Introduction
Dose-escalated radiotherapy is an important
treatment measure for lung cancers (1–3). Excellent
clinical effects have been demonstrated with the treatment of
intensity-modulated radiotherapy (IMRT) in conjunction with
image-guided radiotherapy (IGRT) (4).
IMRT can improve dose conformity, but it requires longer delivery
time (5–8). Compared with IMRT, volumetric modulated
arc therapy (VMAT) has significantly improved the delivery
efficiency and treatment time (7).
IMRT has been previously compared with VMAT in
various types of cancer (7,9–19). Several
studies have suggested that VMAT produces highly conformal dose
distributions, achieves accurate dosimetric delivery and reduces
treatment time (7,10,19–23). For
lung cancer, IMRT and VMAT are currently used and compared
(4,7,10,19–21,23–25).
However, little literature exists that focuses on the comparison of
IMRT and VMAT in different types of lung cancer, peripheral lung
cancer and central lung cancer. In addition, there have been no
studies that compare IMRT and VMAT in two different cases in
central lung cancer, planning target volume (PTV) encompassing and
not-encompassing the mediastinal lymphatic drainage region. In the
present study, IMRT and VMAT plans were compared in quality
parameters and treatment efficiency in central lung cancer and
peripheral lung cancer. Three different plans were employed: Single
360° arc, part of single 360° arc and two arcs. Furthermore, IMRT
and VMAT plans were compared in central lung cancer in cases of PTV
encompassing or PTV not-encompassing the mediastinum.
Patients and methods
Patients and design
A total of 12 patients with lung cancer, who
received radical radiotherapy treatment in the First Hospital
Affiliated to Xi'an Jiao Tong University (Xi'an, China), 12
patients were enrolled in the study from August 2011 to August
2017. Patients with peripheral lung cancer and central lung cancer
were randomly selected. All patients were staged according to the
modified 1997 American Joint Committee on Cancer (AJCC) staging
system. The patient characteristics were listed in Table I. The present study was approved by
the Ethics Committee of the First Hospital Affiliated to Xi'an Jiao
Tong University (Xi'an, China) and written consent was obtained
from the patients.
 | Table I.Characteristics of the patients
(n=12). |
Table I.
Characteristics of the patients
(n=12).
|
Characteristics | Total |
|---|
| Age (years) |
|
|
Median | 54 |
|
Range | 40–68 |
| Sex (no. of
patients) |
|
|
Male | 10 |
|
Female | 2 |
| Disease stage |
|
| II | 6 |
|
IIIa | 4 |
|
IIIb | 2 |
| Central or
peripheral lung cancer (no. of patients) |
|
|
Central | 6 |
|
Peripheral | 6 |
| PTV volumes
(cm3) |
|
|
Median | 95.81 |
|
Range | 48.78–203.97 |
| PTV, planning
target volume. |
|
PTV delineation and dose
prescription
For small cell lung cancer (SCLC), PTV was designed
as Gross tumor volume (GTV) plus 1.5 cm margin, including the
ipsilateral hilum and bilateral mediastinum, and excluding the
contralateral hilum. For non-NSCLC, GTV encompassed the gross
primary tumor and metastatic lymph nodes (LNs) ≥1 cm or
hypermetabolic on PET scan. Clinical target volume (CTV) typically
encompasses the GTV plus 1–1.5 cm margin. PTV added a 0.5–0.8 cm
margin on CTV to account for set-up uncertainties and respiratory
motion. Respiratory tracking or 4D scanning allow for decreased PTV
margins. Each peripheral lung tumor was contoured by two
physicians. The PTV of central lung cancer was defined as GTV
(including primary cancer and metastatic lymph nodes) plus a margin
of 1.5 cm in lateral, anterior and craniocaudal direction, plus the
area within 2 cm of the proximal bronchial tree, which includes the
lower trachea, carina, mainstem bronchi, and the lobar bronchi.
Internal target volume (ITV) was used to encompass all mobile tumor
positions in breathing cycles for treatment accuracy. For each
patient, IMRT plans and VMAT plans were generated simultaneously
and compared. 60 Gy was prescribed as the median dose applied to
the PTV.
Treatment planning and optimizing
IMRT
The IMRT optimization was performed by applying
Direct Machine Parameter Optimization (DMPO) algorithm in our
treatment planning system (Pinnacle3; Philips Radiation Oncology
Systems, Fitchburg, MA, USA), as previously described (13). For each plan, 5 or 7 coplanar beams
were used depending on the tumor location. In the plan generation,
the maximum iterations and maximum number of all segments in the
plan optimizing were 50 and 80. The maximum monitor units (MUs) and
segments area were 5 MU and 5 cm2, respectively. Plans
were generated for the Elekta Beam Modulator with 10 MV.
Single-arc (SA)-VMAT
The VMAT planning was conducted applying the
SmartArc planning algorithm in Pinnacle3 9.2 (research version;
Philips Radiation Oncology Systems). The optimizer (single arc) was
constrained to use one single 360° arc which consisted of 90
control points. The arc was represented by 89 beams each separated
by 4°, which started at 181° and ended at 180°. The
accelerator-used automatic dose rate was selected for each
individual segment of the arc. Plans were generated with 10 MV.
Partial-arc (PA)-VMAT
The plans were optimized in the same planning system
mentioned above. A 180–200° partial arc was generated for
standardization across the studied cased with the range coinciding
with the tumor location while avoiding as much of the contralateral
lung as possible, which started between 170–180° and ended between
0–10°. The arc was represented by 44–49 beams each separated by
4°.
Dual 2PA-VMAT
The plans were generated with two partial arcs, and
each partial arc parameter was the same as the one in the
PA-VMAT.
Plan evaluation
The quality of plans was evaluated by three
radiation oncologists. Dose volume histograms (DVHs) and the
corresponding dose distributions of plans were independently
reviewed by each oncologist. A total of 95% of target volume should
be encompassed by 95% of the prescribed dose. PTV coverage was
evaluated by using Dmax, Dmin, Dmean, the heterogeneity index (HI)
and the conformality index (CI).
HI=D2%–D98%D50%
D2, D98 and D50% represent the dose of 2, 98 and 50%
target volume. Values of HI closer to 0 indicate greater dose
homogeneity within the volume of PTV, while large values indicate
more heterogeneous dose distribution.
CI=VT,refVT×VT,refVref
VT, ref = volume of target
receiving a dose equal to or greater than the reference dose,
VT = volume of target, Vref =
volume receiving a dose equal to or greater than the reference dose
(treated volume). The closer the value of CI to 1.0, the better the
dose conformity.
Statistical analysis
The SAS 21.0 software (SAS Institute Inc., Cary, NC,
USA) was used to analyze and compare the data with randomized block
design. The data of each treatment group were analyzed with
normality test (Shapiro-Wilk test) and homogeneity of variance test
(Levene test). Analysis of variance and Fisher's least significant
test (LSD-t) were used when data obeyed normal distribution and
homogeneity of variance. If the data did not obey the normal
distribution, the variance was not uniform, or the data did not
obey the normal distribution following conversion, the rank sum
test (Friedman's test, also called the M-test) was used for the
randomized block design data; if the rank sum test was
statistically significant, this indicated that the mean of multiple
populations was different or not equal. Then, Friedman's test and
multiple comparison non-parametric tests were used. P<0.05 was
considered to indicate a statistically significant difference.
Results
Dosage comparison between VMAT and
IMRT plans in peripheral lung cancer
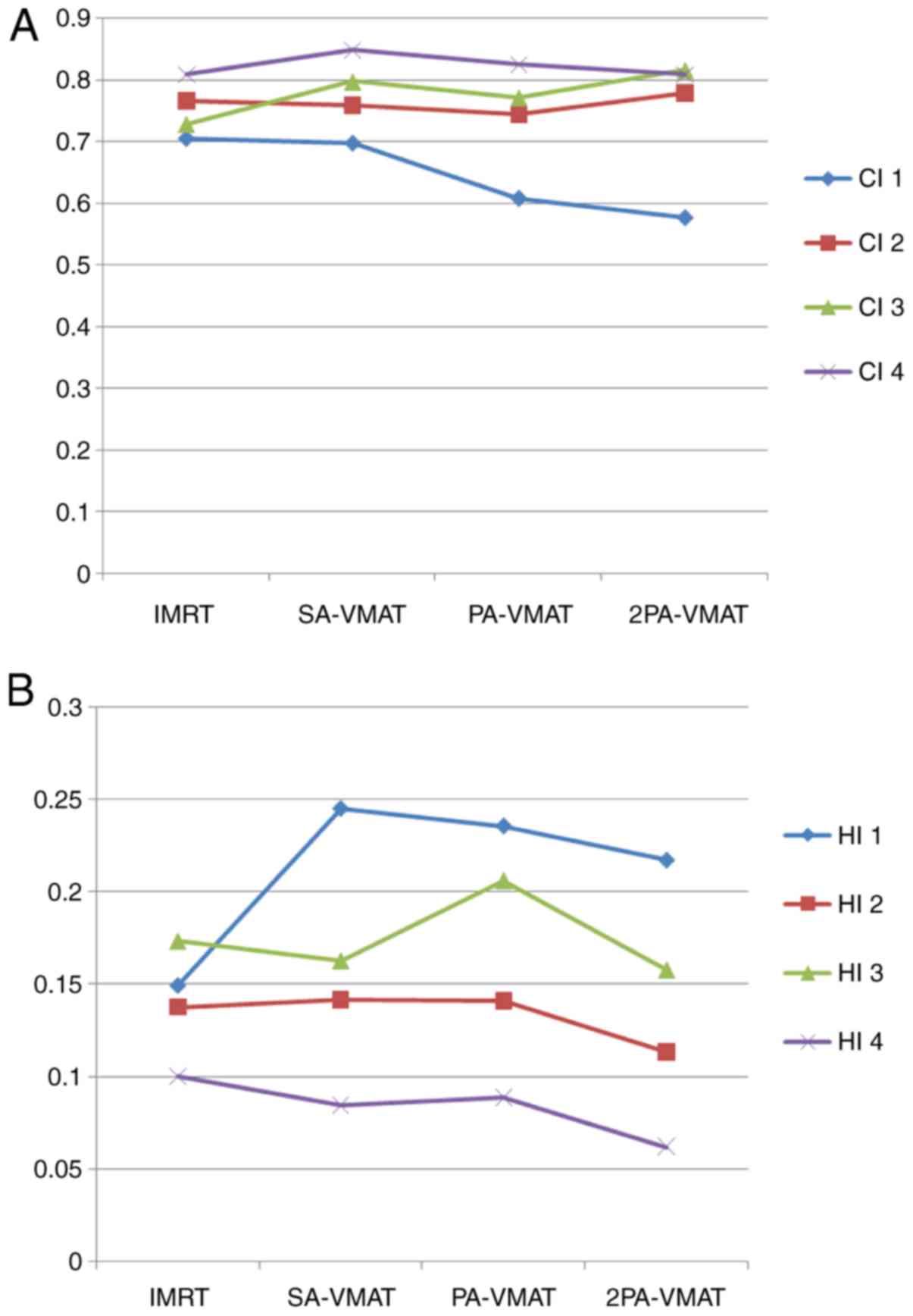
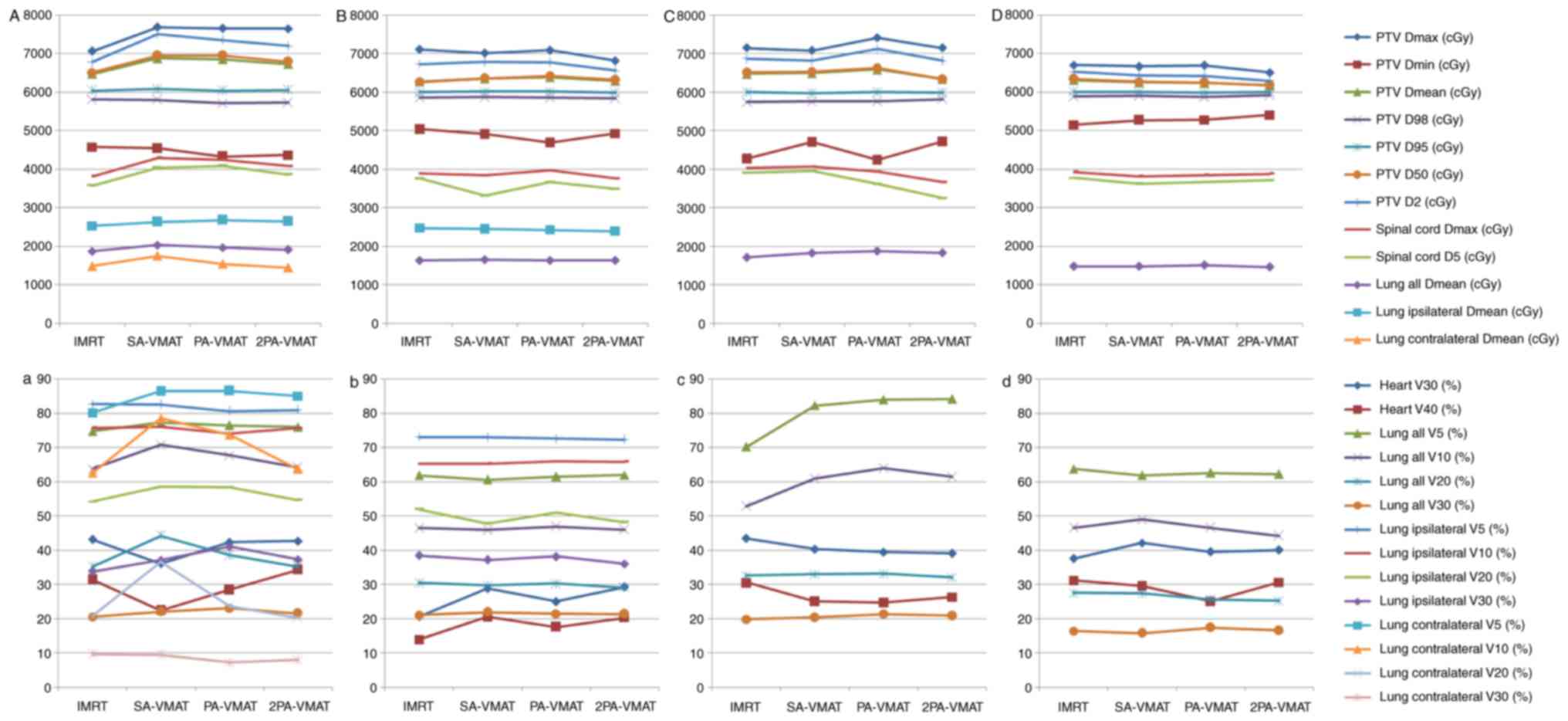
Fig. 1 illustrates the
dose-volume histogram (DVH) and dose distributions of IMRT and VMAT
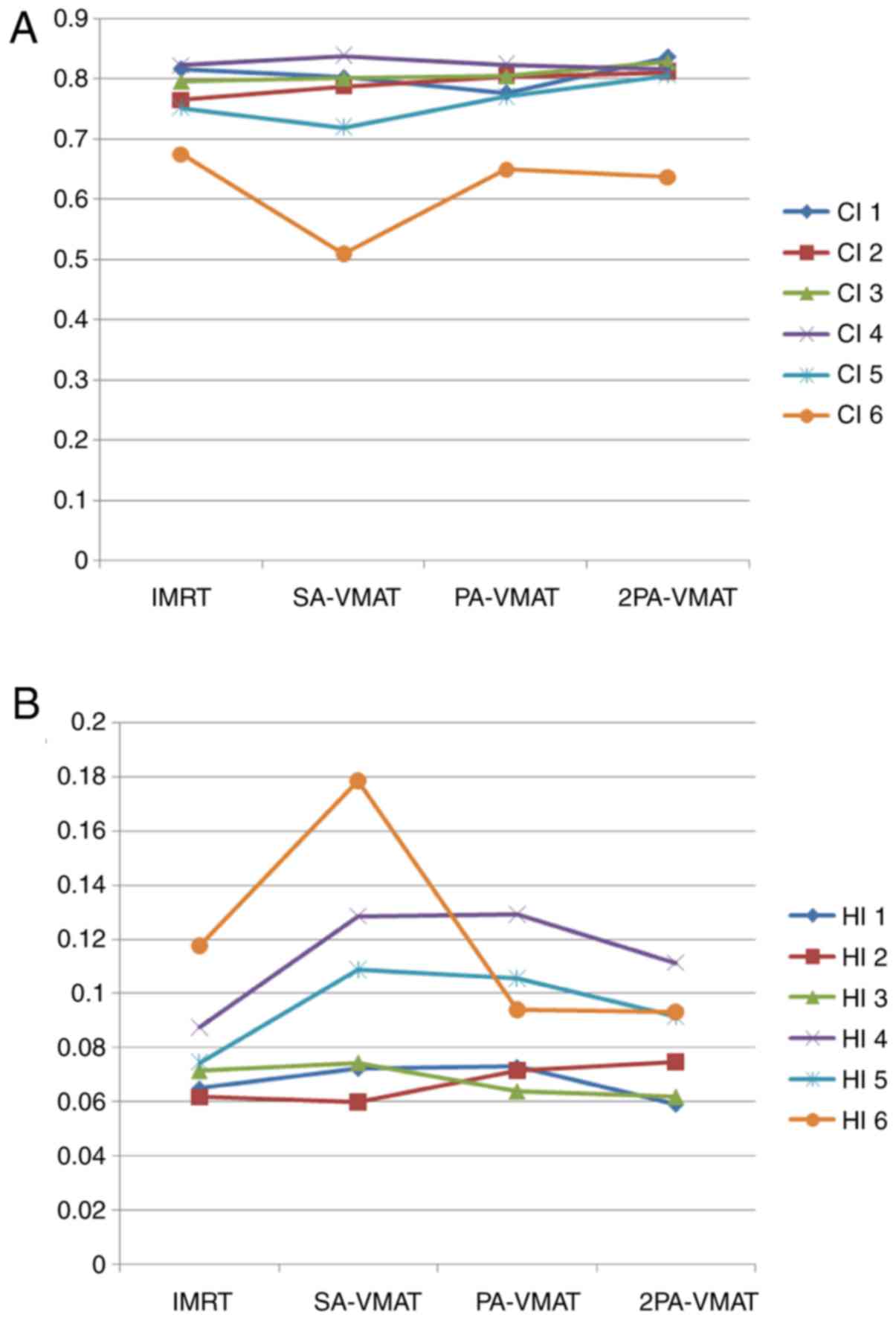
plans of a patient with peripheral lung cancer. Figs. 2 and 3
illustrate the CI, HI and organ at risk (OAR) dosage of each
patient. The average delivery times for IMRT plans and VMAT plans
were 10.5 and 6.1 min respectively. VMAT plans generated larger
numbers of MU compared to IMRT.
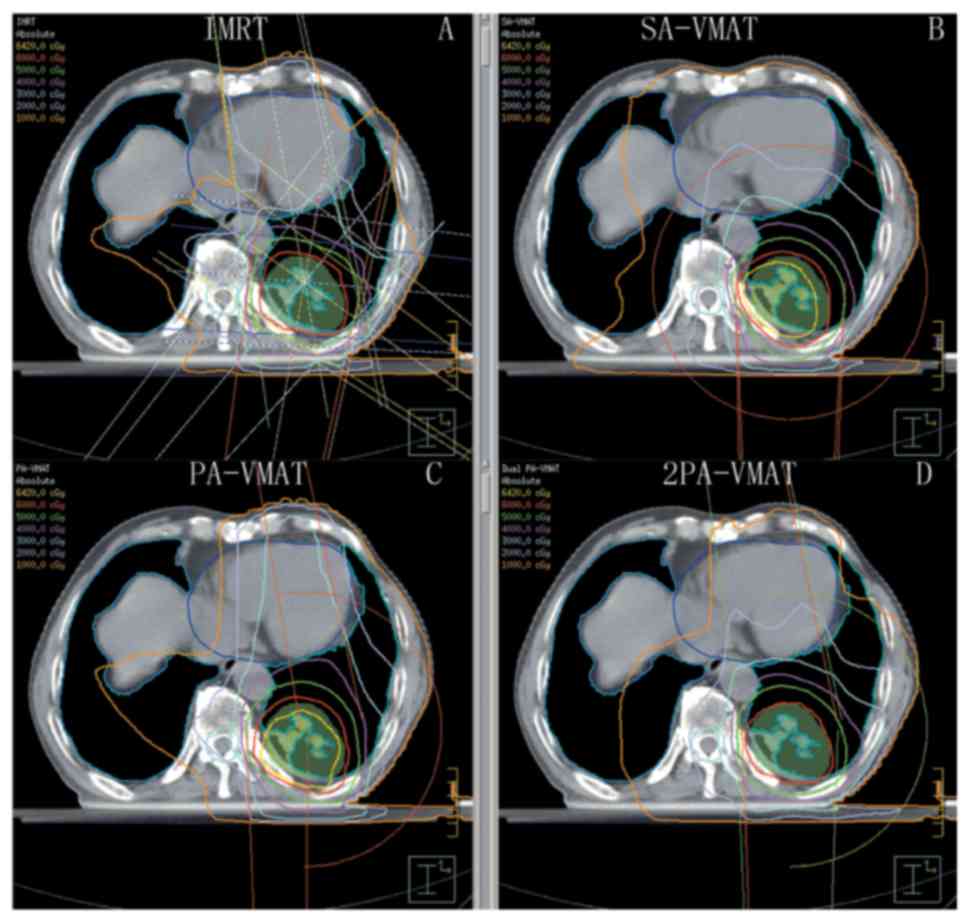
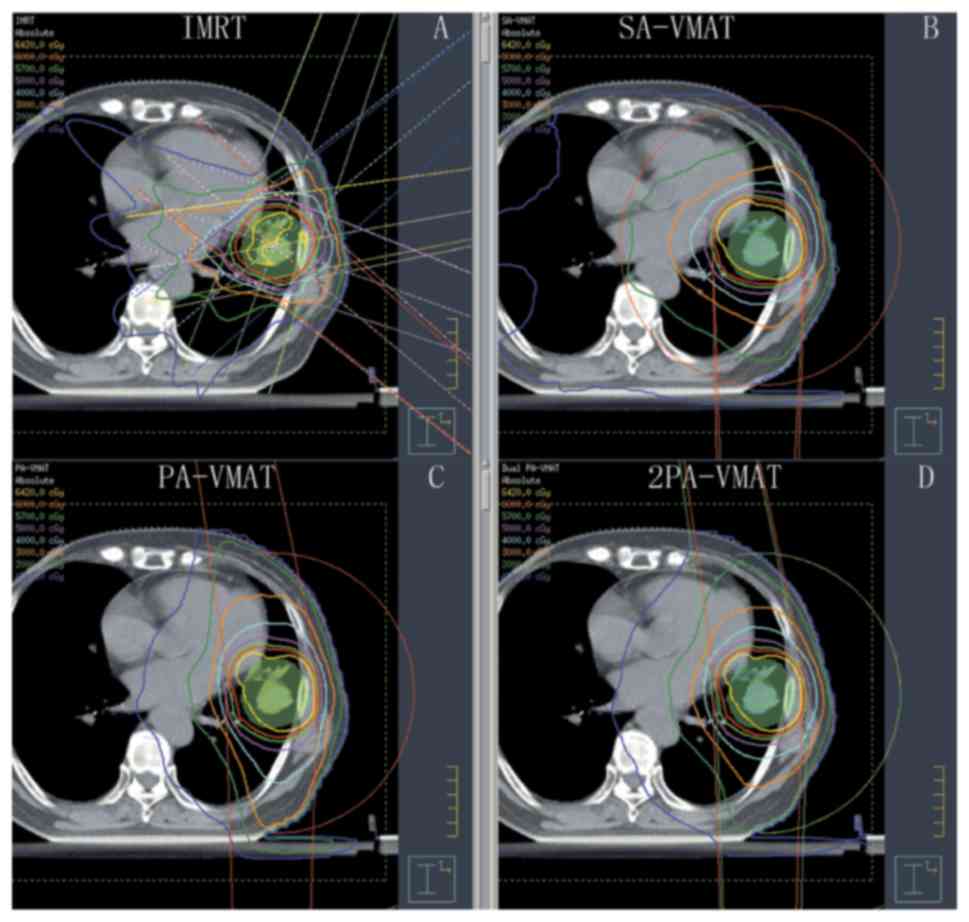 | Figure 1.Isodose curves of IMRT plan and
2PA-VMAT plan in peripheral lung cancer. (A) Isodose curves of IMRT
plan. (B) Isodose curves of SA-VMAT plan. (C) Isodose curves of
PA-VMAT plan. (D) Isodose curves of 2PA-VMAT plan. In peripheral
lung cancer, both IMRT plan and VMAT plan exhibited satisfying
prescribed tumor target coverage and OAR sparing. However, 2PA-VMAT
displayed improved PTV coverage compared with IMRT. 2PA-VMAT
exhibited better sparing of spinal cord, lung-all, and
lung-contralateral compared with IMRT, while IMRT exhibited better
heart and lung-ipsilateral sparing compared with 2PA-VMAT. IMRT had
better dose conformity and homogeneity. IMRT, intensity-modulated
radiation therapy; VMAT, volumetric modulated arc therapy; 2PA,
double partial arc; PA, partial arc; SA, single arc; OAR,
organs-at-risk. |
Table II illustrated
the dosage difference between VMAT and IMRT plans in peripheral
lung cancer. In peripheral lung cancer, there was no significant
difference of CI and HI of PTV among IMRT, SA-VMAT, PA-VMAT, and
2PA-VMAT plans (Figs. 1 and 2). The target area is far away from the
spinal cord and heart, therefore the received dose of the spinal
cord and heart were low; the IMRT plan achieved superior sparing of
spinal cord compared with the VMAT plans, and the radiation dose of
the spinal cord was the highest in the SA-VMAT plan. IMRT plans
exhibited significantly improved sparing of V20, V30 of total lungs
and Dmean, V30 of ipsilateral lung compared with SA-VMAT, PA-VMAT
and 2PA-VMAT plans. PA-VMAT and 2PA-VMAT exhibited significantly
better Dmean, V5 (%), V10 (%) of lung-contralateral compared with
the IMRT plan. V5 (%) of lung-all of SA-VMAT was higher compared
with PA-VMAT. V5 (%) of lung-all of IMRT plan was higher compared
with SA-, PA- and 2PA-VMAT; V20, V30 (%) of lung-all of SA-, PA-,
2PA-VMAT were higher compared with IMRT. V5 (%) of lung-ipsilateral
of IMRT was higher compared with SA-, PA-, 2PA-VMAT. IMRT plans
also exhibited significantly improved sparing of Dmean of
contralateral lung than PA- and 2PA-VMAT plans (Fig. 3).
 | Table II.Dosage comparison between VMAT and
IMRT plans in peripheral lung cancer. |
Table II.
Dosage comparison between VMAT and
IMRT plans in peripheral lung cancer.
| Peripheral lung
cancer | IMRT (mean ±
SD) | SA-VMAT (mean ±
SD) | PA-VMAT (mean ±
SD) | 2PA-VMAT (mean ±
SD) |
|---|
| PTV Dmax |
6,654.70±9.55a |
7,013.00±7.02 |
7,042.40±7.01 |
6,951.40±6.83 |
| PTV Dmin |
5,279.20±5.22 |
5,281.40±5.21 |
5,151.50±5.08 |
5,217.60±5.17 |
| PTV D100 (cGy) |
5,279.20±5.07 |
5,281.40±5.01 |
5,151.50±5.03 |
5,217.60±5.16 |
| PTV D mean
(cGy) |
6,350.00±6.35a |
6,616.40±6.11 |
6,558.70±6.01 |
6,560.80±6.21 |
| PTV D98 (cGy) |
5,860.00±5.11 |
5,790.00±5.13 |
57,770.00±5.07 |
5,775.00±5.14 |
| PTV D95 (cGy) |
6,000.00±5.56 |
6,000.00±5.61 |
6,000.00±5.52 |
6,000.00±5.59 |
| PTV D50 (cGy) |
6,387.00±6.11a |
6,687.00±6.23 |
6,654.00±6.33 |
6,627.00±6.22 |
| PTV D2 (cGy) |
6,557.00±6.15a |
6,971.00±6.32 |
6,967.00±6.42 |
6924±6.25 |
| Heart
V30e (%) |
8.65±0.02a |
11.85±0.03 |
11.94±0.03 |
9.51±0.02c |
| Heart
V40e (%) |
3.19±0.00a |
5.73±0.01 |
5.62±0.01 |
4.55±0.00 |
| Spinalcord D max
(cGy) |
1,330.3±1.32a |
1,692.9±1.43 |
985.7±1.01 |
1,129.7±1.13 |
| Spinalcord
D5 (cGy) |
1,161±1.11a |
1586±1.15 |
900±0.91b |
1,025±1.09c |
| Lung all
V5e (%) |
34.93±0.03a |
37.87±0.03 |
22.64±0.02b |
26.22±0.02c |
| Lung all
V10e (%) |
15.03±0.01 |
22.23±0.01 |
14.1±0.00b |
14.59±
0.01c |
| Lung all
V20e (%) |
7.72±0.00a |
9.63±0.01 |
9.17±0.01 |
9.21±0.01 |
| Lung all
V30e (%) |
5.32±0.01a |
6.37±0.01 |
6.69±0.01 |
6.38±0.01 |
| Lung all D mean
(cGy) |
647.20±0.61 |
761.80±0.71 |
641.60±0.62b |
657.30±0.63c |
| Lung-ipsilateral D
mean (cGy) |
1,072.40±1.02a |
1,235.60±1.21 |
1,215.20±1.20 |
1,209.70±1.19 |
| Lung-ipsilateral V5
(%) |
37.92±0.03a |
41.74±0.04 |
41.49±0.04 |
41.64±0.04 |
| Lung-ipsilateral
V10 (%) |
30.49±0.03 |
33.62±0.03 |
32.51±0.03 |
33.38±0.03 |
| Lung-ipsilateral
V20 (%) |
17.87±0.01a |
22.22±0.02 |
21.22±0.02 |
21.31±0.02 |
| Lung-ipsilateral
V30 (%) |
12.31±0.01a |
14.74±0.01 |
15.47±0.01 |
14.77±0.01 |
| Lung-contralateral
D mean (cGy) |
323.60±0.32a |
401.10±0.04 |
205.00±0.02b |
236.90±0.02c |
| Lung-contralateral
V5 (%) |
32.66±0.03a |
34.93±0.03 |
8.28±0.00b |
14.48±
0.01c |
| Lung-contralateral
V10 (%) |
3.25±0.00a |
13.56±0.00 |
0.09±0.00b |
0.28±0.00c |
| Lung-contralateral
V20 (%) |
0.00±0.00 |
0.05±0.00 |
0.00±0.00 |
0.00±0.00 |
| Lung-contralateral
V30 (%) |
0.00±0.00 |
0.00±0.00 |
0.00±0.00 |
0.00±0.00 |
| HI |
0.11±0.00a |
0.18±0.00 |
0.18±0.00 |
0.17±0.00 |
| CI |
0.69±0.00a |
0.50±0.00 |
0.51±0.00 |
0.52±0.00 |
Dosage comparison between VMAT and
IMRT plans in central lung cancer in which the target volume does
not encompass the mediastinal lymphatic drainage region
For central lung cancer, it remains unanswered when
PTV needs to encompass the mediastinal lymphatic drainage region.
It is hypothesized that this depends on the tumor pathological type
and whether there exist metastatic mediastinal lymph nodes or not.
For SCLC, PTV needs to encompass the mediastinal lymphatic drainage
region. For NSCLC, if there are metastatic mediastinal lymph nodes,
PTV needs to encompass the metastatic lymph nodes. If not, PTV does
not encompass it. Fig. 4 illustrates
the dose-volume histogram DVH and dose distributions of IMRT and
VMAT plans of a patient with central lung cancer whose PTV did not
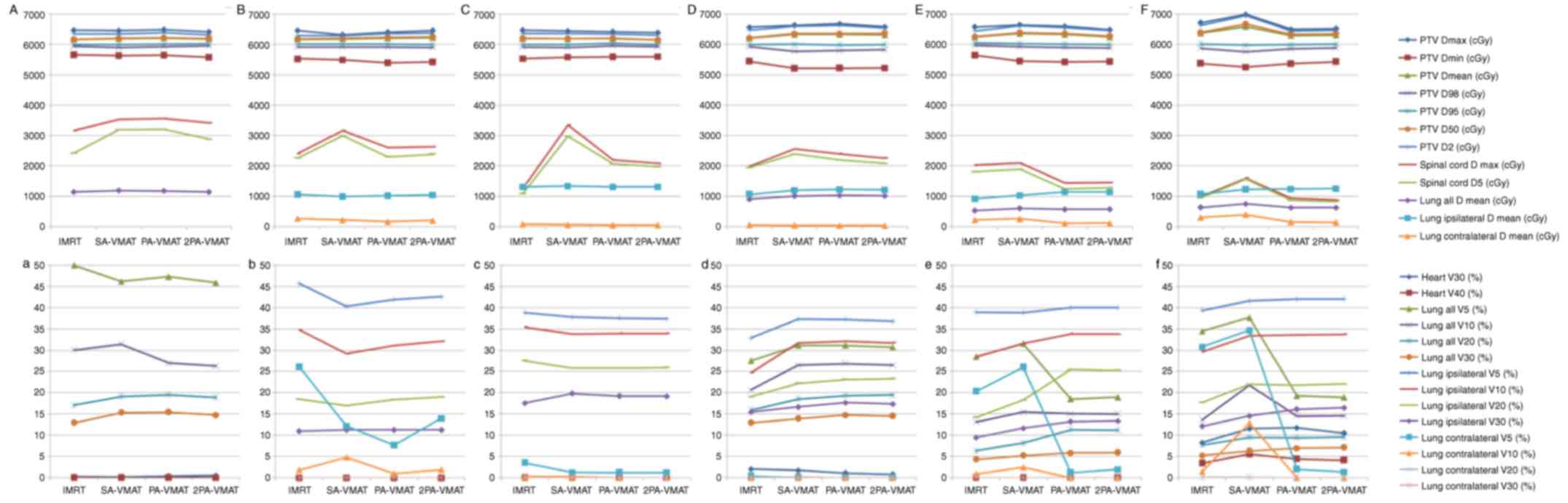
encompass the mediastinum. Figs. 5
and 6 illustrate the CI, HI and OAR
dosage of each patient.
In the case of PTV not encompassing the mediastinum
in central lung cancer, Table III
illustrated the dosage difference between VMAT and IMRT plans.
SA-VMAT exhibited significantly superior CI compared with IMRT, PA-
2PA-VMAT. SA- and 2PA-VMAT exhibited significantly superior HI than
IMRT and PA-VMAT. Dmax of PTV in SA-VMAT and 2PA was significantly
higher compared with IMRT and PA-VMAT. Dmin of PTV in PA-VMAT was
significantly lower compared with IMRT and SA-VMAT. V30, V40 (%) of
heart of SA-VMAT were higher compared with IMRT, PA- and 2PA-VMAT
in most cases. V5, V10, V20 (%) of lung-all of IMRT were higher
compared with SA-, PA-, and 2PA-VMAT in most cases. V10 (%) of
lung-all of 2PA-VMAT was less compared with IMRT, SA- and
PA-VMAT.
 | Table III.Dosage comparison between VMAT and
IMRT plans in central lung cancer in which the target volume does
not encompass the mediastinal lymphatic drainage region. |
Table III.
Dosage comparison between VMAT and
IMRT plans in central lung cancer in which the target volume does
not encompass the mediastinal lymphatic drainage region.
| PTV not
encompassing the mediastinal lymphatic drainage region in central
lung cancer | IMRT (mean ±
SD) | SA-VMAT (mean ±
SD) | PA-VMAT (mean ±
SD) | 2PA-VMAT (mean ±
SD) |
|---|
| PTV Dmax |
6,620.50±6.55 |
6,932.80±6.67 |
7,015.60±7.05 |
6,594.00±6.56 |
| PTV Dmin |
5,192.20±5.55 |
5,012.90±5.05 |
4,897.80±4.87 |
5,164.50±5.11 |
| PTV D100 (cGy) |
5,192.20±5.09 |
5,012.90±5.00 |
4,897.80±4.79 |
5,164.50±5.09 |
| PTV D mean
(cGy) |
6,222.90±6.21 |
6,506.90±6.50 |
6,573.30±6.53 |
6,191.60±6.09c |
| PTV D98 (cGy) |
5,800.00±5.79 |
5,770.00±5.78 |
5,700.00±5.71 |
5,820.00±5.88 |
| PTV D95 (cGy) |
6,000.00±5.58 |
6,000.00±5.59 |
6,000.00±5.61 |
6,000.00±5.63 |
| PTV D50 (cGy) |
6,232.00±6.33 |
6,232.00±6.35 |
6,647.00±6.65 |
6,203.00±6.22 |
| PTV D2 (cGy) |
6,459.00±6.49 |
6,788.00±6.95 |
6,901.00±6.92 |
6,397.00±6.41 |
| Heart
V30e (%) |
14.85±0.02 |
16.00±0.02 |
25.01±0.02b |
11.63±0.02c |
| Heart
V40e (%) |
5.70±0.02 |
6.75±0.02 |
6.58±0.02 |
5.20±0.02 |
| Spinalcord D max
(cGy) |
4,009.60±4.02a |
4,389.30±4.02 |
4,297.70±4.02 |
3,796.00±3.72c |
| Spinalcord
D5 (cGy) |
2,997.00±2.82 |
3,300.00±3.12 |
3,285.00±3.13 |
3,083.00±3.07 |
| Lung all
V5e (%) |
49.59±0.04 |
50.14±0.05 |
49.82±0.05 |
50.03±0.05 |
| Lung all
V10e (%) |
27.66±0.02 |
33.3±0.03 |
31.89±0.03 |
28.23±0.02 |
| Lung all
V20e (%) |
14.83±0.01 |
15.25±0.01 |
15.28±0.02 |
15.01±0.02 |
| Lung all
V30e (%) |
11.90±0.01 |
11.70±0.01 |
11.99±0.01 |
12.17±0.01 |
| Lung all D mean
(cGy) |
1,071.10±1.02 |
1,122.40±1.01 |
1,136.00±1.01 |
1,083.70±1.02 |
| Lung-ipsilateral D
mean (cGy) |
1,843.80±1.71 |
1,892.50±1.79 |
1,918.20±1.91 |
1,873.50±1.81 |
| Lung-ipsilateral V5
(%) |
53.72±0.05 |
53.68±0.04 |
52.36±0.04 |
52.76±0.04 |
| Lung-ipsilateral
V10 (%) |
47.45±0.04 |
48.38±0.04 |
47.69±0.04 |
47.51±0.04 |
| Lung-ipsilateral
V20 (%) |
33.38±0.03 |
34.74±0.03 |
34.59±0.03 |
34.73±0.03 |
| Lung-ipsilateral
V30 (%) |
27.54±0.02 |
27.08±0.02 |
27.76±0.02 |
28.17±0.02 |
| Lung-contralateral
D mean (cGy) |
479.90±0.46 |
531.20±0.52 |
537.00±0.53 |
481.20±0.47c |
| Lung-contralateral
V5 (%) |
46.05±0.04 |
46.97±0.04 |
47.62±0.04 |
47.72±0.04 |
| Lung-contralateral
V10 |
12.43±0.01 |
21.42±0.02 |
19.77±0.01 |
13.61±0.01c |
| Lung-contralateral
V20 |
0.72±0.00 |
0.43±0.00 |
0.60±0.00 |
0.00±0.00 |
| Lung-contralateral
V30 |
0.00±0.00 |
0.00±0.00 |
0.00±0.00 |
0.00±0.00 |
| HI |
0.11±0.00 |
0.16±0.00 |
0.18±0.00 |
0.09±0.00 |
| CI |
0.66±0.00 |
0.51±0.00 |
0.49±0.00 |
0.70±0.00 |
Dosage comparison between VMAT and
IMRT plans in central lung cancer in which the target volume
encompasses the mediastinal lymphatic drainage region
In central lung cancer, in case of either SCLC or
positive metastatic mediastinal lymph nodes, PTV needs to encompass
the mediastinum. Fig. 7 illustrated
the DVH and dose distributions of IMRT and VMAT plans of a patient
with central lung cancer whose PTV encompasses the mediastinum.
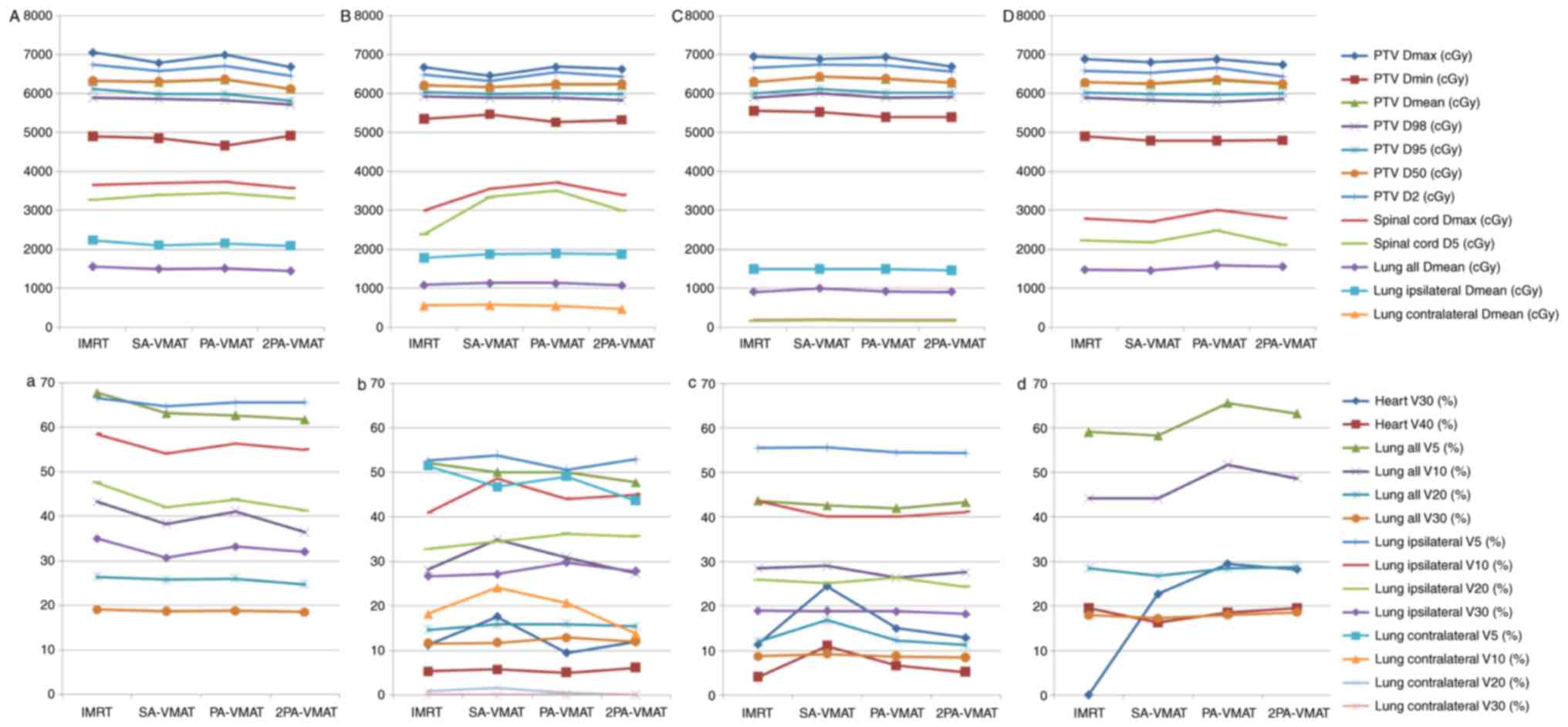
Figs. 8 and 9 illustrate the CI, HI and OAR dosage of
each patient.
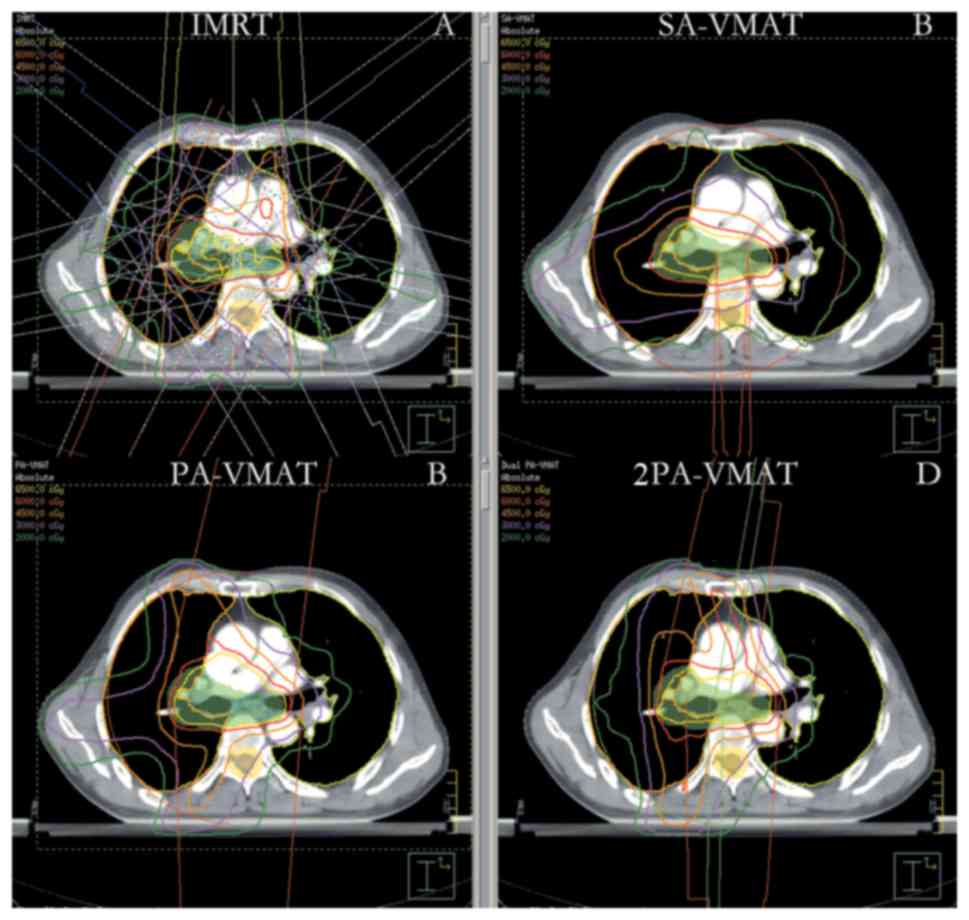 | Figure 7.Isodose curves of 2PA-VMAT and IMRT
plans in central lung cancer in which the target volume encompasses
the mediastinal lymphatic drainage region. (A) Isodose curves of
IMRT plan. (B) Isodose curves of SA-VMAT plan. (C) Isodose curves
of PA-VMAT plan. (D) Isodose curves of 2PA-VMAT plan. In central
lung cancer, when PTV encompasses the mediastinum, 2PA-VMAT
exhibited improved PTV coverage compared with IMRT. IMRT exhibited
better sparing of spinal cord, lung-all, lung-ipsilateral, and
lung-contralateral compared with 2PA-VMAT. In addition, IMRT
exhibited better dose conformity and homogeneity. 2PA, double
partial arc; VMAT, volumetric modulated arc therapy; IMRT,
intensity-modulated radiation therapy; PA, partial arc; SA, single
arc; PTV, planning target volume. |
In the case of PTV encompassing the mediastinum in
central lung cancer, Table IV
illustrated the dosage comparison between VMAT and IMRT plans.
2PA-VMAT exhibited superior HI and CI compared with IMRT, SA- and
PA-VMAT in most cases, but V40 (%) of heart in 2PA-VMAT was higher
compared with IMRT, SA- and PA-VMAT. V5, V30 (%) of lung-all in
2PA-VMAT was higher compared with IMRT. By contrast, V20 (%) of
lung-all in 2PA-VMAT was less compared with IMRT, SA-, and PA-VMAT.
V5, V10 (%) of lung-contralateral in SA-, PA-, and 2PA-VMAT was
higher compared with IMRT, while V20, V30 (%) of lung-contralateral
in 2PA-VMAT was less compared with IMRT.
 | Table IV.Dosage comparison between VMAT and
IMRT plans in central lung cancer in which the target volume
encompassing the mediastinal lymphatic drainage region. |
Table IV.
Dosage comparison between VMAT and
IMRT plans in central lung cancer in which the target volume
encompassing the mediastinal lymphatic drainage region.
| PTV encompassing
the mediastinal lymphatic drainage region in central lung
cancer | IMRT (mean ±
SD) | SA-VMAT (mean ±
SD) | PA-VMAT (mean ±
SD) | 2PA-VMAT (mean ±
SD) |
|---|
| PTV Dmax |
7,063.70±7.05a |
7,689.30±7.65 |
7,651.10±7.55 |
7,644.10±7.55 |
| PTV Dmin |
4,576.80±4.55 |
4,539.10±4.53 |
4,325.10±4.37 |
4,368.30±4.38 |
| PTV D100 (cGy) |
4,576.80±4.56 |
4,539.10±4.51 |
4,325.10±4.31 |
4,368.30±4.37 |
| PTV D mean
(cGy) |
6,473.90±6.50a |
6,875.30±6.79 |
6,847.40±6.83 |
6,725.90±6.71 |
| PTV D98 (cGy) |
5,800.00±5.79 |
5,800.00±5.78 |
5,720.00±5.72 |
5,720.00±5.72 |
| PTV D95 (cGy) |
6,000.00±5.58 |
6,000.00±5.69 |
6,000.00±5.90 |
6,000.00±5.68 |
| PTV D50 (cGy) |
6,513.00±6.37a |
6,950.00±6.68 |
6,948.00±6.78 |
6,792.00±6.68c |
| PTV D2 (cGy) |
6,782.00±6.34a |
7,501.00±7.24 |
7,350.00±7.15 |
7,198.00±7.03c |
| Heart
V30e (%) |
43.07±0.04 |
36.11±0.03 |
42.25±0.04 |
42.66±0.04c |
| Heart
V40e (%) |
31.42±0.03 |
22.42±0.02 |
28.39±0.02 |
34.3±0.03c |
| Spinalcord D max
(cGy) |
4,380.3±4.37a |
4,947.4±4.87 |
5,039.6±5.37 |
4,515.5±4.29c |
| Spinalcord
D5 (cGy) |
3,763.00±3.39 |
4,098.00±4.01 |
4,175.00±4.12 |
3,976.00±3.97 |
| Lung all
V5e (%) |
74.67±0.07 |
77.31±0.07 |
76.43±0.07 |
75.93±0.07 |
| Lung all
V10e (%) |
63.72±0.06 |
70.70±0.07 |
67.66±0.06b |
64.11±0.06c |
| Lung all
V20e (%) |
35.22±0.03 |
44.19±0.04 |
38.47±0.03b |
35.18±0.03c |
| Lung all
V30e (%) |
20.54±0.02a |
22.06±0.02 |
23.02±0.02 |
21.51±0.02 |
| Lung all D mean
(cGy) |
1,869.40±1.78 |
2,034.40±2.02 |
1,968.50±1.98 |
1,913.00±1.99c |
| Lung-ipsilateral D
mean (cGy) |
33.77±0.03a |
37.08±0.03 |
41.03±0.03b |
37.26±0.03 |
| Lung-ipsilateral V5
(%) |
80.64±0.08 |
82.49±0.08 |
80.57±0.08 |
80.69±0.08 |
| Lung-ipsilateral
V10 (%) |
75.11±0.07 |
75.91±0.07 |
73.95±0.07 |
75.55±0.07 |
| Lung-ipsilateral
V20 (%) |
54.19±0.05 |
58.49±0.05 |
58.27±0.05 |
54.60±0.05 |
| Lung-ipsilateral
V30 (%) |
33.77±0.03a |
37.08±0.03 |
41.03±0.03 |
37.26±0.03 |
| Lung-contralateral
D mean (cGy) |
1,483.70±1.37 |
1,751.90±1.67 |
1,540.60±1.56 |
1,446.40±1.46 |
| Lung-contralateral
V5 (%) |
80.07±0.08a |
86.34±0.08 |
86.47±0.08 |
84.96±0.08 |
| Lung-contralateral
V10 |
62.49±0.06 |
78.42±0.07 |
73.59±0.07 |
63.57±0.06c |
| Lung-contralateral
V20 |
20.67±0.02 |
36.62±0.03 |
23.61±0.02 |
20.11±0.02 |
| Lung-contralateral
V30 |
9.60±0.00 |
9.42±0.00 |
7.18±0.00 |
7.95±0.00 |
| HI |
0.15±0.00a |
0.24±0.00 |
0.23±0.00 |
0.22±0.00 |
| CI |
0.70±0.00a |
0.70±0.00 |
0.61±0.00 |
0.58±0.00c |
In conclusion, in peripheral lung cancer, V5 (%) of
the lung in PA-VMAT was less compared with IMRT, SA-, and 2PA-VMAT.
V30 (%) of the lung in IMRT was less compared with SA-, PA- and
2PA-VMAT. In the case of PTV not encompassing the mediastinum in
central lung cancer, the CI and HI of SA-VMAT was improved compared
with IMRT, PA-, and 2PA-VMAT; the received dose of heart in SA-VMAT
was higher compared with IMRT, PA- and 2PA-VMAT. V30 (%) and V5 (%)
of the lung in IMRT was higher compared with SA-, PA- and 2PA-VMAT;
V10 (%) of the lung in 2PA was less compared with IMRT, SA- and
PA-VMAT. In the case of PTV encompassing the mediastinum in central
lung cancer, the HI and CI of 2PA was improved compared with IMRT,
SA- and PA-VMAT. The received dose of heart in 2PA was higher
compared with IMRT, SA- and PA-VMAT. V30 (%) and V5 (%) of the lung
in 2PA-VMAT was higher compared with IMRT, SA- and PA-VMAT. V20 (%)
of the lung in 2PA was lower compared with IMRT, SA- and
PA-VMAT.
Discussion
The differences of dosimetry and clinical characters
between VMAT and IMRT have been studied by many studies (9–16,19,25,27–33)
and VMAT technique has displayed superiority in different types of
solid tumors compared with IMRT, especially tumors with complicated
target volume (1–17). The present study compared dosimetric
differences and treatment efficiency between IMRT and three kinds
of VMAT plans in the following three situations: Peripheral lung
cancer, PTV not encompassing the mediastinal lymphatic drainage
region in central lung cancer, and PTV encompassing the mediastinal
lymphatic drainage region in central lung cancer. To our knowledge,
this is the first study to report dosimetric differences between
IMRT, SA-VMAT, PA-VAMT and 2PA-VMAT plans in these three
situations.
In peripheral lung cancer, there was no significant
difference of CI and HI among IMRT, SA-VMAT, PA-VMAT, and 2PA-VMAT
plans. This is because the targets were small and far away from
normal organs, and radiotherapy plans and dose requirement were
easily achieved, resulting in similar CI and HI for each plan.
Because spinal cords and hearts were far from targets, they
received less radiation. V5 (%) of total lungs in SA-VMAT plans
were higher compared with PA-VMAT plans, because the radiation
regions in SA-VMAT plans were larger compared with PA-VMAT plans.
V5 of total lungs and contralateral lungs in the IMRT plan were
higher compared with SA-, PA- and 2PA-VMAT plans. V5 of ipsilateral
lungs in IMRT plans were lower compared with SA-, PA- and 2PA-VMAT
plans. This was because tumors in most cases located in the lower
lobes, so the radiation fields were relatively concentrated and
focused to the contralateral lung, which led to higher V5 of
contralateral lung and then led to higher V5 of total lungs in IMRT
plans. V20 and V30 of total lungs in SA-, PA- and 2PA-VMAT plans
were higher compared with IMRT plans. This was because tumors in
most cases located near the chest wall, so VMAT plans required a
larger range of radiation angles, which resulted in more dispersed
dose distribution of 20 and 30 Gy.
In the case of PTV not encompassing the mediastinum
in central lung cancer, SA-VMAT plans exhibited better CI and HI
than IMRT, PA- and 2PA-VMAT plans. Because the target volumes were
relatively large and the target located in the center, the
incidence angles of IMRT, PA- and 2PA-VMAT plans were smaller
compared with SA-VMAT. However, V30 and V40 of heart in SA-VMAT
plans were higher compared with IMRT, PA- and 2PA-VMAT plans.
Because the incidence angle of SA-VMAT plan was 360 degree and the
target located near the heart, it resulted in the heart receiving a
higher radiation dose. The spinal cord was far away from the target
thus it received less radiation dose. In most cases, V5, V10 and
V20 of total lungs in IMRT plans were higher compared with SA-, PA-
and 2PA-VMAT plans, because the target was in the center and the
radiation fields of IMRT plans were relatively concentrated. The
concentrated radiation fields focused to the ipsilateral lung and
resulted in a higher dose. V10 and V20 of total lungs in 2PA-VMAT
plans were decreased compared with IMRT, SA- and PA-VMAT plans. The
differences of radiation dose of ipsilateral lungs and
contralateral lungs were slight.
In the case of PTV encompassing the mediastinum in
central lung cancer, the 2PA-VMAT plan in most cases exhibited
improved CI and HI compared with IMRT, SA- and PA-VMAT plans.
Because the target volume in this case was the largest and the
targets were located in the center or near the center, this
increased the difficulty of planning. The 2PA-VMAT plan possesses
the most optimized incidence angles; therefore it achieved the best
CI and HI. Because the optimized incidence angles in 2PA-VMAT plans
induced the largest radiation area of heart, V40 of heart in
2PA-VMAT plans were higher compared with IMRT, SA- and 2PA-VMAT
plans. Since the spinal cord was near the target, planning
optimization for 2PA-VMAT yielded the radiation dose of spinal cord
within the tolerance range. V5, V30 of total lungs in 2PA-VMAT
plans were higher compared with the IMRT plan, whereas V20 of total
lungs in 2PA-VMAT plans were lower compared with IMRT, SA- and
PA-VMAT plans. This is because more incidence angles in the
2PA-VMAT plan resulted in increased areas of low dose in
contralateral lung and total lungs. In addition, more optimized
incidence angles in the 2PA-VMAT plan resulted in lower V20 of
total lungs and similar radiation of ipsilateral lung. V5 and V10
of contralateral lungs in SA-, PA- and 2PA-VMAT plans were higher
compared with the IMRT plan, whereas V20 and V30 of contralateral
lungs in SA-, PA- and 2PA-VMAT plans were lower compared with the
IMRT plan. The reason was the same as the dose comparison of total
lungs.
Is the present data demonstrate that it may be
necessary to classify the radiotherapy plans of lung cancer into
three categories, including peripheral lung cancer, PTV not
encompassing mediastinum of central lung cancer, and PTV
encompassing mediastinum of central lung cancer. Each of IMRT,
SA-VMAT, PA-VMAT, and 2PA-VMAT plan has its individual advantages
and therefore it is important to employ different planning
techniques regarding to different situations. In peripheral lung
cancer, V5 (%) of the lung in PA-VMAT was low. V20 (%) and V30 (%)
of the lung in IMRT was low. Different techniques may be used
according to different requirements of lung dose. In the case of
PTV not encompassing the mediastinum in central lung cancer, the HI
and CI of SA-VMAT was the best, but with a relatively high heart
dose. The received dose of lung was the highest in the IMRT plan.
V10 (%) of the lung in 2PA-VMAT was the lowest. If the pulmonary
function is poor, it may be better to employ 2PA-VMAT instead of
IMRT. In case of PTV encompassing mediastinum in central lung
cancer, if the PTV is big, it is hard to decide on a good
radiotherapy plan. The HI and CI of 2PA-VMAT were good but with
high heart dose. V5 (%) and V30 (%) of the lung in 2PA-VMAT were
high, whereas V20 (%) of the lung was low. Therefore, it may
crucial to employ different planning techniques considering
different OAR requirements.
Radiation-induced pneumonitis (RIP) is one of the
most common and serious complications following radiation of
thoracic malignancies, which produces a considerable effect on
patient morbidity, even leading to death. The incidence of RIP is
closely correlated with the irradiation dose that the lungs
received (28). Studies have
suggested that the dosimetric parameters of the lung DVH, such as
mean lung dose, V20 and V30 of lung, are direct factors which can
affect RIP incidence (34–37).
According to Schallenkamp et al (38), a study of a 92-patient cohort,
V10/13/15 was also significantly correlated to RIP. Wang et
al (39) demonstrated that V5 of
both lung lobes was the only parameter predicting the incidence of
RIP (≥grade 2) in NSCLC patients. The present results suggested
that VMAT plans obviously delivered low irradiation dose to a
larger volume of lung than IMRT, so the VMAT technique may increase
RIP incidence more than IMRT in peripheral lung cancer and PTV not
encompassing mediastinum of central lung cancer.
Multiple studies have confirmed that VMAT has a
significantly shorter delivery time than IMRT (7). The present study also demonstrated that
VMAT had a significant advantage of delivery efficiency and
treatment time. Improved treatment efficiency of VMAT yielded less
scatter dose from reducing MU, which may reduce secondary
malignancies. Less treatment time also enhances patient comfort and
satisfaction and decreases the intra-fraction variation.
With respect to the present study, no one planning
technique was demonstrated to be superior in all aspects. IMRT and
2PA-VMAT plans achieved superior conformal plans in these three
kinds of situations compared with the other plans. As for OARs
sparing, the four planning techniques could achieve superior
sparing of different organs. SA/PA/2PA-VMAT plan were more
effective at dose delivery than other plans.
Several studies have suggested that the VMAT plan is
better than the IMRT plan in lung cancer. Jiang et al
(28) reported that the VMAT plan
gets superior PTV coverage than IMRT plans for locally advanced
lung cancer. Jiang et al (28)
demonstrated that V20, V30 and MLD of the total and contra-lateral
lungs in VMAT plans were significantly lower compared with IMRT
plans. Other studies have demonstrated that VMAT plans achieved the
most objectives on target volumes and OARs for stage III NSCLC
(27,29). There may be two reasons which lead to
the different results. One reason may be related to different
situations of target volume. For example, in the present study,
different results were obtained between the case of PTV
encompassing mediastinal lymphatic drainage region and the case of
PTV not encompassing the mediastinal lymphatic drainage region in
central lung cancer. The other reason may be related to different
planning system, different accelerator and the effort on planning.
The plan quality depends heavily on the amount of effort spent on
the planning and planner's experience (25,40). One
major challenge of VMAT plans is that VMAT requires a much longer
time to optimize compared with IMRT. The long optimization time may
introduce more variations in plan quality due to limits on
planners' time and effort (9,11,14).
Therefore, it is more difficult to assure high-quality of treatment
plans for VMAT than for IMRT.
Concrete analysis should be performed according to
concrete circumstance of each patient to make the best plan. The
suitability of the planning technique could vary depending on the
staging, tumor size, location of tumor, OARs and the dose-tolerance
criteria. However, the data from the present study has limitations,
because the small numbers of patients enrolled renders the results
less reliable. In addition, for IMRT, alternate planning techniques
using more beams could have bene attempted; however, incorporation
of such variation could increase the plan complexity at cost of
treatment time. Further clinical investigation is required in order
to fully address these concerns.
In conclusion, the present results suggest that it
may be necessary to classify the radiotherapy plans of lung cancer
into three categories, including peripheral lung cancer, PTV not
encompassing mediastinum of central lung cancer, and PTV
encompassing mediastinum of central lung cancer. In the present
study, the dosimetric differences and treatment efficiency were
compared between IMRT and VMAT plans in three types of cases. Each
of IMRT, SA-VMAT, PA-VMAT, and 2PA-VMAT plan had individual
advantages, and it may be crucial to employ different planning
techniques in different situations. Different techniques may be
used according to different requirements of lung dose. If the
pulmonary function is poor, it may be better to employ 2PA-VMAT
instead of IMRT. In case of PTV encompassing mediastinum in central
lung cancer, if the PTV is large, it may be hard to decide on a
good radiotherapy plan. Additionally, it may be important to employ
different planning techniques considering different OAR
requirements. The suitability of the planning technique could vary
depending on the staging, tumor size, location of tumor, OARs as
well as the dose-tolerance criteria. Concrete analysis should be
made according to concrete circumstance of each patient to make the
best plan for radiotherapy of lung cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81772793/H1621,
31201060/C0709, 30973175/H1621 and 81172490/H1621), the Program for
New Century Excellent Talents in University (grant no.
NCET-12-0440), the Scientific and Technological Research Foundation
of Shaanxi Province (grant no. 2012K13-01-06), the Research
Foundation of Health Department of Shaan'xi Province (grant no.
2010D41), the Qing Nian Jiao Shi Gen Zong Ji Hua of Xi'an Jiaotong
University, and a Clinical Research Award of the First Affiliated
Hospital of Xi'an Jiaotong University (grant no.
XJTU1AHCR2014-041).
References
|
1
|
Peeters ST, Heemsbergen WD, Van Putten WL,
Slot A, Tabak H, Mens JW, Lebesque JV and Koper PC: Acute and late
complications after radiotherapy for prostate cancer: Results of a
multicenter randomized trial comparing 68 to 78 Gy. Int J Radiat
Oncol. 61:1019–1034. 2005. View Article : Google Scholar
|
|
2
|
Pollack A, Zagars GK, Smith LG, Lee JJ,
von Eschenbach AC, Antolak JA, Starkschall G and Rosen I:
Preliminary results of a randomized radiotherapy dose-escalation
study comparing 70 Gy with 78 Gy for prostate cancer. J Clin Oncol.
18:3904–3911. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kupelian PA, Ciezki J, Reddy CA, Klein EA
and Mahadevan A: Effect of increasing radiation doses on local and
distant failures in patients with localized prostate cancer. Int J
Radiat Oncol Biol Phys. 71:16–22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wolff D, Stieler F, Welzel G, Lorenz F,
Abo-Madyan Y, Mai S, Herskind C, Polednik M, Steil V, Wenz F and
Lohr F: Volumetric modulated arc therapy (VMAT) vs. serial
tomotherapy, step-and-shoot IMRT and 3D-conformal RT for treatment
of prostate cancer. Radiother Oncol. 93:226–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brahme A, Roos JE and Lax I: Solution of
an integral-equation encountered in rotation therapy. Phys Med
Biol. 27:1221–1229. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bratengeier K: 2-Step IMAT and 2-Step IMRT
in three dimensions. Med Phys. 32:3849–3861. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Otto K: Volumetric modulated arc therapy:
IMRT in a single gantry arc. Med Phys. 35:310–317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu CX: Intensity-modulated arc therapy
with dynamic multileaf collimation: An alternative to tomotherapy.
Phys Med Biol. 40:1435–1449. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Palma D, Vollans E, James K, Nakano S,
Moiseenko V, Shaffer R, Mckenzie M, Morris J and Otto K: Volumetric
modulated arc therapy for delivery of prostate radiotherapy:
Comparison with intensity-modulated radiotherapy and
three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol
Phys. 72:996–1001. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cozzi L, Dinshaw KA, Shrivastava SK,
Mahantshetty U, Engineer R, Deshpande DD, Jamema SV, Vanetti E,
Clivio A, Nicolini G and Fogliata A: A treatment planning study
comparing volumetric arc modulation with RapidArc and fixed field
IMRT for cervix uteri radiotherapy. Radiother Oncol. 89:180–191.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoo S, Wu QJ, Lee WR and Yin FF:
Radiotherapy treatment plans with RapidArc for prostate cancer
involving seminal vesicles and lymph nodes. Int J Radiat Oncol Biol
Phys. 76:935–942. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Popescu CC, Olivotto IA, Beckham WA,
Ansbacher W, Zavgorodni S, Shaffer R, Wai ES and Otto K: Volumetric
modulated arc therapy improves dosimetry and reduces treatment time
compared to conventional intensity-modulated radiotherapy for
locoregional radiotherapy of left-sided breast cancer and internal
mammary nodes. Int J Radiat Oncol Biol Phys. 76:287–295. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clivio A, Fogliata A, Franzetti-Pellanda
A, Nicolini G, Vanetti E, Wyttenbach R and Cozzi L:
Volumetric-modulated arc radiotherapy for carcinomas of the anal
canal: A treatment planning comparison with fixed field IMRT.
Radiother Oncol. 92:118–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rao M, Yang WS, Chen F, Sheng K, Ye JS,
Mehta V, Shepard D and Cao DL: Comparison of Elekta VMAT with
helical tomotherapy and fixed field IMRT: Plan quality, delivery
efficiency and accuracy. Med Phys. 37:1350–1359. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scorsetti M, Bignardi M, Clivio A, Cozzi
L, Fogliata A, Lattuada P, Mancosu P, Navarria P, Nicolini G, Urso
G, et al: Volumetric modulation arc radiotherapy compared with
static gantry intensity-modulated radiotherapy for malignant
pleural mesothelioma tumor: A feasibility study. Int J Radiat Oncol
Biol Phys. 77:942–949. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang P, Happersett L, Hunt M, Jackson A,
Zelefsky M and Mageras G: Volumetric modulated arc therapy:
Planning and evaluation for prostate cancer cases. Int J Radiat
Oncol Biol Phys. 76:1456–1462. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu QJ, Yoo S, Kirkpatrick JP, Thongphiew D
and Yin FF: Volumetric arc intensity-modulated therapy for spine
body radiotherapy: Comparison with static intensity-modulated
treatment. Int J Radiat Oncol Biol Phys. 75:1596–1604. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zacarias AS, Brown MF and Mills MD:
Volumetric modulated arc therapy (VMAT) treatment planning for
superficial tumors. Med Dosim. 35:226–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Verbakel WF, Cuijpers JP, Hoffmans D,
Bieker M, Slotman BJ and Senan S: Volumetric intensity-modulated
arc therapy vs. conventional IMRT in head-and-neck cancer: A
comparative planning and dosimetric study. Int J Radiat Oncol Biol
Phys. 74:252–259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lagerwaard FJ, Meijer OW, van der Hoorn
EA, Verbakel WF, Slotman BJ and Senan S: Volumetric modulated arc
radiotherapy for vestibular schwannomas. Int J Radiat Oncol Biol
Phys. 74:610–615. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lagerwaard FJ, van der Hoorn EA, Verbakel
WF, Haasbeek CJ, Slotman BJ and Senan S: Whole-brain radiotherapy
with simultaneous integrated boost to multiple brain metastases
using volumetric modulated arc therapy. Int J Radiat Oncol Biol
Phys. 75:253–259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Verbakel WF, Senan S, Cuijpers JP, Slotman
BJ and Lagerwaard FJ: Rapid delivery of stereotactic radiotherapy
for peripheral lung tumors using volumetric intensity-modulated
arcs. Radiother Oncol. 93:122–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ong CL, Verbakel WF, Cuijpers JP, Slotman
BJ, Lagerwaard FJ and Senan S: Stereotactic radiotherapy for
peripheral lung tumors: A comparison of volumetric modulated arc
therapy with 3 other delivery techniques. Radiother Oncol.
97:437–442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matuszak MM, Yan D, Grills I and Martinez
A: Clinical applications of volumetric modulated arc therapy. Int J
Radiat Oncol Biol Phys. 77:608–616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Quan EM, Chang JY, Liao Z, Xia T, Yuan Z,
Liu H, Li X, Wages CA, Mohan R and Zhang X: Automated volumetric
modulated Arc therapy treatment planning for stage III lung cancer:
How does it compare with intensity-modulated radio therapy? Int J
Radiat Oncol Biol Phys. 84:e69–e76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van't Riet A, Mak AC, Moerland MA, Elders
LH and van der Zee W: A conformation number to quantify the degree
of conformality in brachytherapy and external beam irradiation:
Application to the prostate. Int J Radiat Oncol Biol Phys.
37:731–736. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Scorsetti M, Navarria P, Mancosu P, Alongi
F, Castiglioni S, Cavina R, Cozzi L, Fogliata A, Pentimalli S,
Tozzi A and Santoro A: Large volume unresectable locally advanced
non-small cell lung cancer: Acute toxicity and initial outcome
results with rapid arc. Radiat Oncol. 5:942010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang X, Li T, Liu Y, Zhou L, Xu Y, Zhou X
and Gong Y: Planning analysis for locally advanced lung cancer:
Dosimetric and efficiency comparisons between intensity-modulated
radiotherapy (IMRT), single-arc/partial-arc volumetric modulated
arc therapy (SA/PA-VMAT). Radiat Oncol. 6:1402011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McGrath SD, Matuszak MM, Yan D, Kestin LL,
Martinez AA and Grills IS: Volumetric modulated arc therapy for
delivery of hypofractionated stereotactic lung radiotherapy: A
dosimetric and treatment efficiency analysis. Radiother Oncol.
95:153–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Holt A, van Vliet-Vroegindeweij C, Mans A,
Belderbos JS and Damen EM: Volumetric-modulated arc therapy for
stereotactic body radiotherapy of lung tumors: A comparison with
intensity-modulated radiotherapy techniques. Int J Radiat Oncol
Biol Phys. 81:1560–1567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bertelsen A, Hansen CR, Johansen J and
Brink C: Single Arc volumetric modulated Arc therapy of head and
neck cancer. Radiother Oncol. 95:142–148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guckenberger M, Richter A, Krieger T,
Wilbert J, Baier K and Flentje M: Is a single arc sufficient in
volumetric-modulated arc therapy (VMAT) for complex-shaped target
volumes? Radiother Oncol. 93:259–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shaffer R, Morris WJ, Moiseenko V, Welsh
M, Crumley C, Nakano S, Schmuland M, Pickles T and Otto K:
Volumetric modulated Arc therapy and conventional
intensity-modulated radiotherapy for simultaneous maximal
intraprostatic boost: A planning comparison study. Clin Oncol (R
Coll Radiol). 21:401–407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hernando ML, Marks LB, Bentel GC, Zhou SM,
Hollis D, Das SK, Fan M, Munley MT, Shafman TD, Anscher MS and Lind
PA: Radiation-induced pulmonary toxicity: A dose-volume histogram
analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol
Phys. 51:650–659. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Claude L, Pérol D, Ginestet C, Falchero L,
Arpin D, Vincent M, Martel I, Hominal S, Cordier JF and Carrie C: A
prospective study on radiation pneumonitis following conformal
radiation therapy in non-small-cell lung cancer: Clinical and
dosimetric factors analysis. Radiother Oncol. 71:175–181. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rancati T, Ceresoli GL, Gagliardi G,
Schipani S and Cattaneo GM: Factors predicting radiation
pneumonitis in lung cancer patients: A retrospective study.
Radiother Oncol. 67:275–283. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Graham MV, Purdy JA, Emami B, Harms W,
Bosch W, Lockett MA and Perez CA: Clinical dose-volume histogram
analysis for pneumonitis after 3D treatment for non-small cell lung
cancer (NSCLC). Int J Radiat Oncol Biol Phys. 45:323–329. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schallenkamp JM, Miller RC, Brinkmann DH,
Foote T and Garces YI: Incidence of radiation pneumonitis after
thoracic irradiation: Dose-volume correlates. Int J Radiat Oncol
Biol Phys. 67:410–416. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang S, Liao Z, Wei X, Liu HH, Tucker SL,
Hu CS, Mohan R, Cox JD and Komaki R: Analysis of clinical and
dosimetric factors associated with treatment-related pneumonitis
(TRP) in patients with non-small-cell lung cancer (NSCLC) treated
with concurrent chemotherapy and three-dimensional conformal
radiotherapy (3D-CRT). Int J Radiat Oncol Biol Phys. 66:1399–1407.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chung HT, Lee B, Park E, Lu JJ and Xia P:
Can all centers plan intensity-modulated radiotherapy (IMRT)
effectively? An external audit of dosimetric comparisons between
three-dimensional conformal radiotherapy and IMRT for adjuvant
chemoradiation for gastric cancer. Int J Radiat Oncol Biol Phys.
71:1167–1174. 2008. View Article : Google Scholar : PubMed/NCBI
|