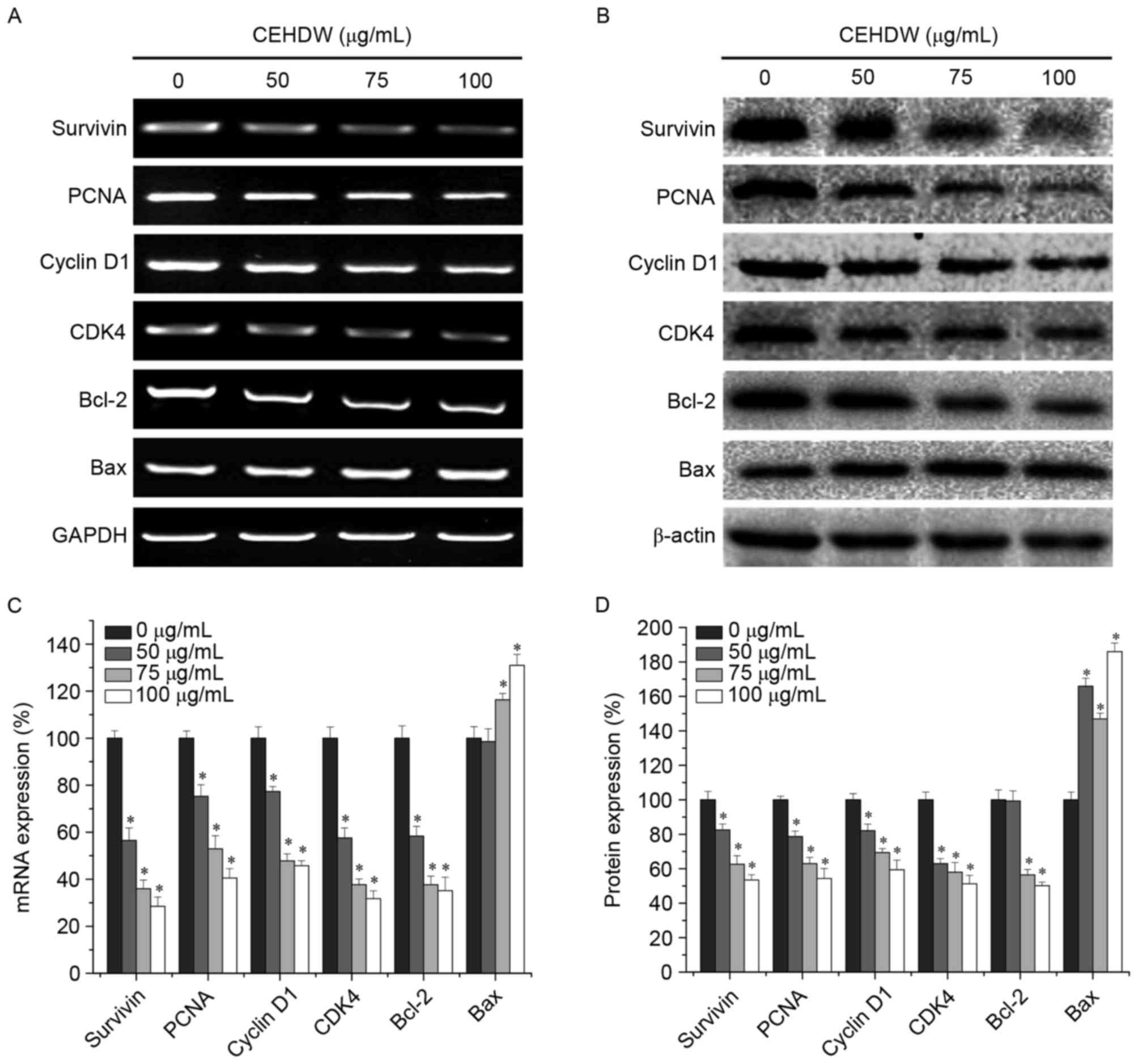 | Figure 4.CEHDW treatment regulates expression
of Survivin, PCNA, cyclin D1, CDK4, Bcl-2 and Bax in SW620 cells.
Cells were treated with various concentrations of CEHDW for 24 h.
(A) mRNA expression and (B) protein expression levels of Survivin,
PCNA, Cyclin D1, CDK4, Bcl-2 and Bax were evaluated by RT-PCR and
Western blot analysis, respectively. GAPDH and β-actin were used as
the internal controls for the RT-PCR and western blotting,
respectively. Densitometric analysis. The data were normalized to
the mean (C) mRNA or (D) protein expression of untreated control
(100%). *P<0.05 vs. internal controls. CEHDW, chloroform extract
of Hedyotis diffusa Willd; PCNA, proliferating cell nuclear
antigen; CDK4, cyclin-dependent kinase 4; Bcl-2, B-cell lymphoma 2;
Bax, Bcl-2-associated X protein; RT-PCR, reverse
transcription-polymerase chain reaction. |
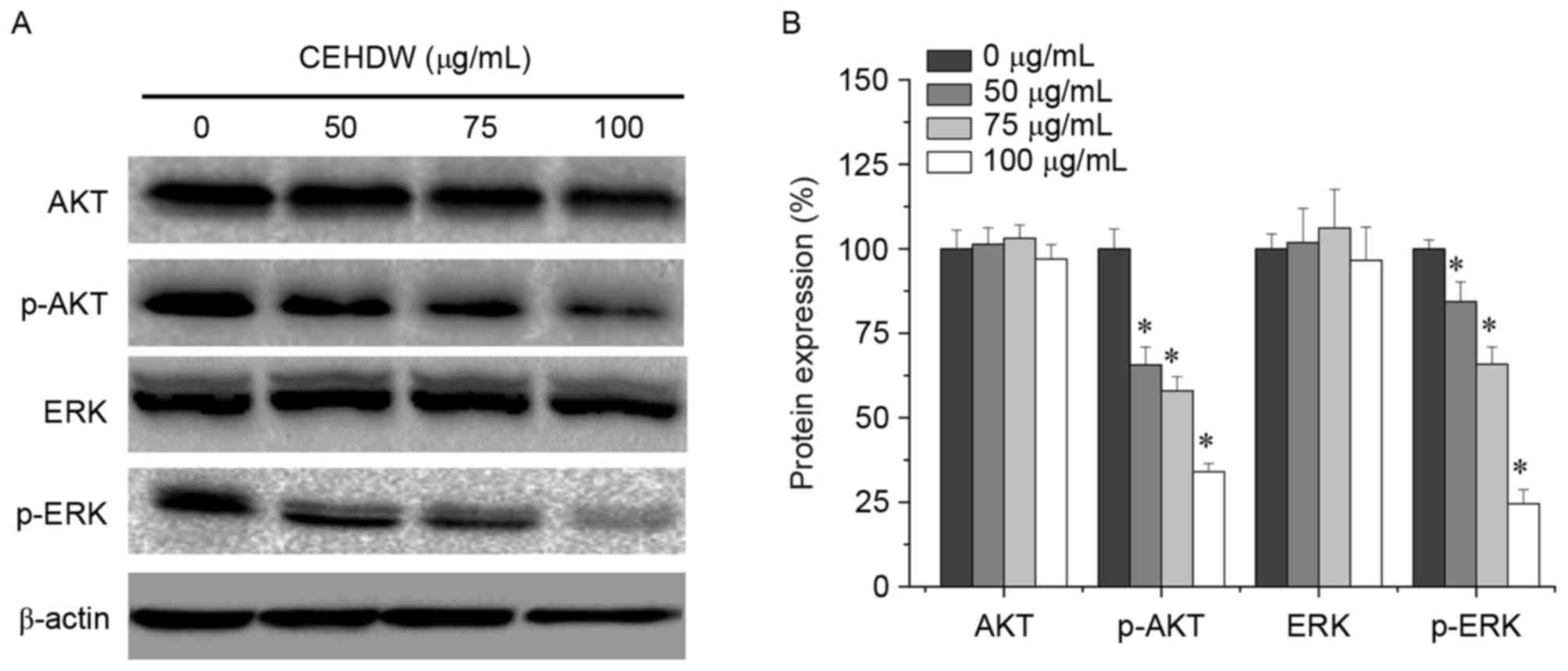
CEHDW inhibits AKT and ERK
phosphorylation in SW620 cells
To gain further insight into the association between
CEHDW and the proliferation and apoptosis of CRC cells, AKT and ERK
signaling molecules were investigated. As presented in Fig. 5A and B, AKT and ERK phosphorylation
were decreased following treatment with 50, 75 and 100 µg/ml CEHDW
for 24 h, indicating that the AKT and ERK signaling pathways may be
involved in CRC cell apoptosis.
Discussion
As a multi-component herb, the active ingredients of
HDW are distinct between various extracts due to different
polarity. Ursolic acid (UA) and oleanolic acid (OA) are
hypothesized to be the major active ingredients of HDW and have
been demonstrated to possess anticancer activity (20–24). It
has been reported that, among CEHDW, EAEHDW and NBEHDW, the highest
levels of UA or OA were observed in CEHDW, which may explain the
discrepancy in the therapeutic effect of different extracts
(25). To verify this hypothesis, in
the present study various organic solvents were used for HDW
extracts, and their anticancer effects were compared. MTT assays
revealed that CEHDW exhibited the most potent anticancer activity
in the CRC cell lines, but whether this was due to the higher
content of UA or OA remains unknown and requires further
investigation.
The unlimited proliferation and apoptosis resistance
of cancer cells facilitates the continuous growth and progression
of tumors (26). The current clinical
therapies including resection and radio- or chemotherapies aim to
remove the majority of solid tumors and to suppress proliferation
of the remaining cancer cells, and therefore cure cancer. However,
they often fail with recurrence, metastasis, drug resistance and
other side effects occurring in patients. The inhibition of
proliferation and promotion of apoptosis remain the standard
approach of anticancer therapy. For improved therapeutic effect and
higher quality of life, multi-target therapy should be emphasized,
and the majority of TCMs including HDW exhibit advantages in this
respect (27,28). HDW has been reported to be clinically
effective with few side effects (5).
In the present study using MTT assays, colony formation assays,
CFDA-SE and annexin V/PI staining, it was established that the
anticancer effect of CEHDW is primarily achieved through the
inhibition of proliferation and promotion of apoptosis.
Numerous disordered genes and aberrant activation of
signaling pathways regulate the growth of cancer. For instance,
Survivin is a protein that is able to block apoptosis to prevent
cell death and prolong cell survival (29). PCNA is a specific marker of cell
division associated with DNA polymerase, synthesized shortly prior
to the S-phase of the cell cycle (30). Cyclin D1 regulates the cell
proliferation through the phosphorylation and inhibition of pocket
proteins by forming an active complex with CDK4 (31). Apoptosis is largely controlled by
Bcl-2 family members, including Bcl-2 and Bax. In particular, the
ERK and AKT signaling pathways are involved in the regulation of
CRC cell apoptosis (32,33); therefore, a rebalance of cell
apoptosis and proliferation by the regulation of AKT and ERK
signaling pathways and the expression of other associated genes is
a promising target for the development of anticancer therapies. In
the present study, using CRC SW620 cells, it was identified that
CEHDW decreased the phosphorylation activation of AKT and ERK,
decreased the expression of Bcl-2, Survivin, PCNA, Cyclin D1 and
CDK4, and increased the expression of Bax.
The results of the present study demonstrate that
CEHDW exhibits a potent inhibitory effect on CRC cell growth, which
is mediated by its pro-apoptotic and anti-proliferative activity.
Furthermore, the effect of CEHDW is mediated through the AKT and
ERK signaling pathways (Fig. 6).
These results provide a strong scientific foundation for the
development of novel anticancer agents from the bioactive
ingredients in CEHDW. HDW may execute anticancer effects by
regulating multiple targets and signaling pathways. Elucidation of
the complete underlying molecular mechanism requires further in
vitro and in vivo investigation.
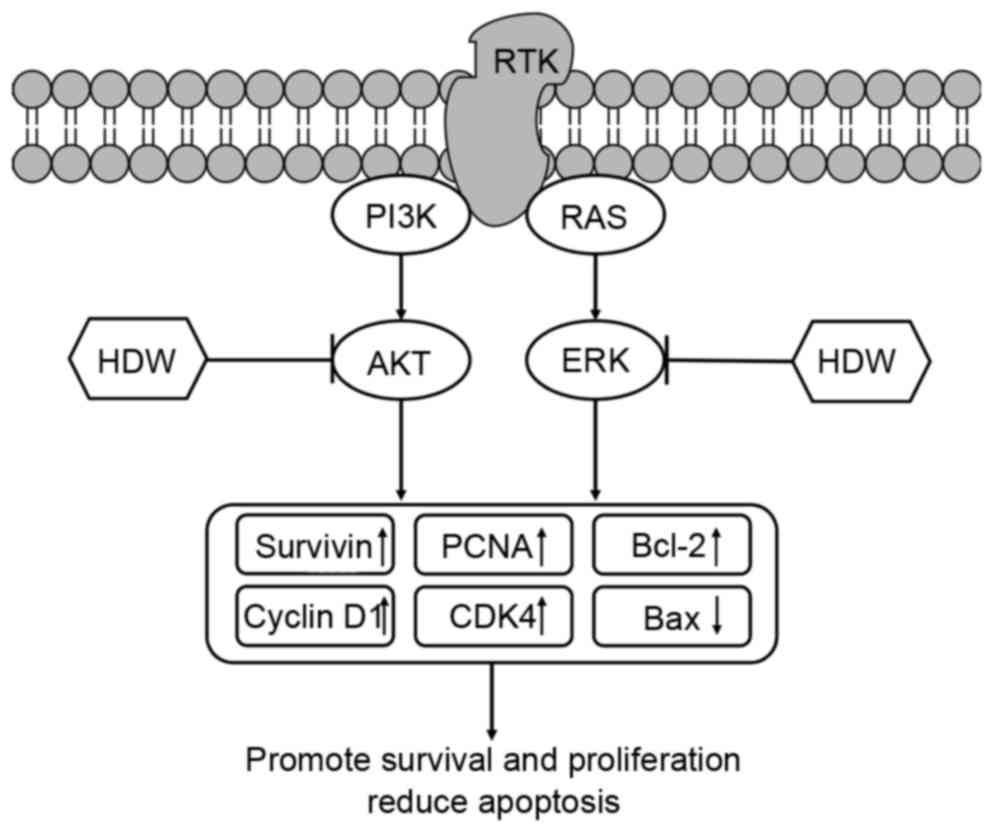 | Figure 6.Schematic diagram of the potential
underlying molecular mechanisms of CEHDW inhibiting proliferation
of SW620 cells. HDW inhibits AKT and ERK phosphorylation, which
subsequently upregulates Survivin, PCNA, Cyclin D1, CDK4, Bcl-2 and
downregulates Bax, promoting survival and proliferation and
decreasing apoptosis of SW620 cells. CEHDW, chloroform extract of
Hedyotis diffusa Willd; HDW, Hedyotis diffusa Willd;
AKT, protein kinase B; ERK, extracellular-signal-regulated kinase;
PCNA, proliferating cell nuclear antigen; CDK4, cyclin-dependent
kinase 4; BCL-2, B-cell lymphoma-2; BAX, Bcl-2-associated X
protein; PI3K, phosphoinositide 3-kinase; RTK, receptor tyrosine
kinase. |
Acknowledgements
The present study was supported by the Research Fund
for the Doctoral Program of Higher Education of China (grant no.
20133519110003), Project Funding for the Training of Young and
Middle-aged Backbone Personnel of Fujian Provincial Health and
Family Planning Commission (grant no. 2016-ZQN-67) and the
Developmental Fund of Chen Keji Integrative Medicine (grant nos.
CKJ2014013 and CKJ2015007).
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
CEHDW
|
chloroform extract of Hedyotis diffusa
Willd
|
|
TCM
|
traditional Chinese medicine
|
|
AKT
|
protein kinase B
|
|
ERK
|
extracellular-signal-regulated
kinase
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McQuade RM, Bornstein JC and Nurgali K:
Anti-colorectal cancer chemotherapy-induced diarrhoea: Current
treatments and side-effects. Int J Clin Med. 05:393–406. 2014.
View Article : Google Scholar
|
|
3
|
Colorectal cancer facts and figures
2014–2016American Cancer Society. Atlanta: 2014
|
|
4
|
Sun Y, Zhao H, Guo Y, Lin F, Tang L, Yao Y
and Abba ML: Clinical study of combining chemotherapy of
oxaliplatin or 5-Fluorouracil/Leucovorin with Hydroxycamptothecine
for advanced colorectal cancer. Clin Oncol Cancer Res. 6:117–123.
2009. View Article : Google Scholar
|
|
5
|
Liu J, Wang S, Zhang Y, Fan HT and Lin HS:
Traditional Chinese medicine and cancer: History, present
situation, and development. Thorac Cancer. 6:561–569. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhuang Q, Hong F, Shen A, Zheng L, Zeng J,
Lin W, Chen Y, Sferra TJ, Hong Z and Peng J: Pien Tze Huang
inhibits tumor cell proliferation and promotes apoptosis via
suppressing the STAT3 pathway in a colorectal cancer mouse model.
Int J Oncol. 40:1569–1574. 2012.PubMed/NCBI
|
|
7
|
Chen Y, Lin Y, Li Y and Li C: Total
flavonoids of Hedyotis diffusa Willd inhibit inflammatory responses
in LPS-activated macrophages via suppression of the NF-kappaB and
MAPK signaling pathways. Exp Ther Med. 11:1116–1122. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao X, Li C, Tang YL, Zhang H and Chan SW:
Effect of Hedyotis diffusa water extract on protecting human
hepatocyte cells (LO2) from H2O2-induced cytotoxicity. Pharm Biol.
54:1148–1155. 2016.PubMed/NCBI
|
|
9
|
Kuo YJ, Lin JP, Hsiao YT, Chou GL, Tsai
YH, Chiang SY, Lin JG and Chung JG: Ethanol extract of hedyotis
diffusa Willd affects immune responses in normal Balb/c mice in
vivo. In Vivo. 29:453–460. 2015.PubMed/NCBI
|
|
10
|
Yeh YC, Chen HY, Yang SH, Lin YH, Chiu JH,
Lin YH and Chen JL: Hedyotis diffusa combined with scutellaria
barbata are the core treatment of Chinese herbal medicine used for
breast cancer patients: A population-based study. Evid Based
Complement Alternat Med. 2014:2023782014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chao TH, Fu PK, Chang CH, Chang SN, Mao
Chiahung F and Lin CH: Evidence-based Chinese medicine research
group: Prescription patterns of Chinese herbal products for
post-surgery colon cancer patients in Taiwan. J Ethnopharmacol.
155:702–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee HZ, Bau DT, Kuo CL, Tsai RY, Chen YC
and Chang YH: Clarification of the phenotypic characteristics and
anti-tumor activity of Hedyotis diffusa. Am J Chin Med. 39:201–213.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin J, Chen Y, Wei L, Chen X, Xu W, Hong
Z, Sferra TJ and Peng J: Hedyotis Diffusa Willd extract induces
apoptosis via activation of the mitochondrion-dependent pathway in
human colon carcinoma cells. Int J Oncol. 37:1331–1338.
2010.PubMed/NCBI
|
|
14
|
Lin J, Li Q, Chen H, Lin H, Lai Z and Peng
J: Hedyotis diffusa Willd. extract suppresses proliferation and
induces apoptosis via IL-6-inducible STAT3 pathway inactivation in
human colorectal cancer cells. Oncol Lett. 9:1962–1970.
2015.PubMed/NCBI
|
|
15
|
Lin J, Wei L, Shen A, Cai Q, Xu W, Li H,
Zhan Y, Hong Z and Peng J: Hedyotis diffusa Willd extract
suppresses Sonic hedgehog signaling leading to the inhibition of
colorectal cancer angiogenesis. Int J Oncol. 42:651–656. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin J, Wei L, Xu W, Hong Z, Liu X and Peng
J: Effect of hedyotis diffusa Willd extract on tumor angiogenesis.
Mol Med Rep. 4:1283–1288. 2011.PubMed/NCBI
|
|
17
|
Lin M, Lin J, Wei L, Xu W, Hong Z, Cai Q,
Peng J and Zhu D: Hedyotis diffusa Willd extract inhibits HT-29
cell proliferation via cell cycle arrest. Exp Ther Med. 4:307–310.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang L, Cai Q, Lin J, Fang Y, Zhan Y,
Shen A, Wei L, Wang L and Peng J: Chloroform fraction of
Scutellaria barbata D. Don promotes apoptosis and suppresses
proliferation in human colon cancer cells. Mol Med Rep. 9:701–706.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhong ZF, Tan W, Wang SP, Qiang WA and
Wang YT: Anti-proliferative activity and cell cycle arrest induced
by evodiamine on paclitaxel-sensitive and -resistant human ovarian
cancer cells. Sci Rep. 5:164152015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Furtado RA, Rodrigues EP, Araujo FR,
Oliveira WL, Furtado MA, Castro MB, Cunha WR and Tavares DC:
Ursolic acid and oleanolic acid suppress preneoplastic lesions
induced by 1,2-dimethylhydrazine in rat colon. Toxicol Pathol.
36:576–580. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Guo WJ and Yang QY: Effects of
ursolic acid and oleanolic acid on human colon carcinoma cell line
HCT15. World J Gastroenterol. 8:493–495. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin J, Chen Y, Wei L, Shen A, Sferra TJ,
Hong Z and Peng J: Ursolic acid promotes colorectal cancer cell
apoptosis and inhibits cell proliferation via modulation of
multiple signaling pathways. Int J Oncol. 43:1235–1243. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Prasad S, Yadav VR, Sung B, Reuter S,
Kannappan R, Deorukhkar A, Diagaradjane P, Wei C,
Baladandayuthapani V, Krishnan S, et al: Ursolic acid inhibits
growth and metastasis of human colorectal cancer in an orthotopic
nude mouse model by targeting multiple cell signaling pathways:
Chemosensitization with capecitabine. Clin Cancer Res.
18:4942–4953. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kassi E, Papoutsi Z, Pratsinis H,
Aligiannis N, Manoussakis M and Moutsatsou P: Ursolic acid, a
naturally occurring triterpenoid, demonstrates anticancer activity
on human prostate cancer cells. J Cancer Res Clin Oncol.
133:493–500. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Chen D, Lin J and Peng J: Analysis
the difference chemical constituents among the different solvent
extracts from Hedyotis Diffusa Willd. Fujian Analysis &
Testing. 8–12. 2015.(in Chinese).
|
|
26
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X, Feng Y, Wang N, Cheung F, Tan HY,
Zhong S, Li C and Kobayashi S: Chinese medicines induce cell death:
The molecular and cellular mechanisms for cancer therapy. Biomed
Res Int. 2014:5303422014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li-Weber M: Targeting apoptosis pathways
in cancer by Chinese medicine. Cancer Lett. 332:304–312. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Casati C, Dalerba P, Rivoltini L, Gallino
G, Deho P, Rini F, Belli F, Mezzanzanica D, Costa A, Andreola S, et
al: The apoptosis inhibitor protein survivin induces tumor-specific
CD8+ and CD4+ T cells in colorectal cancer patients. Cancer Res.
63:4507–4515. 2003.PubMed/NCBI
|
|
30
|
Guzinska-Ustymowicz K, Pryczynicz A,
Kemona A and Czyzewska J: Correlation between proliferation
markers: PCNA, Ki-67, MCM-2 and antiapoptotic protein Bcl-2 in
colorectal cancer. Anticancer Res. 29:3049–3052. 2009.PubMed/NCBI
|
|
31
|
Muntean AG, Pang L, Poncz M, Dowdy SF,
Blobel GA and Crispino JD: Cyclin D-Cdk4 is regulated by GATA-1 and
required for megakaryocyte growth and polyploidization. Blood.
109:5199–5207. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ye Q, Cai W, Zheng Y, Evers BM and She QB:
ERK and AKT signaling cooperate to translationally regulate
survivin expression for metastatic progression of colorectal
cancer. Oncogene. 33:1828–1839. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feng M, Li J, Wang J, Ma C, Jiao Y, Wang
Y, Zhang J, Sun Q, Ju Y, Gao L, et al: High glucose increases
LPS-induced DC apoptosis through modulation of ERK1/2, AKT and
Bax/Bcl-2. BMC Gastroenterol. 14:982014. View Article : Google Scholar : PubMed/NCBI
|