Introduction
Brenner tumors are uncommon surface
epithelial-stromal tumors of the ovary, frequently presented in
women between their fifth and seventh decades of life. The majority
of Brenner tumors are benign, and these tumors predominantly
present as solid on imaging and pathological examination, although
in <30% of cases, serous and mucinous-associated cystadenomas
may account for the cystic appearance when the Brenner tumor itself
is very small or visually inseparable from the coexisting cystic
neoplasm (1). There is little
information regarding their appearance on computed tomography (CT)
examination (1,2). Although ovarian tumors have similar
clinical and radiological features, predominant or specific imaging
features may be present in certain types of ovarian tumors.
Characterization of an ovarian mass is of the utmost importance by
CT in the preoperative evaluation of an ovarian neoplasm (3). It enables the surgeon to anticipate
carcinoma of the ovary prior to surgery so that adequate procedures
are planned. Therefore, familiarity with the clinical and imaging
features of various ovarian tumors is important in determining the
likelihood of a tumor being benign or malignant. The aim of the
present study was to describe the clinical and CT characteristics
of Brenner tumors.
Materials and methods
Patients
Patients with a histological diagnosis of a Brenner
tumor of the ovary who had undergone preoperative CT examination
prior to surgical removal of the mass between June 2003 and
December 2012 from the clinical databases of the Department of
Obstetrics and Gynecology, Huzhou Central Hospital (Huzhou, China)
were retrospectively analyzed. The study protocol was approved by
the Institutional Review Board of Ethics Committee of Huzhou
Central Hospital and written informed consent was obtained from all
the patients.
CT examinations
CT examinations were performed with Toshiba
Aquilionor (Toshiba Medical Systems, Otawara, Japan) or Philips
Brilliance 16-slice CT scanners (Philips Medical Systems,
Amsterdam, The Netherlands). All patients were placed in the supine
position and given an appropriate amount of water to drink to fill
the bladder prior to the scan. The CT scan covered the entire
primary lesion from the illum attachment and ischial tuberosity
attachment, and the scan range was adjusted according to the lesion
size in certain cases. Contrast-enhanced CT images were obtained
following the injection of 80–100 ml of a nonionic iodinated
contrast material at a concentration of 370 mg/ml [iopamidol
(Iopamiron 370; Bayer AG, Leverkusen, Germany)] or 300 mg/ml
[iohexol (Omnipaque 300; Daiichi-Sankyo Health Care, Tokyo, Japan)]
at a rate of 2.5–3.0 ml/sec. The parameters of the CT scan were
120–150 kV, 120 mAs, slice thickness of 1.0–2.0 mm. Arterial and
venous phase images were obtained during 25–30 sec and 65–80 sec
delay, respectively.
Pathological examination
Pathological samples were stored in 4%
paraformaldehyde solution at 4°C overnight, dehydrated through a
series of graded ethanol solutions (70, 90, 95 and 100% ethanol and
each for 5 min at room temperature), cleared in xylene (3 times for
5–10 min each), embedded in paraffin and cut into 5 µm thickness
sections. Sections were then stained with hematoxylin (1% w/v; 1–2
min at room temperature) and eosin (0.5% w/v; 2–5 sec at room
temperature) for general examination under a Leica microscope
(Leica DMI3000 B; Leica, Solms, Germany; ×100 magnification). All
the pathological sections were reviewed by a senior pathologist for
diagnosis confirmation. The following histological parameters were
recorded on the basis of histological analysis and review of the
original pathologic reports: Gross configuration (solid or cystic);
tumor size (in centimeters); gross tumor color; microscopic
calcifications; and the presence of coexisting ovarian neoplasms or
tumor-like lesions.
Results
Patient demographical data
Details of the demographical data of these 9
patients are listed in Table I. The
mean age of patients were 57.2 years, range, 24–80 years. The
clinical manifestations were as follows: 2 cases were
postmenopausal and presented with irregular vaginal bleeding; 2
cases presented with abdominal swelling pain; 3 cases exhibited
uterine leiomyomas; and 2 were detected at the time of physical
examination.
 | Table I.Clinical features of the 9 cases of
ovarian Brenner tumor. |
Table I.
Clinical features of the 9 cases of
ovarian Brenner tumor.
| Characteristics | No. of patients
(n=9) |
|---|
| Postmenopausal | 2 |
| Postmenopausal
bleeding | 2 |
| Abdominal swelling
pain | 2 |
| Uterine
leiomyomas | 3 |
Clinical features of ovarian Brenner
tumors
All 9 cases of ovarian Brenner tumor were unilateral
lesions with clear borders (Table
II). Among these cases, 3 were located to the right and 6 were
located to the left. Seven cases presented with round- or
oval-shaped tumors, and 2 cases presented with irregular- and
lobulated-shaped tumors. The largest and smallest size of lesions
was 8.1×7.2×3.2 and 1.5×1.2×0.8 cm, respectively, and the mean size
was 4.3 cm. Upon morphological analysis, 2 cases presented with
uterine enlargement and endometrium thickening, 3 were combined
with uterine leiomyomas, 1 with uterine leiomyomas and endometrium
thickening, 2 combined with an adeno-cystic mass, and 1 combined
with an ovarian cyst.
 | Table II.Detailed clinical features and
computed tomography manifestation of the ovarian Brenner tumor. |
Table II.
Detailed clinical features and
computed tomography manifestation of the ovarian Brenner tumor.
|
|
|
|
|
|
| CT value of the solid
mass (HU) |
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Patient no. | Age (years) | Location | Morphology | Size (cm) | Type | Plain scan | Arterial | Venous | Calcification | Comorbidity |
|---|
| 1 | 54 | Left | Oval | 6.5×5 | Solid | 55 | 64 | 73 | Multiple and
scattered shape | Intramural and
subserous uterine leiomyomas combined with adenomyosis |
| 2 | 51 | Right | Round | 1.5×1.2×0.8 | Solid | 48 | 67 | 86 | None | Uterine
leiomyomas |
| 3 | 80 | Left | Oval | 6×5×4.5 | Solid | 50 | 89 | 90 | Dot-like shape | Intramural leio
leiomyomas, post menopausal endometrial hyperproliferation
response |
| 4 | 48 | Left | Lobulated | 4.5×3.8×2.7 | Solid | 53 | 75 | 83 | Multiple and
scattered shape | Uterine adenomyosis
and endometrial hyperplasia |
| 5 | 81 | Right | Oval | 8.1×7.2×3.2 | Cystic-solid | 56 | 80 | 91 | Dot- and disk-like
shape | Endometrial
hyperproliferation response |
| 6 | 41 | Left | Oval | 6.1×5.3×4.7 | Cystic-solid | 46 | 68 | 73 | None | Left ovarian mucinous
cystic adenoma |
| 7 | 24 | Right | Lobulated | 3.7×2.2×2.0 | Cystic-solid | 52 | 69 | 78 | Dot-like shape | Ovarian simple
cyst |
| 8 | 66 | Left | Oval | 7.5×3.2×4.4 | Cystic | 30 | 38 | 60 | None | Right ovarian
mucinous cystic adenoma |
| 9 | 70 | Left | Oval | 6.2×4.2×3.8 | Solid | 45 | 63 | 81 | None | Intramural uterine
leiomyomas combined with adenomyosis |
CT manifestation
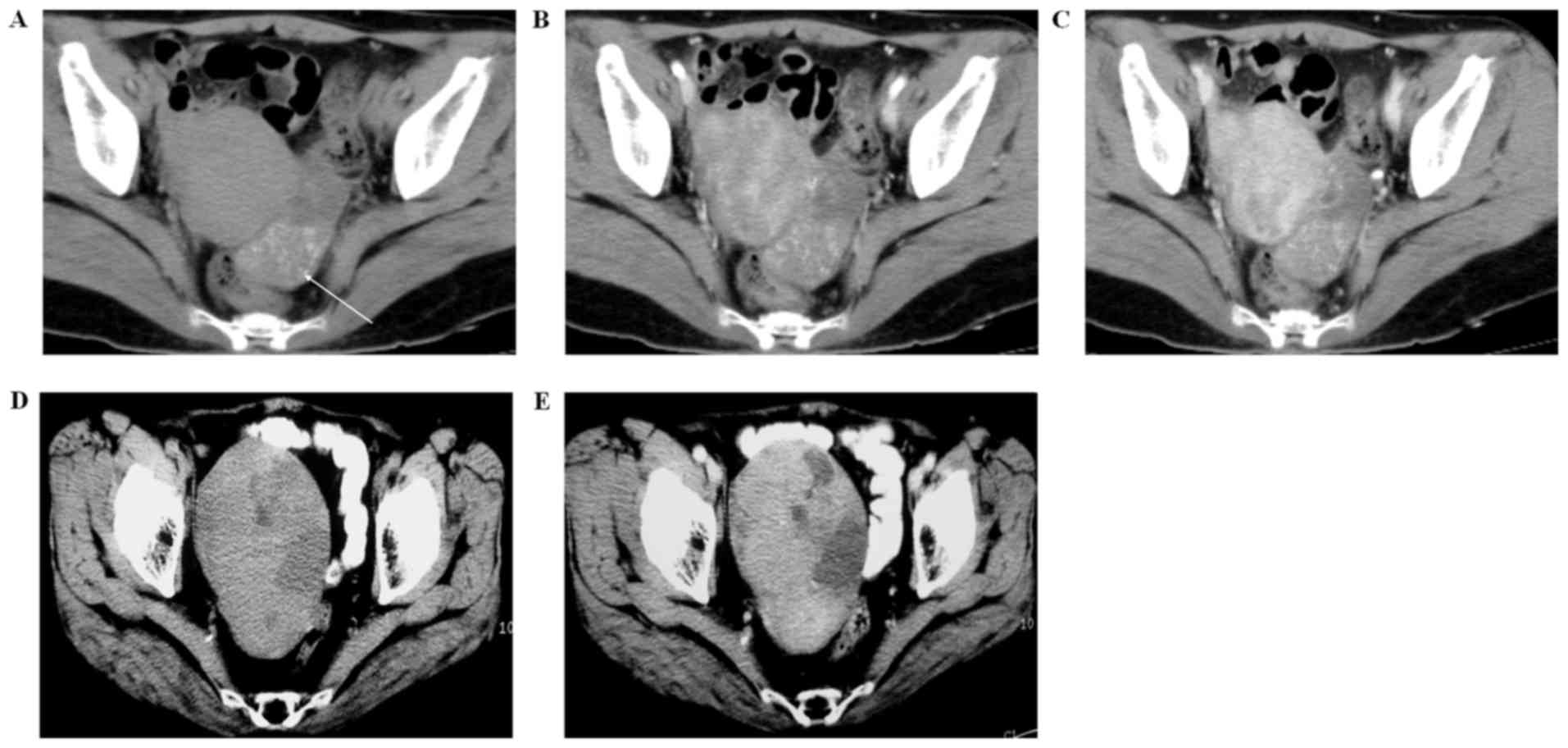
The masses were classified according to the cystic
and solid ratio inside the lesion as follows: i) 5 cases were solid
mass (Fig. 1). A small amount of
low-density necrosis region was observed inside the lesions.
Multiple scattered or dot-like calcification with evident
enhancement was observed in 3 cases of lesions. ii) Cystic-solid
lesion in 3 cases (Fig. 1). Similar
ratios of the cystic and solid component were observed in these
cystic-solid mixed lesions. The CT value of the cystic part was
18–20 HU and no enhancement was identified following contrast
medium administration, whereas moderate enhancement was observed in
the solid part. iii) Cystic lesion in 1 case (Fig. 2). The CT manifestations included
single cystic lesion with papillary projections and slight
enhancement was observed following contrast medium administration.
The detailed CT features of each case of ovarian Brenner tumor are
listed in Table II.
Post-operation pathology features
The post-operation pathology features of the 9
Brenner tumors were as follows: 9 cases with an intact capsule and
smooth surface; 5 cases with a hard texture solid mass; 3 with a
tenacious solid mass; and 1 with a soft cystic lesion. The
cross-section of the mass revealed gray-white or gray-red coloring,
and calcification with a gritty texture was observed in some of the
mass. Furthermore, different degrees of cystic change and necrosis
were observed in these masses. Water- or jelly-like translucent
mucous with a coffee-colored cystic liquid and a smooth cystic wall
was observed inside the cystic or cystic-solid mass. Furthermore,
nodular or papillary projections were observed inside the inner
wall of the cystic lumen.
The microscopic observation revealed that the
Brenner tumors were epithelial cell nests composed by transitional
urothelial-like cells and fibrous stroma, and close or merged
epithelial nests with a limited amount of mesenchyme were observed
in some of the regions. The epithelial nest exhibited various
morphologies, the majority of which were of solid composition
(Fig. 3). Sieve and adenoduct-like
morphologies were observed in the center of certain epithelial
nests and 5 cases of nests demonstrated an adeno-like lumen
(Fig. 4). No nuclear mitosis or
mesenchyme infiltration was observed in any case and the
histological diagnosis confirmed benign Brenner tumor in all
cases.
Discussion
Brenner tumors are uncommon epithelial-stromal
tumors that originate from the surface of the ovaries (4) and were first described in detail by
Fritz Brenner in 1907 (5).
Histologically, Brenner tumors exhibit epithelial cell nests
growing in a fibrous stroma (6).
These epithelial cells have the similar appearance to urothelial
cells, and therefore Brenner tumors were classified as a
transitional cell tumor type by the World Health Organization.
Brenner tumors are divided into three primary types, including
benign, borderline and invasive. Benign Brenner tumors may be found
in women of any age, but are predominantly seen in women aged ~50
years old, while women with borderline or malignant Brenner tumors
are typically 10 years older (7). The
mean age of patients who participated in the present study was 57.2
years, and all cases were benign Brenner tumors. Almost all Brenner
tumors are asymptomatic and discovered by accident. In ~30% of
cases, a second tumor is identified in the same ovary, most often a
serous or mucinous cystadenoma, or occasionally a teratoma
(5,7,8). In the
present study, 3 cases were identified with coexisting uterine
leiomyomas, 2 with abdominal swelling pain and 2 with
postmenopausal irregular bleeding. The post-operative histological
examination confirmed 2 cases with differing degrees of endometrium
thickening, 3 with uterine leiomyomas, 1 with uterine leiomyomas
and endometrium thickening, 2 with ovarian cystadenoma, and 1 with
ovary cysts. To the best of our knowledge, no literature has
reported an association between ovarian Brenner tumors and
coexisting comorbidities. Patients that also present with pleural
fluid and ascites are said to have Meigs' syndrome (9). Ovarian Brenner tumors combined with
Meigs's syndrome are e common tumor types. Here, no case was
identified with pleural fluid and ascites, which was attributed to
the limited number of cases investigated.
The CT manifestations of Brenner tumor include
adnexal solid or solid-cystic, or cystic masses with a clear
border, amorphous in appearance, with round, oval, irregular or
lobulated morphologies. Benign Brenner tumors are typically small
solid tumors with small cysts (10).
Furthermore, grossly visible amorphous calcifications in the solid
mass with mild or moderate enhancement are typical CT
manifestations. Histologically, minimal cystic changes, necrosis
and varying degrees of calcium crystal are often identified in the
solid mass (11). In the current
study, 5 cases had solid masses, of which 2 were with multiple
scattered calcification and 1 with dot-like calcification. In
addition, 3 cases were with evident enhancement and 1 with mild
enhancement. According to the aforementioned description,
borderline or invasive Brenner tumors are characterized by the
presence of solid-cystic or cystic structures with papillary
structures, and mild or moderate enhancement in the solid mass. In
a study by Wang et al (12),
ovarian Brenner tumors were described are typically unilateral and
often accompanied by other tumor components, such as solid tissue
in ovarian cancer. When a tumor is of uniform component, the CT
imaging often reveals a homogeneous solid tumor with homogeneous or
heterogeneous density.
When a tumor is accompanied by other tumor
components, it may be solid-cystic or cystic and has partial
calcification. Following enhancement, a benign Brenner tumor is
slightly enhanced, while the borderline of the Brenner tumor is
moderately/highly enhanced. In the present study, following the
pre-surgery histology, 3 cases were confirmed as cystic-solid mass,
of which 2 were borderline tumors and 1 was an invasive tumor.
According to the post-surgery histology, epithelial nest with an
intact border had no heterogeneous cell or mesenchyme infiltration.
Furthermore, a cystic mass with a papillary nodular shadow (1/9
cases) was revealed on the CT scan. We hypothesized that the
residue cystic or necrosis tumor resulted in an artifact shadow,
thereby generating a papillary nodular shadow. We concluded that CT
scans combined with a histology should be performed when diagnosing
Brenner tumors. The observations of the current study are
consistent with the previous description by Wang et al
(12). Generally, malignant tumors
should be considered when a cystic mass of the ovary with enhanced
solid nodules is present (13);
however, Brenner tumors do not adhere to this rule. Furthermore,
the possibility of a Brenner tumor should be considered when there
is the presence of combined dot-like calcifications in a
cystic-solid lesion. The main CT manifestations of Brenner tumors
were as follows: A solid mass in a small sized tumor; cystic-solid
mass with multiple cystic changes during tumor growth; and single
cystic lesions with a solid border when the presence of increasing
degree of cystic changes, necrosis and septa destroy.
The differential diagnosis of Brenner tumor includes
fibroma, cystoadenocarcinoma, Krukenberg tumor and leiomyomas.
Fibroma presents as a homogeneous solid mass with a smooth border
and minimal enhancement, but occasionally involves cystic changes
and necrosis calcification (3).
Cystoadenocarcinoma presents as an adnexal cystic-solid mass with
an unclear border and cystic-solid borderline, an unequally
thickened cystic wall with multiple nodules and septa, and is
usually accompanied by peritoneal lumen implantation metastasis
(14). Krukenberg tumors are usually
bilateral with additional findings of primary malignancy, and
frequently accompanied by abdominal and pelvic effusion or
metastasis (15). Leiomyomas present
with a limited amount of dot- or eggshell-like calcification with a
similar degree of enhancement as Brenner tumors (16). Furthermore, the difference of blood
supplies may be detected between Brenner tumor and leiomyomas
(17).
In conclusion, the present study demonstrated that
the CT features of benign ovarian Brenner tumors include multiple,
scattered calcification focused in the solid component, and we
propose that histological examination is necessary. These results
aid in improving the understanding of the clinical manifestation,
histological and CT features of ovarian Brenner tumors, which may
aid in its diagnosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YZ and XM conceived and designed the study; YZ, XM
and LY performed the study; YZ, XM LY and JS analyzed the data; YZ
and LY contributed reagents/materials/analysis tools; YZ, XM, LY
and JS contributed to the writing of the manuscript; XM and JS gave
the final approval of the version to be published.
Ethics approval and consent to
participate
The study protocol was approval by the Institutional
Review Board or Ethics Committee and written informed consent was
obtained from all the patients.
Consent for publication
Written informed consent was obtained from all the
patients for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Green GE, Mortele KJ, Glickman JN and
Benson CB: Brenner tumors of the ovary: Sonographic and computed
tomographic imaging features. J Ultrasound Med. 25:1245–1251. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buy J, Ghossain M, Sciot C, Bazot M,
Guinet C, Prevot S, Hugol D, Laromiguiere M, Truc J, Poitout P, et
al: Epithelial tumors of the ovary: CT findings and correlation
with US. Radiology. 178:811–818. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jung SE, Lee JM, Rha SE, Byun JY, Jung JI
and Hahn ST: CT and MR imaging of ovarian tumors with emphasis on
differential diagnosis. Radiographics. 22:1305–1325. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dierickx I, Valentin L, van Holsbeke C,
Jacomen G, Lissoni AA, Licameli A, Testa A, Bourne T and Timmerman
D: Imaging in gynecological disease (7): Clinical and ultrasound
features of Brenner tumors of the ovary. Ultrasound Obstet Gynecol.
40:706–713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Balasa RW, Adcock LL, Prem KA and Dehner
LP: The Brenner tumor: A clinicopathologic review. Obstet Gynecol.
50:120–121. 1977.PubMed/NCBI
|
|
6
|
Ordóñez NG and MacKay B: Brenner tumor of
the ovary: A comparative immunohistochemical and ultrastructural
study with transitional cell carcinoma of the bladder. Ultrastruct
Pathol. 24:157–167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Silverberg SG: Brenner tumor of the ovary.
A clinicopathologic study of 60 tumors in 54 women. Cancer.
28:588–596. 1971.
|
|
8
|
van der Westhuizen A and Tiltman AJ:
Brenner tumours-a clinicopathological study. S Afr Med J.
73:98–101. 1988.PubMed/NCBI
|
|
9
|
Cuatrecasas M, Catasus L, Palacios J and
Prat J: Transitional cell tumors of the ovary: A comparative
clinicopathologic, immunohistochemical, and molecular genetic
analysis of Brenner tumors and transitional cell carcinomas. Am J
Surg Pathol. 33:556–567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kurman RJ: Blaustein's pathology of the
female genital tract. Springer Science & Business Media;
2013
|
|
11
|
Scully RE, Bonfiglio TA, Kurman RJ,
Silverberg SG and Wilkinson E: Histological typing of female
genital tract tumours. Springer Science & Business Media;
2012
|
|
12
|
Wang XY, Dai JR, Zhu Z, Zhao YF and Zhou
CW: CT features of ovarian Brenner tumor and a report of 9 cases.
Zhonghua Zhong Liu Za Zhi. 32:359–362. 2010.(In Chinese).
PubMed/NCBI
|
|
13
|
Levine D, Brown DL, Andreotti RF,
Benacerraf B, Benson CB, Brewster WR, Coleman B, DePriest P,
Doubilet PM, Goldstein SR, et al: Management of asymptomatic
ovarian and other adnexal cysts imaged at US: Society of
radiologists in ultrasound consensus conference statement 1.
Radiology. 256:943–954. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moon WJ, Koh BH, Kim SK, Kim YS, Rhim HC,
Cho OK, Hahm CK, Byun JY, Cho KS and Kim SH: Brenner tumor of the
ovary: CT and MR findings. J Comput Assisted Tomogr. 24:72–76.
2000. View Article : Google Scholar
|
|
15
|
Imaoka I, Wada A, Kaji Y, Hayashi T,
Hayashi M, Matsuo M and Sugimura K: Developing an MR imaging
strategy for diagnosis of ovarian masses. Radiographics.
26:1431–1448. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bristow R and Montz F: Leiomyomatosis
peritonealis disseminata and ovarian Brenner tumor associated with
tamoxifen use. Int J Gynecol Cancer. 11:312–315. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boyle KJ and Torrealday S: Benign
gynecologic conditions. Surg Clin North Am. 88:245–264. 2008.
View Article : Google Scholar : PubMed/NCBI
|