Introduction
The immune system is a complex, balanced and organic
entity. Under normal conditions, cells of the immune system
recognize foreign antigens and destroy them, while playing a
continuous role in immune regulation, it is vital to maintain a
balanced immune response. Cytokines are small secreted proteins
which function as the mediators of cell differentiation,
specificity, inflammation, immunopathology and immune responses
(1–3).
The interleukin-1 (IL-1) family cytokines play a central role in
mediating the activation of innate and adaptive immune responses
(4). So far, eleven IL-1 family
members have been identified, including seven receptor agonists
(IL-1α, IL-1β, IL-18, IL-33, IL-36α, IL-36β and IL-36γ), three
ligands with antagonist activity (IL-1Ra, IL-36Ra and IL-38) and a
newly renamed anti-inflammatory cytokine, IL-37 (4–6). IL-37,
originally known as IL-1 family member 7 (IL-1F7), is the seventh
member of the IL-1 family discovered by computational cloning in
2000 and was renamed in 2010 (7–9). IL-37 has
been investigated as a natural inhibitor of immune responses in
chronic inflammatory and autoimmune disorders and cancer. In this
review, we will summarize the current knowledge of the location,
structure, expression and regulation of IL-37. Furthermore, we will
discuss the function and potential clinical application of IL-37
for identifying novel therapeutic targets and developing new
IL-37-based therapies for the treatment of human diseases.
Location and structure of IL-37
The human IL-37 gene cluster is located on
chromosome 2 (9,10). The IL-37 gene seems to be absent in
mice as no cDNA or genomic sequence related to human IL-37 has been
reported (9,11). The IL-37 gene size is 3.617 kb and
includes six exons which encode a 17–26 KDa protein (9,12). The
IL-37 gene undergoes alternative splicing; five different splice
variants of IL-37 have been identified and termed IL-37a-e, of
which IL-37b is the largest (218 amino acids) and best
characterized isoform (Fig. 1)
(8,9,13,14). The transcript variant encoded by
IL-37b contains exons 1 and 2 and has an N-terminal prodomain,
which includes a potential caspase-1 cleavage site, leading to the
IL-37b precursor being spliced into mature IL-37b (9,11). In
addition, IL-37b also encodes exons 4–6 which contain the 12
putative β-strands necessary for forming the IL-1-like β-trefoil
secondary structure (9,15). IL-37b shares a β-barrel structure
which is commonly present in other members of the IL-1 family, and
binds to the IL-18 receptor (IL-18R) α chain (16,17).
IL-37a has a distinctive start codon in exon 3 which encodes a
unique N-terminus and is absent in IL-37c, IL-37d and IL-37e, which
is then spliced in exons 4 to 6 (9,16). The
sequence encoded by exon 3 results in a prodomain which is
processed into the mature form of IL-37a protein (10). IL-37c, first reported by Busfield
et al, is similar to IL-37b (18). The IL-37c transcript variant comprises
exons 1 and 2 followed by exons 5 and 6 (9). It seems that IL-37c could not represent
a functional form a cytokine (9).
Exon 2 of IL-37d is missing, instead it contains only exon 1
followed by exons 4–6 and may function as a cytokine (9). IL-37e comprises only exons 1, 5 and 6
and can not bind to IL-18R as a result of lacking exon 4 (9). Interestingly, a chimeric transcript
including exons 1, 4 and 5 of IL-37 has been found to be cleaved
into the 5′UTR of the full length IL-36γ message (9,10).
Expression and regulation of IL-37
Expression
IL-37a, IL-37b, and IL-37c are found to be expressed
in a variety of normal cells and tissues including natural killer
(NK) cells, stimulated B cells, monocytes, skin keratinocytes,
epithelial cells, lymphnode, thymus, lung, colon, uterus and bone
marrow (9,18–25).
However, some IL-37 isoforms are expressed in a tissue specific
manner. Brain only expresses IL-37a, kidney only IL-37b, heart only
IL-37c, and bone marrow and testis only IL-37d (9,10,26,27).
IL-37b was first discovered in bone marrow, and was synthesized by
neutrophils (28). Following this,
IL-37b has been found mainly in blood cells, skin keratinocytes,
and the respiratory and gastrointestinal tracts (28). The chimeric transcript including exons
1, 4 and 5 of IL-37 is also found in testis and placenta, but its
function remains unclear (9).
It is estimated that IL-37 protein translocates to
the nucleus, redistributes between intracellular and extracellular
sites and affects cellular responses, which may explain why the
IL-37 level is reduced in serum (28,29). What
is noteworthy is that IL-37 expression is seemingly dependent on
the inflammatory milieu and inflammatory cells (11). Emerging evidence demonstrates that
IL-37 is expressed at low levels in human cells and tissues but
upregulated by inflammatory stimuli and pro-cytokines including
several toll-like receptor (TLR) agonists, IL-18, interferon
(IFN)γ, IL-1b, transforming growth factor β1 and tumor necrosis
factor (TNF) (Table I) (17,30,31). Other
factors, such as granulocyte-macrophage colony-stimulating factor
(GM-CSF) plus IL-4, suppress IL-37 expression (17). Li et al proved that IL-37 is
increased after stimulation by TLR agonists in monocytes (28). Interestingly, Nold et al
clarified that IL-37b protein expression increased dose-dependently
after stimulation with LPS in a mouse macrophage RAW cell line that
stably expresses human IL-37b (17).
 | Table I.Factors and signal pathways involved
in IL-37 expression. |
Table I.
Factors and signal pathways involved
in IL-37 expression.
| Cell type | Triggering
factor | Regulation | Signal pathway
involved | (Refs.) |
|---|
| Monocytes | TLR agonists | Increased | Unclear | (28) |
| RAW cell line | LPS | Increased | Unclear | (17) |
| THP cells | Triptolide,
triptonide | Increased | ERK1/2, p38 | (30) |
| Human cells | TNF-a | Increased | MAPK, PI3K, NF-kB,
AP-1 | (28) |
| Epithelial
cells | Mannose-capped
lipoarabinomannan | Increased | ERK1/2, p38 | (40) |
| Keratinocytes | Human
β-defensin-3 | Increased | CCR6 | (43) |
| Human cells | IL-18, IFN-γ,
IL-1b, TGF-β1 | Increased | Unclear | (17,30,31) |
| Human cells | GM-CSF, IL-4 | Iecreased | Unclear | (17) |
| Endothelial
cells | ICAM-1, NF-κB | Iecreased | TLR2
activation | (45) |
| BMDMs | M-CSF, GM-CSF,
IL-6 | Iecreased | Unclear | (49) |
| HUVECs | VEGF | Iecreased | Unclear | (75) |
To further investigate the intracellular expression
pattern of IL-37b, Sharma et al generated fusion proteins of
IL-37b, with either yellow fluorescence protein (YFP) at the C
terminus (IL-37b-YFP) or cyan fluorescence protein (CFP) at the N
terminus (IL-37b-CFP) by using protein fusing techniques (29). They then transfected mouse RAW cells
with these two different IL-37b fusion proteins, intracellular
expression of which were found to be at low levels. However, after
LPS stimulation, only IL-37b-YFP translocated into the nucleus,
which suggests that the N terminus of IL-37b is processed before
nuclear translocation (29).
Therefore, only the post-cleavage mature form of the IL-37b
precursor, but not the N terminal fragment, specifically
translocates to the nucleus after LPS exposure (29). Thus, after stimulation, IL-37 is
processed from pro-protein to its mature form (9).
Regulation
The mitogen activated protein kinases (MAPK) signal
pathway is one of the main signaling pathways involved in immune
responses, with vital roles in the regulation of cytokine and
chemokine responses (32). There are
three main components in the MAPK signaling pathway: The Jun
N-terminal kinase (JNK 1/2/3), extracellular signal-regulated
kinase (ERK1/2, ERK 3/4, ERK5, ERK 7/8,) and p38 MAPKs (p38α/β/γ/δ)
(33–36). Once stimulated, the MAPKs, which are
expressed in all cell types, are activated and participate in
various physiological and biologic processes including
inflammation, growth, differentiation, survival and apoptosis of
cells (35–37). Phosphatidylinositol 3-kinases (PI3Ks)
are a family of lipid kinases that play important roles in
intracellular signal transduction and regulation of inflammatory
and immune processes (38,39). Based on protein structure and
substrate specificity, PI3Ks are classified into three classes,
PI3K I–III (39).
Recent studies have identified MAPK and PI3K
signaling pathways as mediators of regulating various agents in
IL-37 expression. Triptolide and triptonide, two active components
extracted from the herb Tripterygium wilfordii Hook F
(TwHF), upregulate IL-37 expression and this expression is
suppressed by inhibitors of the ERK1/2 and p38 signal pathways in
THP cells (30). TNF-α induces IL-37b
mRNA expression by activating MAPK and PI3K signaling pathways and
the transcription factors NF-κB and AP-1 (28). Mannose-capped lipoarabinomannan
purified from Mycobacterium tuberculosis induces IL-37
production in a time- and dose-dependent manner via upregulating
TLR2 expression and enhancing p38 and ERK1/2 phosphorylation in
human type II alveolar epithelial cells (40).
A study in human PBMCs has shown that IL-1F7
expression is markedly increased by activation of each TLR, except
TLR7 and TLR8 (41). Optimization of
the TLR5 response causes a significant increase in IL-37 mRNA and
protein expression in intestinal epithelial cells (42). In a recent study, it has been
demonstrated that human β-defensin-3 (hBD-3) upregulates IL-37
expression via CCR6 in human keratinocytes (43). In addition, hBD-3 also induces the
release of IL-37 into the culture supernatants. However, the
signaling pathways participating in IL-37 expression remain to be
defined, and the mechanisms of IL-37 regulation will continue to
attract further attention.
The function of IL-37
The biological function of IL-37 is just beginning
to be explored (Fig. 2) (26). There is still a long way to go before
the specific role of IL-37 is completely elucidated, but so far,
the anti-inflammatory effect of IL-37 has been comprehensively
reported. As an inhibitor of both innate and adaptive immunity and
inflammatory responses, IL-37 plays a pivotal role in the
antimicrobial response, including antiviral, antibacterial,
neutralization of endotoxins and anti-immune and tumor regulation,
mainly by changing the permeability of bacterial cells (28).
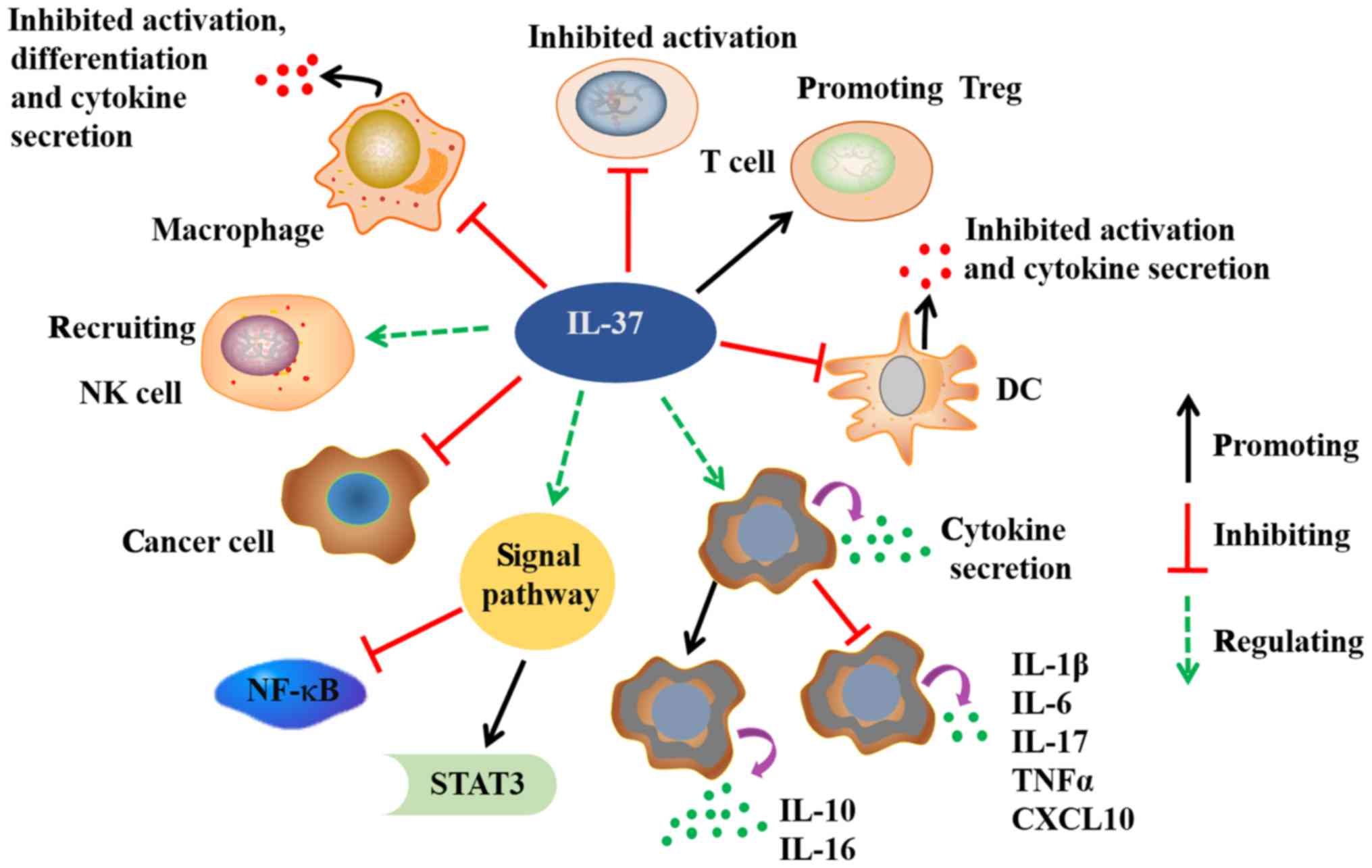 | Figure 2.Possible biological functions of
IL-37. IL-37 exerts significant anti-inflammatory, anticancer,
immune deviatory, immunosuppressive, and metaboregulatory effects.
IL-37 dramatically reduces the cytokines secretion in macrophages
and DCs. The activation and differentiation of macrophages, DCs and
T cells are also inhibited by IL-37. In addition to healthy
tissues, IL-37 is variably expressed in many cancer cells. IL-37
exerts antitumor immune responses through recruiting NK cells into
tumors tissues. The binding of IL-37 to its receptor activates
STAT-3, and inhibits NF-kB signals. IL, interleukin; STAT-3, signal
tranducer and activator of transcription 3; NF-kB, nuclear factor
kB; DC, dendritic cell; Treg, T regulatory cell; TNFα, tumor
necrosis factor α; CXCL10, C-X-C motif chemokine 10. |
IL-37 significantly decreases proinflammatory
cytokines secreted by macrophages and dendritic cells (DCs),
inhibits their activation and macrophages differentiation (11). SiRNA knockdown of IL-37 in PBMCs and
human renal tubular epithelial cells increases the production of
IL-6, TNF-α and IL-1β induced by inflammatory stimuli and cytokines
(44). In human coronary artery
endothelial cells (HCAECs), IL-37 suppresses both NF-κB and ICAM-1
expression upon TLR2 activation (45). Moreover, Li et al demonstrated
that epithelial cell-derived IL-37 inhibits T cell and DCs
activation in the inflammatory mucosa of inflammatory bowel disease
(IBD), possibly by reducing CD86 and major histocompatibility
complex (MHC) II surface expression in DCs (28). IL-37 induction of tolerogenic DCs may
help to induce regulatory T cells (Tregs) (11). Nold et al found that IL-37
functions partly via the IL-37-smad3 complex in the nucleus and
smad3 knockdown reduces the activity of IL-37 (17). McNamee et al proved that
transgenic expression of human IL-37 (IL-37tg) remarkably protects
against LPS-induced shock in a mouse model (31).
In addition, IL-37tg mice subjected to
dextransulfate sodium-induced colitis show a lower level of colonic
inflammation, decreased IL-1β and TNF-α secretion, but increased
IL-10 production (31). Furthermore,
IL-10 is not necessary for IL-37 function as an IL-10 receptor
blocking antibody has no effect on IL-37-mediated anti-inflammatory
effects (31). rIL-37tg mice also
exhibit inhibition of cytokine and chemokine (IL-6, IL-1a, IL-13,
GM-CSF, etc) expression after spinal cord injury (46). Therefore, IL-37 plays a vital role in
modulation of intestinal and spinal cord inflammation. Besides,
IL-37 strongly inhibit TNF-α-induced IP-10 expression (47). Except for its anti-inflammatory
effects, Zhao et al indicated that IL-37 is also a negative
regulator of immune responses in Liseria monocytogenes (Lm)
infection due to reduced production of colony-stimulating factors
and increased macrophage apoptosis (48).
Similar to IL-33 and IL-1α, IL-37 also translocates
into the nucleus in a caspase-1-dependent manner, decreases
cytokine production and affects innate and adaptive immune
responses (29,49). Li et al reported an
extracellular function of the IL-37 precusor, which suppresses
LPS-induced IL-6 production in human M1 differentiated macrophages
(49). IL-37 acts as an extracellular
cytokine by binding to the IL-18 receptor and requires the IL-1
family decoy receptor IL-1R8 for its anti-inflammatory function
(49) It has been shown that these
pro-inflammatory cytokines (TNF-α, IL-1α, IL-1β, IFN-γ) could play
pivotal roles in experimental autoimmune thyroiditis, multiple
sclerosis, insulin-dependent diabetes mellitus or experimental
autoimmune diabetogenesis, which may indirect suggest the
biological and potentially therapeutic relevance of IL-37 to these
diseases (50–55). However, it remains unknown whether
nuclear translocation of IL-37 is the only mechanism that leads to
reduction in cytokine expressions. The specific mechanism of
IL-37-mediated suppression of the adaptive immunity also remains
unclear. Therefore, further studies on IL-37 function are
needed.
IL-37 expression in human diseases
A growing body of literature has demonstrated that
IL-37 is expressed and exerts anti-inflammatory effects in a
variety of diseases including melanoma, rheumatoid arthritis,
morbid obesity, contact hypersensitivity, atopic dermatitis, liver
inflammatory injury, systemic lupus erythematosus (SLE) and IBD,
among others (Table II) (13,14,28,56–65).
In addition, as IL-37 has a strong effect on inhibiting
inflammatory responses, many studies have focused on proving an
association between IL-37 expression levels and the severity of
inflammatory and autoimmune disease (66,67).
 | Table II.Expression and clinical significance
of IL-37 in human disorders. |
Table II.
Expression and clinical significance
of IL-37 in human disorders.
| Author | Year | Disease | No. of samples | Methods | Expression
level | Association | (Refs.) |
|---|
| Liu et
al | 2014 | Children with
allergic rhinitis | 40 | ELISA | Decreased | The efficacy of
intranasal steroid therapy | (14) |
| Li et
al | 2014 | Graves'
disease | 40 | RT-PCR and
ELISA | Increased | TNF-a, IL-6, IL-17
and disease activity | (13) |
| Li et
al | 2014 | Inflammatory bowel
disease | 27 | ELISA | Decreased | UC activity | (28) |
| Højen et
al | 2015 | Chronic
HIV-1-infected individuals | 60 | Quantitative
RT-PCR | Increased | The size of the
total viral HIV-1 reservoir | (56) |
| Wan et
al | 2014 | Intervertebral disc
degeneration | 14 | RT-PCR and western
blotting | Decreased | Disease
aggravation | (57) |
| Günaltay et
al | 2014 | Microscopic and
ulcerative colitis | 31 | Quantitative
RT-PCR | Decreased | Possible UC
remission | (58) |
| Chen et
al | 2015 | Ankylosing
spondylitis | 46 | RT-PCR and
ELISA | Increased | Disease
activity | (59) |
| Ye et
al | 2014 | Systemic lupus
erythematosus | 66 | RT-PCR and
ELISA | Increased | Disease
activity | (60) |
| Ji et
al | 2014 | Acute coronary
syndrome | 257 | ELISA | Increased | The onset of
ACS | (61) |
| Wang et
al | 2015 | Myocardial
infarction | 56 | Immunoblotting | Decreased | Possible leukocytic
inflammation | (62) |
| Yu et
al | 2016 | Arterial
calcification | 125 |
Immunohistochemistry and ELISA | Increased | The onset of
arterial calcification | (63) |
| Zhao et
al | 2014 | Hepatocellular
carcinoma | 163 | Immunohistochemical
staining | Decreased | Tumor size, OS,
DFS, the density of tumor-infiltrating NK cells | (64) |
| Ge et
al | 2016 | Non-small cell lung
cancer | 182 | Immunohistochemical
staining and RT-PCR | Decreased | Tumor
angiogenesis | (65) |
Chronic inflammatory and autoimmune
disorders
In recent years, it has been reported that IL-37
expression is apparently related to IBDs. The IL-37 expression
level is significantly upregulated in macrophages of Crohn's
disease (CD) lesions and in the skin lesions of psoriasis patients
(9). Increased epithelial IL-37b
protein expression has also been identified in the inflamed mucosa
of IBD patients (22). Serum IL-37
levels are significantly reduced in ulcerative colitis (UC) and CD
patients compared with healthy subjects (28). IL-37 is expressed in intestinal
epithelial and inflammatory cells and serum IL-37 levels show an
inverse correlation with UC activity (28). Immunohistochemistry and western blot
analyses prove that IL-37 protein expression levels are higher in
UC and CD patients than in healthy people, and are highest in
samples from UC patients compared with that of CD patients
(28). Nevertheless, the function of
IL-37 is still not completely understood in different inflammatory
diseases and the inflammation in microscopic colitis (MC) is more
subtle than in UC and CD (58).
Günaltay et al further found that UC remission patients
demonstrated increased expression levels of IL-37 mRNA, suggesting
that IL-37 may be involved in inflammation and therefore
contributing to UC remission (56).
On the contrary, low IL-37 expression may contribute to the
chronicity of colonic inflammation in MC and UC patients (56).
IL-37 protein is higher in synovial cells of
rheumatoid arthritis patients compared with those of healthy donors
(17,28). Luo et al proved that IL-37
expression in DCs impairs the activation of effector T-cell
responses, induces Tregs and regulates adaptive immunity in contact
hypersensitivity (68). Ankylosing
spondylitis (AS) is a common chronic, progressive, immune-mediated
inflammatory disorder characterized by sacroileitis and axial
inflammation (69). Chen et al
showed that IL-37 secretion and mRNA levels are significantly
higher in PBMCs isolated from AS patients compared with healthy
controls (59). IL-37 level is also
correlated with the activity of AS, particularly with the primary
pro-inflammatory cytokines involved in AS (59). Furthermore, human recombinant IL-37
dramatically impairs LPS-stimulated IL-6, IL-17, IL-23 and TNF-α
production in PBMCs from AS patients (59).
A recent study showed that over-expressed IL-37 mRNA
levels in adipose tissue may lead to better insulin sensitivity and
protect against insulin resistance in obesity-related inflammation
(70). Additionally, a remarkable
positive correlation between IL-37 mRNA level and the size of the
HIV-1 reservoir has been elucidated (56). LPS exposure increases IL-37 mRNA
expression to higher levels in HIV-1-infected patients compared
with non-infected individuals (56).
Previously it has been reported that the monocyte inflammatory
marker sCD14 is associated with increased mortality in HIV-1
infection, and high levels of sCD163 were observed among
HIV-1-infected patients (71,72). However, recently a positive
correlation between sCD14 and IL-37 mRNA, rather than sCD163, has
been observed in a cross-sectional cohort study, which implies a
functional link between IL-37 and monocyte activation (65).
Li et al verified that the levels of IL-37 in
PBMCs and serum are remarkably increased in patients with Graves'
disease (GD) (13). Higher IL-37 mRNA
and serum protein levels are positively correlated with the
activity of GD (13). Moreover, serum
IL-37 is positively associated with free triiodothyronine (FT3) and
free thyroxine (FT4) but negatively correlated with thyrotropin
(TSH) (13). Serum IL-37 is also
significantly associated with TSH receptor antibody (TRAB), IL-6,
IL-17 and TNF-α (13). Surprisingly,
IL-37 inhibits the production of TNF-α, IL-6 and IL-17 in PBMCs of
GD patients during GD pathogenesis (13). Wan et al reported that
decreased IL-37 expression leads to the increased secretion of
pro-inflammatory cytokines including IL-16 and IL-1β in
degenerative intervertebraldisc, which suggests a function for
IL-37 in delaying the progression of intervertebral disc
degeneration (57). It has also been
shown that IL-37 expression is significantly higher in the plasma
of patients with SLE and that IL-37 suppresses the secretion of
pro-inflammatory cytokines in PBMCs of SLE patients (60,73,74).
IL-37b expression levels in serum and nasal lavage are
significantly increased in children with allergic rhinitis (AR)
(14). Furthermore, IL-37b decreased
Th2 cytokine secreted by PBMCs via MAPK and PI3K signal pathways
(14). Conversely, Imaeda et
al found that IL-37b suppresses the Th1-chemokine, CXCL10,
which implies a possible function of IL-37b in inhibiting Th1
inflammation (22).
In conclusion, these results suggest complicated
biological functions of IL-37 in different diseases. IL-37
expression in autoimmune diseases seems to decrease excessive
inflammatory immune responses. However, further detailed study
remains necessary to explore the specific mechanisms and potential
immunosuppressive functions of IL-37 in inflammatory and autoimmune
diseases.
Cardiac diseases
IL-37 expression is found to be increased in
patients with acute coronary syndrome (61,75,76).
Inflammationis an important step and the NF-κB signaling pathway is
activated after acute myocardial infarction (AMI). Moreover,
inhibition of the NF-κB signaling pathway improves cardiac function
after AMI through decreasing the left ventricular shortening
fraction (77–79). IL-37 expression level is normally low
in PBMCs, being mainly expressed in DCs and monocytes, but rapidly
increases in the context of inflammation following AMI (61). Plasma IL-37 expression is decreased in
patients with acute ST-segment elevation myocardial infarction
(ASTEMI) (62). In patients with
arterial calcification, high concentrations of IL-37 have been
detected and IL-37 is positively correlated with age, fasting
glucose, alkaline phosphatase, IL-6, TNF-α, C-reactive protein and
Agatston scores (63).
Excessive myocardial inflammatory responses to
endotoxemia frequently leads to cardiac dysfunction. Expression of
IL-37 suppresses LPS-induced MCP-1 and ICAM-1 production and NF-κB
activation in cardiac microvascular endothelial cells (80). In addition, Xu et al found that
IL-37 suppresses MPO expression and recombinant IL-37 effectively
suppresses activation of the NF-κB signaling pathway, and finally
results in an anti-inflammatory effect in AMI mice (77).
Cancer
Transcripts of IL-37 have been detected in human
cancers and human cancer cell lines including THP-1, U937 and A431
(30,64). However, the biological role of IL-37
in cancers and the relationship between this cytokine and cancer is
largely unknown.
To explore IL-37 expression, Zhao et al
examined a relatively large series of hepatocellular carcinoma
(HCC) clinical specimens by immunohistochemistry (64). IL-37 is decreased in tumor tissues
compared with adjacent non-tumor tissues and normal liver samples
(64). The expression level of IL-37
is negatively correlated with tumor size and high IL-37 expression
is linked to disease-free survival (DFS) and better overall
survival (OS) in HCC patients, which suggests that IL-37 may be a
potentially valuable prognostic marker for HCC patients (64). Wang et al offered evidence that
IL-37 inhibits the proliferation and invasion of cervical cancer
(CC) cells via the signal transducer and activator of transcription
3 (STAT3) signaling pathway (12).
IL-37 upregulated STAT3 expression at the gene and protein levels
and reduced STAT3 phosphorylation (12). After transfection with siSTAT3, CC
cell proliferation and invasion inhibited by IL-37 was
significantly reversed. STAT3 overexpression restored the CC cell
growth and invasion, and increased the transcription of TNF-α and
IL-1β (12).
IL-37 expression is upregulated in breast carcinoma
tissues, which indicates that this cytokine may have a role in
tumor progression (9,81). However, IL-37 expression is
downregulated in lung cancer tissues and, it suppresses
tumorigenesis in non-small cell lung cancer (NSCLC) in vivo.
IL-37 may thus have an inhibitory function in NSCLC development
(65,82). However, the specific mechanism and
signaling pathways involved in the IL-37-induced immune responses
in cancer remain unclear and need further exploration. Infiltration
of NK cells into the tumor area is necessary for the activation of
potent antitumor immunity (83).
IL-37 expression in HCC is positively linked to the density of
CD57-positive NK cells, and consequently IL-37-overexpressing HCC
cells significantly inhibit tumor growth and recruit more NK cells
into tumor tissues in vivo mice experiments (64). Thus, IL-37 may be involved in
antitumor immune responses via regulating NK cells in the tumor
microenvironment.
Potential roles of IL-37 in clinical
therapy
A comprehensive knowledge of the function of
cytokines in the pathogenesis of human disorders has led to the
exploration of new therapies targeted at neutralizing specific
cytokines or inhibiting their signaling pathways (3,84–87). IL-37 plays a vital role in innate and
adaptive immunity and may be a useful molecule for effective
cytokine therapy.
It has been shown that IL-37 suppresses the innate
immunity to infection-mediated inflammation, which may be of
therapeutic value in reducing pulmonary damage in bacterial
diseases (8). Zhao et al and
Gao et alfound that intra-tumoral injection of IL-37 leads
to strong inhibition of tumor growth and this effect is dependent
on T cells and B cells as it is reversed in IL-12-, IFN-γ- or Fas
ligand-deficient mice and in nude and SCID mice (64,88). Yin
et al showed that a single nucleotide polymorphism in the
IL-37 gene (rs3811047) is significantly associated with
coronary artery disease (CAD), which suggests that IL37 represents
a new susceptibility gene for CAD (89). However, there have been no clinical
trials to date to prove the effect of IL-37 on disease treatment.
Therefore, it is of great significance to evaluate whether IL-37
can be implicated in cytokine therapy for curing diseases in the
future.
Conclusion
In summary, as a new anti-inflammatory inhibitor,
IL-37 plays important roles in immune responses, protects from
inflammatory and autoimmune diseases, and holds great potential for
clinical applications. As such, IL-37 research continues to receive
increasing attention. However, several IL-37 mysteries remain
unclear, and further detailed study remains necessary to fully
determine the possible functions of IL-37. While challenges and
opportunities still coexist for IL-37, this cytokine may emerge as
a new target for diagnosis and therapy of cancer, inflammatory and
autoimmune diseases in the near future.
Acknowledgements
The present study is supported by Funds for the
Natural Science Foundation of Shandong Province (grant no.
ZR2014HM077), the Key Research Project program of Shandong Province
(grant no. 2016GSF201056), Young Scholars of National Natural
Science Foundation of China (no. 81402353) and the Medical Health
Science and Technology Development Plan of Shandong Province (no.
2014WS0287), China Postdoctoral Science Foundation (no.
2015M580594) and Postdoctoral Innovation Foundation of Shandong
Province (grant no. 201502008).
Glossary
Abbreviations
Abbreviations:
|
IL-1
|
interleukin-1
|
|
IL-37
|
interleukin-37
|
|
IL-1F7
|
IL-1 family member 7
|
|
IL-18R
|
IL-18 receptor
|
|
NK
|
natural killer
|
|
TLR
|
toll-like receptor
|
|
IFN
|
interferon
|
|
TGF-β1
|
transforming growth factor β1
|
|
TNF
|
tumor necrosis factor
|
|
GM-CSF
|
granulocyte-macrophage
colony-stimulating factor
|
|
YFP
|
yellow fluorescence protein
|
|
CFP
|
cyan fluorescence protein
|
|
MAPK
|
mitogen activated protein kinases
|
|
JNK
|
the Jun N-terminal kinase
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
PI3Ks
|
Phosphatidylinositol 3-kinases
|
|
TwHF
|
Tripterygium wilfordii Hook
F
|
|
hBD-3
|
human β-defensin-3
|
|
DCs
|
dendritic cells
|
|
HCAECs
|
human coronary artery endothelial
cells
|
|
MHC
|
major histocompatibility complex
|
|
Tregs
|
regulatory T cells
|
|
DSS
|
dextransulfate sodium
|
|
UC
|
ulcerative colitis
|
|
CD
|
Crohn's disease
|
|
MC
|
microscopic colitis
|
|
AS
|
Ankylosing spondylitis
|
|
GD
|
Graves' disease
|
|
FT3
|
free triiodothyronine
|
|
FT4
|
FT4, freethyroxine
|
|
TSH
|
thyrotropin
|
|
TRAB
|
thyrotropin receptor antibody
|
|
Lm
|
Liseria monocytogenes
|
|
SLE
|
systemic lupuserythematosus
|
|
IBD
|
inflammatory bowel disease
|
|
AR
|
allergic rhinitis
|
|
AMI
|
acute myocardial infarction
|
|
ASTEMI
|
acute ST-segment elevation myocardial
infarction
|
|
HCC
|
hepatocellular carcinoma
|
|
DFS
|
disease-free survival
|
|
OS
|
overall survival
|
|
CC
|
cervical cancer
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
NSCLC
|
non-small cell lung cancer
|
|
CAD
|
coronary artery disease
|
References
|
1
|
Moran EM and Mastaglia FL: Cytokines in
immune-mediated inflammatory myopathies: Cellular sources, multiple
actions and therapeutic implications. Clin Exp Immunol.
178:405–415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hoeppli RE, Wu D, Cook L and Levings MK:
The environment of regulatory T cell biology: Cytokines,
metabolites and the microbiome. Front Immunol. 6:612015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Venkatesha SH, Dudics S, Acharya B and
Moudgil KD: Cytokine-modulating strategies and newer cytokine
targets for arthritis therapy. Int J Mol Sci. 16:887–906. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Garlanda C, Dinarello CA and Mantovani A:
The interleukin-1 family: Back to the future. Immunity.
39:1003–1018. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dinarello CA: Interleukin-1 in the
pathogenesis and treatment of inflammatory diseases. Blood.
117:3720–3732. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smith DE: The biological paths of IL-1
family members IL-18 and IL-33. J Leukoc Biol. 89:383–392. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dinarello C, Arend W, Sims J, Smith D,
Blumberg H, O'Neill L, Goldbach-Mansky R, Pizarro T, Hoffman H,
Bufler P, et al: IL-1 family nomenclature. Nat Immunol. 11:9732010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moretti S, Bozza S, Oikonomou V, Renga G,
Casagrande A, Iannitti RG, Puccetti M, Garlanda C, Kim S, Li S, et
al: IL-37 inhibits inflammasome activation and disease severity in
murine aspergillosis. PLoS Pathog. 10:e10044622014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boraschi D, Lucchesi D, Hainzl S, Leitner
M, Maier E, Mangelberger D, Oostingh GJ, Pfaller T, Pixner C,
Posselt G, et al: IL-37: A new anti-inflammatory cytokine of the
IL-1 family. Eur Cytokine Netw. 22:127–147. 2011.PubMed/NCBI
|
|
10
|
Taylor SL, Renshaw BR, Garka KE, Smith DE
and Sims JE: Genomic organization of the interleukin-1 locus.
Genomics. 79:726–733. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ye L and Huang Z: IL-37 restrains
autoimmune diseases. Oncotarget. 6:21775–21776. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang S, An W, Yao Y, Chen R, Zheng X, Yang
W, Zhao Y, Hu X, Jiang E, Bie Y, et al: Interleukin 37 expression
inhibits STAT3 to Suppress the proliferation and invasion of human
cervical cancer cells. J Cancer. 6:962–969. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Wang Z, Yu T, Chen B, Zhang J, Huang
K and Huang Z: Increased expression of IL-37 in patients with
Graves' disease and its contribution to suppression of
proinflammatory cytokines production in peripheral blood
mononuclear cells. PLoS One. 9:e1071832014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu W, Deng L, Chen Y, Sun C, Wang J, Zhou
L, Li H and Luo R: Anti-inflammatory effect of IL-37b in children
with allergic rhinitis. Mediators Inflamm. 2014:7468462014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murzin AG, Lesk AM and Chothia C:
Beta-Trefoil fold. patterns of structure and sequence in the kunitz
inhibitors interleukins-1 beta and 1 alpha and fibroblast growth
factors. J Mol Biol. 223:531–543. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Banchereau J, Pascual V and O'Garra A:
From IL-2 to IL-37: The expanding spectrum of anti-inflammatory
cytokines. Nat Immunol. 13:925–931. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nold MF, Nold-Petry CA, Zepp JA, Palmer
BE, Bufler P and Dinarello CA: IL-37 is a fundamental inhibitor of
innate immunity. Nat Immunol. 11:1014–1022. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Busfield SJ, Comrack CA, Yu G, Chickering
TW, Smutko JS, Zhou H, Leiby KR, Holmgren LM, Gearing DP and Pan Y:
Identification and gene organization of three novel members of the
IL-1 family on human chromosome 2. Genomics. 66:213–216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smith DE, Renshaw BR, Ketchem RR, Kubin M,
Garka KE and Sims JE: Four new members expand the interleukin-1
superfamily. J Biol Chem. 275:1169–1175. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ross R, Grimmel J, Goedicke S, Möbus AM,
Bulau AM, Bufler P, Ali S and Martin MU: Analysis of nuclear
localization of interleukin-1 family cytokines by flow cytometry. J
Immunol Methods. 387:219–227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Akdis M, Burgler S, Crameri R, Eiwegger T,
Fujita H, Gomez E, Klunker S, Meyer N, O'Mahony L, Palomares O, et
al: Interleukins, from 1 to 37 and interferon-γ: Receptors,
functions and roles in diseases. J Allergy Clin Immunol.
127:701–721. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Imaeda H, Takahashi K, Fujimoto T, Kasumi
E, Ban H, Bamba S, Sonoda H, Shimizu T, Fujiyama Y and Andoh A:
Epithelial expression of interleukin-37b in inflammatory bowel
disease. Clin Exp Immunol. 172:410–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pan G, Risser P, Mao W, Baldwin DT, Zhong
AW, Filvaroff E, Yansura D, Lewis L, Eigenbrot C, Henzel WJ and
Vandlen R: IL-1H, an interleukin 1-related protein that binds IL-18
receptor/IL-1Rrp. Cytokine. 13:1–7. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bufler P, Gamboni-Robertson F, Azam T, Kim
SH and Dinarello CA: Interleukin-1 homologues IL-1F7b and IL-18
contain functional mRNA instability elements within the coding
region responsive to lipopolysaccharide. Biochem J. 381:503–510.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kumar S, McDonnell PC, Lehr R, Tierney L,
Tzimas MN, Griswold DE, Capper EA, Tal-Singer R, Wells GI, Doyle ML
and Young PR: Identification and initial characterization of four
novel members of the interleukin-1 family. J Biol Chem.
275:10308–10314. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dunn E, Sims JE, Nicklin MJ and O'Neill
LA: Annotating genes with potential roles in the immune system: Six
new members of the IL-1 family. Trends Immunol. 22:533–536. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bufler P, Azam T, Gamboni-Robertson F,
Reznikov LL, Kumar S, Dinarello CA and Kim SH: A complex of the
IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18
activity. Proc Natl Acad Sci USA. 99:pp. 13723–13728. 2002;
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Y, Wang Y, Liu Y, Wang Y, Zuo X, Li Y
and Lu X: The possible role of the novel cytokines il-35 and il-37
in inflammatory bowel disease. Mediators Inflamm. 2014:1363292014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sharma S, Kulk N, Nold MF, Gräf R, Kim SH,
Reinhardt D, Dinarello CA and Bufler P: The IL-1 family member 7b
translocates to the nucleus and down-regulates proinflammatory
cytokines. J Immunol. 180:5477–5482. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He L, Liang Z, Zhao F, Peng L and Chen Z:
Modulation of IL-37 expression by triptolide and triptonide in
THP-1 cells. Cell Mol Immunol. 12:515–518. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McNamee EN, Masterson JC, Jedlicka P,
McManus M, Grenz A, Collins CB, Nold MF, Nold-Petry C, Bufler P,
Dinarello CA and Rivera-Nieves J: Interleukin 37 expression
protects mice from colitis. Proc Natl Acad Sci USA. 108:pp.
16711–16716. 2011; View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Su S, Duan J, Chen T, Huang X, Shang E, Yu
L, Wei K, Zhu Y, Guo J, Guo S, et al: Frankincense and myrrh
suppress inflammation via regulation of the metabolic profiling and
the MAPK signaling pathway. Sci Rep. 5:136682015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo C, Xia Y, Niu P, Jiang L, Duan J, Yu
Y, Zhou X, Li Y and Sun Z: Silica nanoparticles induce oxidative
stress, inflammation and endothelial dysfunction in vitro via
activation of the MAPK/Nrf2 pathway and nuclear factor-κB
signaling. Int J Nanomedicine. 10:1463–1477. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fang HU, Zhang HH, Yang BX, Huang JL, Shun
JL, Kong FJ, Peng-Xu, Chen ZG and Lu JM: Cdk5 contributes to
inflammation-induced thermal hyperalgesia mediated by the p38 MAPK
pathway in microglia. Brain Res. 1619:166–175. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lei YY, Wang WJ, Mei JH and Wang CL:
Mitogen-activated protein kinase signal transduction in solid
tumors. Asian Pac J Cancer Prev. 15:8539–8548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grossi V, Peserico A, Tezil T and Simone
C: p38α MAPK pathway: A key factor in colorectal cancer therapy and
chemoresistance. World J Gastroenterol. 20:9744–9758. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jha SK, Jha NK, Kar R, Ambasta RK and
Kumar P: p38 MAPK and PI3K/AKT signalling cascades inparkinson's
disease. Int J Mol Cell Med. 4:67–86. 2015.PubMed/NCBI
|
|
38
|
Lupia E, Pigozzi L, Goffi A, Hirsch E and
Montrucchio G: Role of phosphoinositide 3-kinase in the
pathogenesis of acute pancreatitis. World J Gastroenterol.
20:15190–15199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yip PY: Phosphatidylinositol
3-kinase-AKT-mammalian target of rapamycin (PI3K-Akt-mTOR)
signaling pathway in non-small cell lung cancer. Transl Lung Cancer
Res. 4:165–176. 2015.PubMed/NCBI
|
|
40
|
Huang Z, Zhao GW, Gao CH, Chi XW, Zeng T,
Hu YW, Zheng L and Wang Q: Mannose-capped lipoarabinomannan from
mycobacterium tuberculosis induces Il-37 production via
upregulating ERK1/2 and p38 in human type II alveolar epithelial
cells. Int J Clin Exp Med. 8:7279–7287. 2015.PubMed/NCBI
|
|
41
|
Rudloff I, Cho SX, Lao JC, Ngo D, McKenzie
M, Nold-Petry CA and Nold MF: Monocytes and dendritic cells are the
primary sources of interleukin 37 in human immune cells. J Leukoc
Bio. 101:901–911. 2016. View Article : Google Scholar
|
|
42
|
Gunaltay S, Ghiboub M, Hultgren O and
Hörnquist EH: Reduced IL-37 production increases spontaneous
chemokine expressions in colon epithelial cells. Dig Dis Sci.
62:1204–1215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Smithrithee R, Niyonsaba F, Kiatsurayanon
C, Ushio H, Ikeda S, Okumura K and Ogawa H: Human β-defensin-3
increases the expression of interleukin-37 through CCR6 in human
keratinocytes. J Dermatol Sci. 77:46–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang Y, Zhang ZX, Lian D, Haig A,
Bhattacharjee RN and Jevnikar AM: IL-37 inhibits IL-18-induced
tubular epithelial cell expression of pro-inflammatory cytokines
and renal ischemia-reperfusion injury. Kidney Int. 87:396–408.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xie Y, Li Y, Cai X, Wang X and Li J:
Interleukin-37 suppresses ICAM-1 expression in parallel with NF-κB
down-regulation following TLR2 activation of human coronary artery
endothelial cells. Int immunopharmacolo. 38:26–30. 2016. View Article : Google Scholar
|
|
46
|
Coll-Miró M, Francos-Quijorna I,
Santos-Nogueira E, Torres-Espin A, Bufler P, Dinarello CA and
López-Vales R: Beneficial effects of IL-37 after spinal cord injury
in mice. Proc Natl Acad Sci USA. 113:pp. 1411–1416. 2016;
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu M, Guo S, Hibbert JM, Jain V, Singh N,
Wilson NO and Stiles JK: CXCL10/IP-10 in infectious diseases
pathogenesis and potential therapeutic implications. Cytokine
Growth Factor Rev. 22:121–130. 2011.PubMed/NCBI
|
|
48
|
Zhao M, Hu Y, Shou J, Su SB, Yang J and
Yang T: IL-37 impairs host resistance to listeria infection by
suppressing macrophage function. Biochem Biophys Res Commun.
485:563–568. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li S, Neff CP, Barber K, Hong J, Luo Y,
Azam T, Palmer BE, Fujita M, Garlanda C, Mantovani A, et al:
Extracellular forms of IL-37 inhibit innate inflammation in vitro
and in vivo but require the IL-1 family decoy receptor IL-1R8. Proc
Natl Acad Sci USA. 112:pp. 2497–2502. 2015; View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zaccone P, Fehervari Z, Blanchard L,
Nicoletti F, Edwards CK III and Cooke A: Autoimmune thyroid disease
induced by thyroglobulin and lipopolysaccharide is inhibited by
soluble TNF receptor type I. Eur J Immunol. 32:1021–1028. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dujmovic I, Mangano K, Pekmezovic T,
Quattrocchi C, Mesaros S, Stojsavljevic N, Nicoletti F and Drulovic
J: The analysis of IL-1 beta and its naturally occurring inhibitors
in multiple sclerosis: The elevation of IL-1 receptor antagonist
and IL-1 receptor type II after steroid therapy. J Neuroimmunol.
207:101–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nicoletti F, Patti F, DiMarco R, Zaccone
P, Nicoletti A, Meroni P and Reggio A: Circulating serum levels of
IL-1ra in patients with relapsing remitting multiple sclerosis are
normal during remission phases but significantly increased either
during exacerbations or in response to IFN-beta treatment.
Cytokine. 8:395–400. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nicoletti F, Zaccone P, Di Marco R,
Lunetta M, Magro G, Grasso S, Meroni P and Garotta G: Prevention of
spontaneous autoimmune diabetes in diabetes-prone BB rats by
prophylactic treatment with antirat interferon-gamma antibody.
Endocrinology. 138:281–288. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Nicoletti F, Di Marco R, Barcellini W,
Magro G, Schorlemmer HU, Kurrle R, Lunetta M, Grasso S, Zaccone P
and Meroni P: Protection from experimental autoimmune diabetes in
the non-obese diabetic mouse with soluble interleukin-1 receptor.
Eur J Immunol. 24:1843–1847. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Akdis M, Aab A, Altunbulakli C, Azkur K,
Costa RA, Crameri R, Duan S, Eiwegger T, Eljaszewicz A, Ferstl R,
et al: Interleukins (from IL-1 to IL-38), interferons, transforming
growth factor β and TNF-α: Receptors, functions and roles in
diseases. J Allergy Clin Immunol. 138:984–1010. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Højen JF, Rasmussen TA, Andersen KL,
Winckelmann AA, Laursen RR, Gunst JD, Møller HJ, Fujita M,
Østergaard L, Søgaard OS, et al: Interleukin-37 expression is
increased in chronic HIV-1-Infected individuals and is associated
with inflammation and the size of the total viral reservoir. Mol
Med. 21:337–345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wan ZY, Sun Z, Song F, Chen YF, Zhang WL,
Wang HQ and Luo ZJ: Downregulated interleukin 37 expression
associated with aggravation of intervertebral disc degeneration.
Int J Clin Exp Pathol. 7:656–662. 2014.PubMed/NCBI
|
|
58
|
Günaltay S, Nyhlin N, Kumawat AK, Tysk C,
Bohr J, Hultgren O and Hultgren Hörnquist E: Differential
expression of interleukin-1/Toll-like receptor signaling regulators
in microscopic and ulcerative colitis. World J Gastroenterol.
20:12249–12259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chen B, Huang K, Ye L, Li Y, Zhang J,
Zhang J, Fan X, Liu X, Li L, Sun J, et al: Interleukin-37 is
increased in ankylosing spondylitis patients and associated with
disease activity. J Transl Med. 13:362015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ye L, Ji L, Wen Z, Zhou Y, Hu D, Li Y, Yu
T, Chen B, Zhang J, Ding L, et al: IL-37 inhibits the production of
inflammatory cytokines in peripheral blood mononuclear cells of
patients with systemic lupus erythematosus: Its correlation with
disease activity. J Transl Med. 12:692014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ji Q, Zeng Q, Huang Y, Shi Y, Lin Y, Lu Z,
Meng K, Wu B, Yu K, Chai M, et al: Elevated plasma IL-37, IL-18 and
IL-18BP concentrations in patients with acute coronary syndrome.
Mediators Inflamm. 2014:1657422014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang X, Cai X, Chen L, Xu D and Li J: The
evaluation of plasma and leukocytic IL-37 expression in early
inflammation in patients with acute ST-segment elevation myocardial
infarction after PCI. Mediators Inflamm. 2015:6269342015.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yu K, Min X, Lin Y, Huang Y, Huang S, Liu
L, Peng Y, Meng K, Li D, Ji Q and Zeng Q: Increased IL-37
concentrations in patients with arterial calcification. Clin Chim
Acta. 461:19–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhao JJ, Pan QZ, Pan K, Weng DS, Wang QJ,
Li JJ, Lv L, Wang DD, Zheng HX and Jiang SS: Interleukin-37
mediates the antitumor activity in hepatocellular carcinoma: Role
for CD57+ NK cells. Sci Rep. 4:51772014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ge G, Wang A, Yang J, Chen Y, Yang J, Li Y
and Xue Y: Interleukin-37 suppresses tumor growth through
inhibition of angiogenesis in non-small cell lung cancer. J Exp
Clin Cancer Res. 35:132016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wu BW, Zeng QT, Meng K and Ji QW: The
potential role of IL-37 in atherosclerosis. Pharmazie. 68:857–860.
2013.PubMed/NCBI
|
|
67
|
Sakai N, Van Sweringen HL, Belizaire RM,
Quillin RC, Schuster R, Blanchard J, Burns JM, Tevar AD, Edwards MJ
and Lentsch AB: Interleukin-37 reduces liver inflammatory injury
via effects on hepatocytes and non-parenchymal cells. J
Gastroenterol Hepatol. 27:1609–1616. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Luo Y, Cai X, Liu S, Wang S, Nold-Petry
CA, Nold MF, Bufler P, Norris D, Dinarello CA and Fujita M:
Suppression of antigen-specific adaptive immunity by IL-37 via
induction of tolerogenic dendritic cells. Proc Natl Acad Sci USA.
111:pp. 15178–15183. 2014; View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Jethwa H and Bowness P: The interleukin
(IL)-23/IL-17 axis in ankylosing spondylitis: New advances and
potentials for treatment. Clin Exp Immunol. 138:30–36. 2016.
View Article : Google Scholar
|
|
70
|
Ballak DB, van Diepen JA, Moschen AR,
Jansen HJ, Hijmans A, Groenhof GJ, Leenders F, Bufler P,
Boekschoten MV, Müller M, et al: IL-37 protects against
obesity-induced inflammation and insulin resistance. Nat Commun.
5:47112014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Burdo TH, Lentz MR, Autissier P, Krishnan
A, Halpern E, Letendre S, Rosenberg ES, Ellis RJ and Williams KC:
Soluble CD163 made by monocyte/macrophages is a novel marker of HIV
activity in early and chronic infection prior to and after
anti-retroviral therapy. J Infect Dis. 204:154–163. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sandler NG, Wand H, Roque A, Law M, Nason
MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S, et al:
Plasma levels of soluble CD14 independently predict mortality in
HIV infection. J Infect Dis. 203:780–790. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wu GC, Li HM, Wang JB, Leng RX, Wang DG
and Ye DQ: Elevated plasma interleukin-37 levels in systemic lupus
erythematosus patients. Lupus. 25:1377–1380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Song L, Qiu F, Fan Y, Ding F, Liu H, Shu
Q, Liu W and Li X: Glucocorticoid regulates interleukin-37 in
systemic lupus erythematosus. J Clin Immunol. 33:111–117. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yang T, Fang F, Chen Y, Ma J, Xiao Z, Zou
S, Zheng N, Yan D, Liao S, Chen S, et al: Elevated plasma
interleukin-37 playing an important role in acute coronary syndrome
through suppression of ROCK activation. Oncotarget. 8:9686–9695.
2017.PubMed/NCBI
|
|
76
|
Liu K, Tang Q, Zhu X and Yang X: IL-37
increased in patients with acute coronary syndrome and associated
with a worse clinical outcome after ST-segment elevation acute
myocardial infarction. Clin Chim Acta. 468:140–144. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Xu D, Wang A, Jiang F, Hu J and Zhang X:
Effects of interleukin-37 on cardiac function after myocardial
infarction in mice. Int J Clin Exp Pathol. 8:5247–5251.
2015.PubMed/NCBI
|
|
78
|
Zhang XQ, Tang R, Li L, Szucsik A, Javan
H, Saegusa N, Spitzer KW and Selzman CH: Cardiomyocyte-specific p65
NF-kappaB deletion protects the injured heart by preservation of
calcium handling. Am J Physiol Heart Circ Physiol. 305:H1089–H1097.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zeng M, Wei X, Wu Z, Li W, Li B, Zhen Y,
Chen J, Wang P and Fei Y: NF-κB-mediated induction of autophagy in
cardiac ischemia/reperfusion injury. Biochem Biophys Res Commun.
436:180–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Li J, Zhai Y, Ao L, Hui H, Fullerton DA,
Dinarello CA and Meng X: Interleukin-37 suppresses the inflammatory
response to protect cardiac function in old endotoxemic mice.
Cytokine. 95:55–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ding VA, Zhu Z, Xiao H, Wakefield MR, Bai
Q and Fang Y: The role of IL-37 in cancer. Med Oncol. 33:682016.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Abulkhir A, Samarani S, Amre D, Duval M,
Haddad E, Sinnett D, Leclerc JM, Diorio C and Ahmad A: A protective
role of IL-37 in cancer: A new hope for cancer patients. J Leukoc
Biol. 101:395–406. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Moretta L, Pietra G, Montaldo E, Vacca P,
Pende D, Falco M, Del Zotto G, Locatelli F, Moretta A and Mingari
MC: Human NK cells: From surface receptors to the therapy of
leukemias and solid tumors. Front Immunol. 5:872014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kunz M and Ibrahim SM: Cytokines and
cytokine profiles in human autoimmune diseases and animal models of
autoimmunity. Mediators Inflamm. 2009:9792582009. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Semerano L, Assier E and Boissier MC:
Anti-cytokine vaccination: A new biotherapy of autoimmunity?
Autoimmun Rev. 11:785–786. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Taylor PC and Williams RO: Combination
cytokine blockade: The way forward in therapy for rheumatoid
arthritis? Arthritis Rheumatol. 67:14–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Striz I, Brabcova E, Kolesar L and
Sekerkova A: Cytokine networking of innate immunity cells: A
potential target of therapy. Clin Sci (Lond). 126:593–612. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Gao W, Kumar S, Lotze MT, Hanning C,
Robbins PD and Gambotto A: Innate immunity mediated by the cytokine
IL-1 homologue 4 (IL-1H4/IL-1F7) induces IL-12-dependent adaptive
and profound antitumor immunity. J Immunol. 170:107–113. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yin D, Naji DH, Xia Y, Li S, Bai Y, Jiang
G, Zhao Y, Wang X, Huang Y, Chen S, et al: Genomic Variant in IL-37
confers a significant risk of coronary artery disease. Scientific
reports. 7:421752017. View Article : Google Scholar : PubMed/NCBI
|
















