Introduction
Gastric cancer is one of the most common types of
malignant tumor, with gastric cancer-associated mortality
accounting for 23.03% of all malignant tumors (1), which is second only to lung cancer and
ranks first in digestive tract tumors (2,3).
The early diagnosis of gastric cancer improves the
prognosis and the postoperative 5-year survival rate of early
gastric cancer (EGC) has been recorded at 90% (4). EGC refers to cancer with an invasion
depth limited to the mucosa or mucosa and submucosa, regardless of
metastasis in the lymph nodes (5).
The median duration of EGC prior to mortality (i.e., the survival
time post-diagnosis) is 44 months (6)
and if early cancerous lesions in the stomach are identified during
this period, the prognosis of patients may be improved. Therefore,
the effects of increasing the detection rate of EGC require study
(7).
On the basis of the Paris endoscopic classification
(8) and according to the gross
morphology of lesions, EGC is divided into three types: Protruded
type (0–I), flat type (0–II) and depression type (0–III). According
to the morphology, the 0–II type is divided into three subtypes:
Superficial protruded type (0–IIA), flat type (0–IIB) and
superficial depression type (0–IIC). Type III may be more easily
identified by gastroscopy and cancer cells may be determined by
performing biopsy of tissues. In the progressive stage, gastric
cancer may be observed under a gastroscope and cancer cells may be
easily determined by biopsy.
According to the staging method by Shinji et
al (9), the ulcer course may be
divided into three stages: Active (A), healing (H) and scarring
(S). Furthermore, each stage may be divided into two phases to
provide the following: A1, A2, H1, H2, S1 and S2.
A previous study identified that gastric ulcers are
a gastric precancerous disease (10),
but were primarily aimed at situations where gastric ulcers are
difficult to heal. Some researchers believe that endoscopic
follow-up of benign gastric ulcers is hypothesized to be of limited
value; for instance, Thomopoulos et al (11) demonstrated that during the 6-year
follow-up of 731 patients with benign gastric ulcers, no case of
gastric cancer was identified. In addition, Bustamante et al
(12) revealed that no gastric cancer
case was identified in 289 patients with benign gastric ulcers.
However, in our 10 years of clinical work, (Ma et al,
unpublished data), gastric cancer was occasionally identified. In
an experimental group of 232 cases of patients with gastric ulcers,
in which 9 patients were diagnosed with cancer, 5 cases of gastric
cancers were detected from the edges of ulcers at 1, 14, 18, 19 and
22 months later, respectively. In another 4 cases, gastric cancers
were detected from the base of ulcers at 1, 6, 17 and 24 months
later, respectively. By repeated gastroscopic biopsy of the healed
gastric ulcer site and was diagnosed as EGC by surgery and
pathology. Mañas et al (13)
identified that 8/452 patients with benign gastric ulcers were
diagnosed with gastric cancer (detection rate, 1.8%) during
follow-up. Therefore, we hypothesized that for healed or currently
healing chronic gastric ulcers, the biopsy of the ulcer base during
gastroscopic follow-up may improve the diagnosis of EGC compared
with the traditional method of the biopsy of gastric ulcer
margins.
Patients diagnosed with benign gastric ulcer at the
Endoscopy Center of the Affiliated Huaian Hospital of Xuzhou
Medical University (Huai'an, China), were randomly divided into two
groups: An experimental group and a control group. Furthermore, the
patients underwent regular gastroscopic rechecks to assess whether
the biopsy of the ulcer margins and base may improve the detection
rate of EGC compared with the biopsy of ulcer margins alone.
Patients and methods
Selection of subjects and
criteria
The present study was approved by the Ethics
Committee of the Affiliated Huaian Hospital of Xuzhou Medical
University and all patients provided written informed consent.
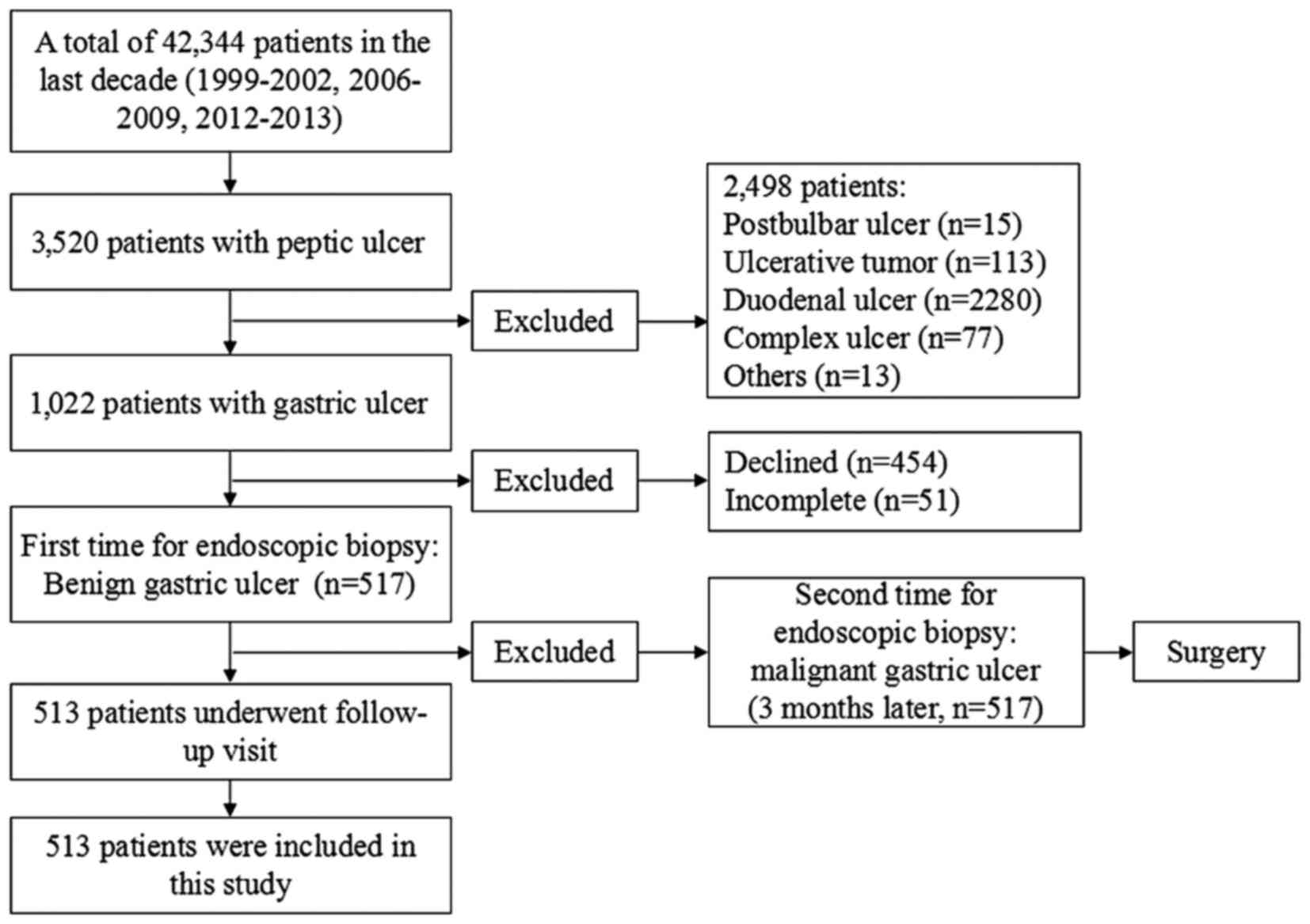
Between January 1999 and December 2002 14, 202 patients (Fig. 1) underwent gastroscopic examinations
in the Endoscopy Center and gastric ulcer patients were rechecked
at least twice by gastroscopy. Patients were followed-up for
between 1 week and 24 months. A total of 281 patients were selected
as controls and these patients underwent biopsy of the ulcer
margins only. Following diagnosis with a benign ulcer, patients
underwent 8 weeks of regular medical treatment and gastroscopic
recheck started from the second week. If the ulcer began to heal,
biopsy was no longer conducted. If the ulcer did not heal, only a
biopsy of the ulcer margins was conducted. Between January 2006 and
December 2009 and between March 2012 and December 2013, 28,142
patients (Fig. 1) underwent
gastroscopic examinations in the Endoscopy Center. Gastric ulcer
patients were rechecked at least three times by gastroscopy and
were followed up for between 1 week and 24 months. A total of 243
patients from this period were included in the experimental group,
11 of whom were lost to follow-up, and so 232 patients completed
the study. Patients in the experimental group underwent biopsy of
the ulcer margins. When diagnosed with a benign ulcer, patients
underwent 8 weeks of regular medical treatment and gastroscopic
recheck began from the second week. If the ulcer healed, only
biopsy of the ulcer margins was conducted; however, if the ulcer
healed or was currently healing, biopsy of the ulcer base was
conducted. Clinical, endoscopic and pathological examination data
were collected from the two groups of patients. During the last 4
weeks prior to gastroscopic examination, all patients did not use
proton pump inhibitors, Bismuth agents, H2 receptor
antagonists or antibiotics.
Tissue samples
All 513 selected patients (281 patients in the
control group and 232 patients in the experimental group) underwent
regular gastroscopic biopsy. Patients in the control group
underwent biopsy of the ulcer margins only. Patients in the
experimental group underwent a biopsy of the ulcer margins during
the active stage of the ulcer, and a biopsy of the ulcer margins
and base if the ulcer healed or was currently healing. Between 4
and 6 sections of biopsy samples were obtained using disposable
biopsy forceps, and were sent to the Department of Pathology of The
Affiliated Huaian Hospital of Xuzhou Medical University for
histopathological diagnosis. The gastroscopy and biopsy records of
all patients were complete.
Exclusion criteria of disease
cases
Patients with the following were excluded: Acute
erosive gastritis, cancerous ulcers, compound ulcers and multiple
gastric ulcers; patients diagnosed with benign ulcers by visual
observation, but diagnosed with gastric cancer during the initial
biopsy; and patients with gastric ulcers occurring after gastric
cancer surgery.
Research methods
The epidemiological features of patients included
the following: Sex, age, smoking history and the administration of
non-steroidal anti-inflammatory drugs (NSAIDs).
Investigation method of the
administration history of NSAIDs
Regardless of dose and duration, the oral
administration of NSAIDs prior to gastroscopic examination was
defined as the administration of NSAIDs.
Helicobacter pylori detection using
rapid urease
The white square silicone oil slip sheet of the
H. pylori test paper was removed and biopsy samples were
placed in the center of the round yellow paper. Subsequently, the
adhesive paper was fixed on the plastic in order to fix the test
paper with biopsy tissues to the plastic. The results were observed
under normal temperature conditions. The alteration in the color of
the margins of the biopsy tissue on the test paper determined the
judgment criteria, as follows: Strong positive result, yellow to
red within 1 min; weak positive result, yellow to red within 3 min;
and negative result, no change. The outcome was invalid if a change
occurred after 3 min.
Helicobacter pylori detection by
13C expiration
The investigation was performed on patients either
in the morning, when stomach was empty, or following >2 h
fasting. Patients filled out the required information on the two
labeled air bags and subsequently blew air into the bags in a
normal manner until the bags were full. The bag covers were then
immediately tightened. The air collected was defined as the ‘0-min
expiration air’. The subjects took one urea 13C capsule
with between 80 and 100 ml cold drinking water and sat quietly.
After 30 min, expiration air was collected from the subjects as
aforementioned, and the air collected was defined as the ‘30-min
expiration air’. ‘0-min expiration air’ and ‘30-min expiration air’
were used for 13CO2 detection, with δ‰
(parts/thousand) used to represent the results. The following
formula was used: δ‰=(13C abundancedetermined
sample-13C abundancereference sample)
×1000/13C abundancereference sample. The diagnosis of
H. pylori was determined on the basis of the difference
between the δ‰ values of the ‘0-min expiration air’ minus and that
of the ‘30-min expiration air’ (detection value=δ‰ at 30 min-δ‰ at
0 min). When the positive judgment value was ≥4.0±0.4 (mean ±
standard deviation), subjects were defined as H.
pylori-positive.
Judgment criteria of H. pylori
infection
When the urease and 13C expiration tests
presented positive results, or when urease was negative and
13C was positive, the patient was identified as infected
with H. pylori. If the urease and 13C expiration
tests presented negative results, or if the urease test was
positive and 13C expiration test was negative, the
patient was identified to be negative for H. pylori.
Medical treatment scheme for gastric
ulcer
Treatment for H. pylori-positive gastric
ulcers was as follows: Rabeprazole sodium enteric-coated capsules,
20 mg, twice/day for 14 days; colloidal bismuth pectin dry
suspension, 150 mg, three times/day for 14 days; clarithromycin
sustained-release tablets, 500 mg, twice/day for 14 days; and
amoxicillin, 1,000 mg, twice/day for 14 days. After 14 days of
treatment, clarithromycin sustained-release tablets and amoxicillin
were ceased, and the administration of rabeprazole sodium
enteric-coated capsules and colloidal bismuth pectin dry suspension
was continued for 6 weeks. Treatment for H. pylori-negative
gastric ulcers was as follows: Rabeprazole sodium enteric-coated
capsules, 20 mg per day for 8 weeks; and colloidal bismuth pectin
dry suspension, 150 mg, three times/day for 8 weeks
Gastroscopy
All patients were examined using the Olympus
GIF-XQ240 and GI-XQ260 series electronic gastroscope (Olympus
Corporation, Tokyo, Japan) and all examinations were completed by
two experienced endoscopic physicians.
Preparation prior to the
examination
All patients were examined with an empty stomach in
the early morning. A total of 10 mg racanisodamine hydrochloride
was intramuscularly injected 5 min prior to the examination.
Subsequently, 1 bottle of dyclonine hydrochloride mucilage was
orally taken (0.1 g, 10 ml).
Examination steps
A conventional endoscope was inserted and reached
the descending part of the duodenum, and the pyloric canal, gastric
antrum, gastric body and gastric fundus were observed during the
withdrawal of the gastroscope. If suspected lesions were
identified, lesion morphology, margins and color distinctions of
the surrounding area were preliminarily observed and images were
acquired. The majority of benign ulcers were round or elliptical,
although certain ulcers were linear with smooth margins. The bottom
was covered with yellowish gray or grayish white exudate, the
surrounding mucosa presented with congestion and edema, and the
plicae around the ulcer were centrally located. Biopsy tissues were
obtained from a number of lesion sites using disposable biopsy
forceps for pathological examination.
Sampling
Patients in the control group underwent biopsy of
the ulcer margins only. When diagnosed with a benign ulcer,
patients underwent 8 weeks of regular medical treatment with
gastroscopic recheck beginning from the second week. If the ulcer
began to heal, biopsy was no longer conducted; however, if the
ulcer did not heal, biopsy of the ulcer margins was conducted. The
duration of follow-up by gastroscopy ranged between 1 week and 24
months. Patients in the experimental group underwent biopsy of the
ulcer margins alone in the initial gastroscopy, and following
diagnosis with a benign ulcer, the patients underwent 8 weeks of
regular medical treatment, with gastroscopic recheck beginning from
the second week. During the healing process of the ulcer, the ulcer
margins were prioritized as the site of gastroscopic biopsy. If the
ulcers healed or were currently healing, biopsy samples were
obtained from the ulcer base or the healed sites. Gastroscopy
rechecks in the experimental group were conducted on weeks 2, 4 and
8, and thereafter once a year. When gastric cancer was identified,
patients were surgically treated, followed by gross
histopathological examination. From each ulcer lesion, between 4
and 6 biopsy samples were obtained.
Pathological examination
All biopsy samples were placed on filter paper,
fixed in 10% formalin solution and sent to the Department of
Pathology of The Affiliated Huaian Hospital of Xuzhou Medical
University (Huai'an, China) for histopathological diagnosis. All
biopsies were conducted by experienced pathologists following
fixation in 10% formalin solution, then dehydration, clearing, and
infiltrating were finished in the machine overnight (12 h),
embedding in paraffin (~2 h at 58°C). Sections were sliced at a
thickness of ~4 µm. Subsequently, sections were subjected to
hematoxylin eosin staining: i) The sections were deparaffinized,
flamed using a burner and then placed in the >99% xylene. This
step was repeated 2 times; ii) the tissues were hydrated by passing
through decreasing concentrations of alcohol and water (100, 90, 80
and 70%); iii) the sections were stained in hematoxylin (1 g) for 5
min at room temperature; iv) and were then washed with tap water
for ≤5 min, until a blue color change was observed; v) excess dye
was removed and the clear staining effect achieved following the
addition of 1% acid alcohol (1% HCL in 70% alcohol) for 5 min; vi)
and were then washed in running tap water until the sections were
again blue by dipping in an alkaline solution followed by another
tap water wash; vii) the sections were stained with 1% eosin for 10
min; viii) and were then washed in tap water for 5 min; ix) the
sections were dehydrated in increasing concentrations (85, 90, 95,
10 and 100%) of alcohols and placed in >99% xylene; and x) a
neutral balsam neutral gum (Sinopharm Chemical Reagent Co., Ltd.,
Shanghai, China) was used to seal the sections.
Statistical analysis
Data were recorded using Windows Excel (2007;
Microsoft Corporation, Redmond, WA, USA) and analyzed using Stata
for Windows (version 12.0; StataCorp LP, College Station, TX, USA).
Data are expressed as the mean ± standard deviation and count data
were expressed as percentages. Continuous variables, including age,
were compared using Student's t-test and discrete variables were
compared using the χ2 test. The inspection level was
α=0.05 and P<0.05 was considered to indicate a statistically
significant difference.
Results
Epidemiological characteristics of
patients in the two groups
Sex and age
In the control group, 185/281 patients were male and
96/281 patients were female, with a male-to-female ratio of 1.93:1.
The age of patients in the control group ranged between 30 and 80
years, with a mean age of 53.9±12.7 years. In the experimental
group, 139/232 patients were male and 93/232 patients were female,
with a male-to-female ratio of 1.49:1. The age of patients in the
experimental group ranged between 30 and 83 years, with a mean age
of 55.8±12.0 years.
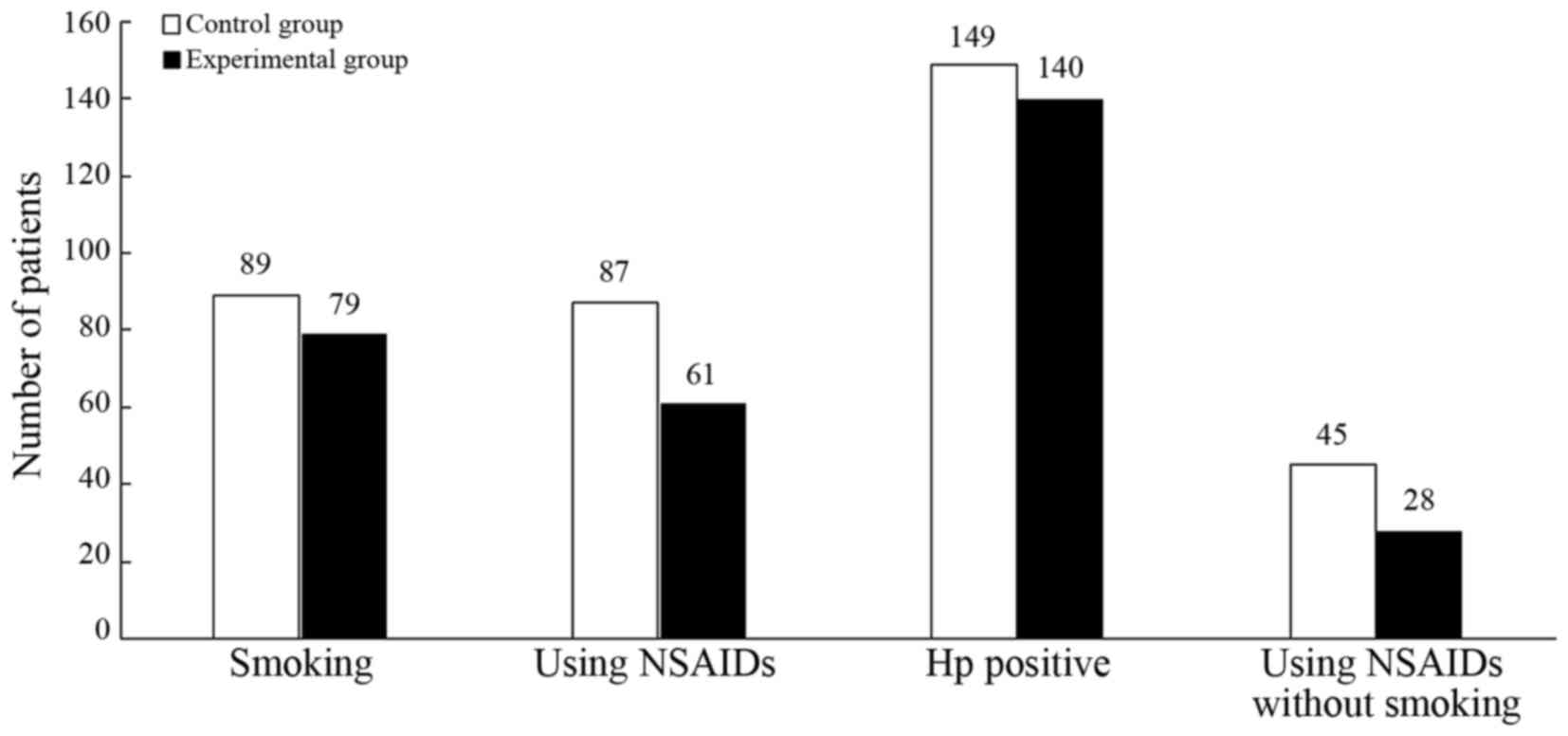
Previous medical history
In the control group, 89 patients (31.7%) exhibited
a history of smoking, 87 patients (31.0%) possessed a history of
NSAID administration, 149 patients (53.0%) had a H.
pylori-positive history, and 45 patients (16.0%) had never
smoked and never used NSAIDs. In the experimental group, 79
patients (34.1%) exhibited a history of smoking, 61 patients
(26.3%) possessed a history of NSAID administration, 140 patients
(60.3%) have a H. pylori-positive history, and 28 patients
(12.1%) have never smoked and never used NSAIDs (Fig. 2).
Statistical analysis of
epidemiological characteristics
Differences between the epidemiological
characteristics of the two groups of patients were not identified
to be statistically significant (P>0.05; Table I).
 | Table I.Statistical analysis of
epidemiological features between the experimental group and the
control group. |
Table I.
Statistical analysis of
epidemiological features between the experimental group and the
control group.
| Feature | Experimental group
(n=232) | Control group
(n=281) | P-value |
|---|
| Sex, n |
|
|
|
|
Male | 139 | 185 | 0.167a |
|
Female | 93 | 96 |
|
| Mean age,
years |
55.8±12.0b | 53.9±12.7 | 0.080c |
| Smoking history,
n |
|
|
|
|
Smoking | 79 | 89 | 0.568a |
| No
smoking | 153 | 192 |
|
| NSAID use, n |
|
|
|
|
Yes | 61 | 87 | 0.245a |
| No | 171 | 194 |
|
| Hp infection,
n |
|
|
|
|
Yes | 140 | 149 | 0.096a |
| No | 92 | 132 |
|
| NSAID use without
smoking, n |
|
|
|
|
Yes | 28 | 45 | 0.201a |
| No | 204 | 236 |
|
Gastric cancer cases determined in
patients
In the experimental and control groups overall, 12
(2.34%) patients with a gastric ulcer were diagnosed with gastric
cancer. Furthermore, gastric cancer was identified in 9 patients in
the experimental group (detection rate, 3.88%), with 5 of these
patients diagnosed with gastric cancer at 1, 14, 18, 19 and 22
months, respectively, following the diagnosis of gastric ulcer.
Diagnoses were validated by biopsies obtained from the margins of
the ulcer that had healed, or almost healed. The remaining 4
patients were diagnosed with gastric cancer at 1, 6, 17 and 24
months, respectively, following the diagnosis of the gastric ulcer,
which was subsequently confirmed by biopsy of the base of the
healed ulcers (Table II). All 9
patients with gastric cancer underwent surgical treatment and were
validated as patients with EGC by postoperative pathology. In the
control group, 3/281 patients were diagnosed with gastric cancer
(detection rate, 1.07%) at 3, 15 and 16 months, respectively,
following the diagnosis of gastric ulcer, and were validated by
biopsy of the margins of the almost healed ulcers (Table III). All 3 patients with gastric
cancer underwent surgical treatment and were diagnosed with EGC by
postoperative pathology.
 | Table II.Clinical features of the 9 patients
diagnosed with gastric cancer in the experimental group. |
Table II.
Clinical features of the 9 patients
diagnosed with gastric cancer in the experimental group.
|
| Patient no. |
|---|
|
|
|
|---|
| Feature | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|
| Sex | M | M | F | M | F | F | M | F | M |
| Age, years | 65 | 57 | 48 | 70 | 63 | 49 | 56 | 67 | 55 |
| Hp infection | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| History of NSAIDs
use | No | No | No | Yes | No | Yes | No | Yes | No |
| Biopsy blocks,
n | 5 | 4 | 6 | 5 | 4 | 4 | 6 | 7 | 5 |
| Gastric cancer
location in ulcer | Base | Margin | Base | Margin | Margin | Base | Margin | Margin | Base |
 | Table III.Clinical features of 3 patients
diagnosed with gastric cancer in the control group. |
Table III.
Clinical features of 3 patients
diagnosed with gastric cancer in the control group.
|
| Patient no. |
|---|
|
|
|
|---|
| Feature | 1 | 2 | 3 |
|---|
| Sex | M | F | M |
| Age, year | 63 | 55 | 61 |
| Hp infection | Yes | Yes | Yes |
| History of NSAIDs
use | No | No | Yes |
| Biopsy blocks,
n | 5 | 4 | 6 |
| Gastric cancer
location in ulcer | Margin | Margin | Margin |
Statistical comparison of EGC cancer
detection rate between the two groups
The detection rate of gastric cancer in patients in
the experimental group and control group was 3.88 and 1.07%,
respectively. The difference was identified to be statistically
significant (P<0.05; Table
IV).
 | Table IV.Comparison of gastric cancer
detection rate between the control and experimental groups. |
Table IV.
Comparison of gastric cancer
detection rate between the control and experimental groups.
| Group | No. of positive
gastric cancer cases | No. of negative
gastric cancer cases | Total | Detection rate,
% |
P-valuea |
|---|
| Experimental | 9 | 223 | 232 | 3.88 | 0.034 |
| Control | 3 | 278 | 281 | 1.07 |
|
Comparison of H. pylori infection rate
between patients with EGC and non-gastric cancer patients
A total of 12/513 patients with gastric ulcers in
the two groups were diagnosed with EGC, with all 12 patients
identified to be infected with H. pylori (infection rate,
100%). Among the gastric ulcer patients who were not diagnosed with
gastric cancer, 277/501 patients were diagnosed with H.
pylori infection (infection rate, 55.29%). The difference
between the two groups was identified to be statistically
significant (P<0.05; Table V).
 | Table V.Comparison of the Helicobacter
pylori infection rate between patients with early gastric
cancer and patients without gastric cancer. |
Table V.
Comparison of the Helicobacter
pylori infection rate between patients with early gastric
cancer and patients without gastric cancer.
| Patients | No. of Hp positive
cases | No. of Hp negative
cases | Total | Hp-positive rate,
% |
P-valuea |
|---|
| Patients with
EGC | 12 | 0 | 12 | 100.0 | 0.002 |
| Non-gastric cancer
patients | 277 | 224 | 501 | 55.29 |
|
Discussion
With the decreasing incidence of gastric cancer and
the wide use of NSAIDs following identification of benign gastric
ulcers by gastroscopic observation and biopsy pathology, the
necessity of regular follow-ups by gastroscopy remains
controversial in Western countries (13–15). A
previous study revealed that in 144 patients with a benign gastric
ulcer, ~7% patients were eventually diagnosed with gastric cancer
(16). Todd et al (17) hypothesized that, if ulcers were
diagnosed to be benign by endoscopic observation and were
pathologically confirmed, all patients with gastric ulcer require
follow-up by gastroscopy in order to assess ulcer healing and the
occurrence of malignant transformation. Furthermore, the British
Society of Gastroenterology guidelines (18) emphasize the value of follow-up by
gastroscopy for patients with gastric ulcers. Eckardt et al
(19) identified that 8/452 patients
with benign gastric ulcers were diagnosed with gastric cancer
(detection rate, 1.8%) during follow-up and demonstrated that
regular follow-up by gastroscopy may improve the detection rate of
EGC. Hopper et al (20)
identified that follow-up by gastroscopy may enable earlier
detection of gastric cancer, improving the survival time of
patients with gastric cancer. In addition, Podolsky et al
(21) revealed that 6 patients with
gastric ulcers were diagnosed with gastric cancer during follow-up
by gastroscopy and that 2 of these patients exhibited EGC, with
lesions limited to the mucosa and submucosa and no lymph node
metastasis. Furthermore, a previous study revealed that, despite
the use of typical treatments (including the eradication of H.
pylori), a number of patients with benign gastric ulcer
exhibited disease progression and developed an ulcer with an
undetermined nature, A limited number of these patients may have a
gastric cancer type, including lymphoma (22).
It has been previously demonstrated that the
likelihood of determining whether a benign ulcer will exhibit
canceration during follow-up of patients is low; however, the
cost-benefit ratio is high (11,12,14).
Furthermore, a previous study identified patients to exhibit ulcer
canceration during the follow-up, but their prognosis did not
improve (19). In addition, a
previous study revealed that it is inadequate to diagnose the
nature of a disease only by visual inspection under an endoscope;
therefore, biopsy is required (23).
In previous studies, the detection rate of gastric cancer in
gastric ulcer patients, according to gastroscopy and pathology
during follow-up, was between 1.3 and 13.3% (16,24).
Therefore, it is necessary to perform regular gastroscopy on
gastric ulcer patients in order to detect early canceration. A
previous study revealed that gastric ulcers may heal as a result of
effective anti-ulcer drug treatment (5). In addition, sampling from the base of
the healed ulcer for biopsy may assist in detecting gastric cancer
at an earlier stage (25). Banerjee
et al (26) hypothesized that
a biopsy was necessary after the ulcer healed, that samples may be
obtained from the base of the ulcer, that patients may be followed
up for >1 year and that it was incorrect to consider a healed
ulcer as a benign lesion. Additionally, Esmadi et al
(27) hypothesized that gastric
ulcers may be cured and demonstrated that patients should undergo
regular gastroscopic recheck. Podolsky et al (21) identified that a biopsy of the healed
sites of the gastric ulcer was required. However, to date, there
have been no large-sample studies in which, following healing of a
gastric ulcer, EGC has been determined using gastroscopy,
pathological follow-up examinations and biopsies of the healed
site.
In the present study, 513 patients with gastric
ulcers were followed up for between 1 week and 24 months. In the
experimental group, 9/232 patients developed EGC and H.
pylori infection, with a gastric cancer detection rate of
3.88%, which was consistent with the results found by Ogura et
al (28). Furthermore, after the
ulcers had almost or completely healed, 9 cases of gastric cancer
were determined by biopsy of the margins or base of the ulcer, and
the detection rate of EGC was increased compared with that of the
control group. The results of the present study suggested that the
additional biopsy of the ulcer base during the healing process may
improve the detection rate of EGC.
In the present study, the H. pylori infection
rate was 56.3% and patients with EGC exhibited a significantly
increased H. pylori infection rate compared with patients
with gastric ulcers alone, suggesting that H. pylori
infection is associated with gastric cancer. The results of the
present study suggest that, under the same conditions of H.
pylori infection, atrophic gastritis, intestinal metaplasia and
atypical hyperplasia, gastric cancer may develop not only on the
ulcer margins, but also on the ulcer base.
An endoscope with advanced auxiliary functions,
including magnifying endoscopy, narrow-band imaging endoscopy,
staining endoscopy, ultrasound endoscopy and confocal
endomicroscopy, would enable the local mucosal structure and layer
structures of tissues to be observed. Additionally, experience of
gastroscopy technology and the endoscopic biopsy technique
continues to increase, which may improve the detection rate of EGC.
In the present study, the biopsy of the ulcer base is hypothesized
to be of increased importance since it may be more effective and
enable the detection of gastric cancer at an earlier stage. To the
best of our knowledge, there are no studies on its underlying
molecular mechanism of occurrence on a global scale. It is
hypothesized that a gastric ulcer may occur on the basis of EGC. If
the malignant degree of cancer cells is decreased, the invasion and
damage effects of cancer cells are less than the proliferation
effect of the cancerous tissue itself, however, if cancerous
tissues are submucosal infiltrating growth, the ulcer may shrink
and even completely heal. Therefore, early cancer lesions may be
identified in healed gastric ulcers, which may explain clinical
ulcer repair in type II and III gastric ulcers, but does not
explain the occurrence of EGC in healed ulcer sites. However, the
occurrence of early cancer lesions in the gastric ulcer healed
sites may be due to the mutation of submucosal cells in the ulcer
base. During the process of ulcer repair, cancer cells (EGC)
gradually transition to the mucosal surface where, over time and
following alterations in the internal and external environment,
these cells progress to advanced gastric cancer. This hypothesis
may explain EGC that is identified following the repair of large
gastric ulcers.
In the experimental and control groups, 9/232 and
3/281 patients were diagnosed with EGC, respectively (detection
rates, 3.88 and 1.07%, respectively), and the difference between
the two groups was identified to be statistically significant. The
results of the present study suggested that gastroscopy and
pathological follow-up of chronic gastric ulcers, during and after
the healing process, is required and is of greater importance in
regions with an increased incidence of gastric cancer. In addition,
biopsy of the base or margins of healing or healed ulcers in
gastroscopic follow-ups is important. At present, in China,
patients with chronic gastric ulcer may not fully understand the
seriousness of ulcerated lesions. Clinicians may not realize the
importance of gastroscopic biopsy and the biopsy site of healing or
healed gastric ulcers. Therefore, additional studies on this topic
and large-scale multicenter randomized controlled trials of chronic
gastric ulcers are required.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Bureau of Huai'an city (grant no. has2010016).
Availability of data and materials
The datasets generated and analyzed in the present
study are included in this published article.
Authors' contributions
SH, XM and JW were responsible to study design and
method selection. JW, SL and DX performed gastroscopy. JW and SF
performed data collection, and JW and SF performed data
arrangement. JW, SF, SH and XM performed statistical analysis. JW,
SH and XM reviewed and revised the manuscript. HC and JZ were
responsible for the registration of the patients and the collection
of the specimens.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of the Affiliated Huaian Hospital of Xuzhou Medical
University. All patients provided written informed consent to
participate.
Consent for publication
All patients consented to the publication of this
research.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Birner P, Schindl M, Obermair A, Plank C,
Breitenecker G and Oberhuber G: Over expression of
hypoxia-inducible factor 1alpha is a marker for an unfavorable
prognosis in early-stage invasive cervical cancer. Cancer Res.
60:4693–4696. 2000.PubMed/NCBI
|
|
2
|
Forman D and Burley VJ: Gastric cancer:
Global pattern of the disease and an overview of environmental risk
factors. Best Prac Res Clin Gastroenterol. 20:633–649. 2006.
View Article : Google Scholar
|
|
3
|
Brenner H, Rothenbacher D and Arndt V:
Epidemiology of stomach cancer. Methods Mol Biol. 472:467–477.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dong ZW: The guidelines for the screening
and early diagnosis and treatment of cancer in China. Peking Univ
Med Press. 1–77. 2005.
|
|
5
|
Xu SF and Zhao ZQ: Current therapy evolved
of early gastric cancer. Med J CASC. 3:74–76. 2001.
|
|
6
|
Tsukuma H, Oshima A, Narahara H and Morii
T: Natural history of early gastric cancer: A non-concurrent, long
term, follow up study. Gut. 47:618–621. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gadducci A, Ferdeghini M, Prontera C,
Moretti L, Mariani G, Bianchi R and Fioretti P: The concomitant
determination of different tumor markers in patients with
epithelial ovarian cancer and benign ovarian masses: Relevance for
differential diagnosis. Gynecol Oncol. 44:147–154. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
The Paris Endoscopic Classification of
Superficial Neoplastic Lesions: Esophagus, stomach, and colon:
November 30 to December 1, 2002. Gastrointest Endosc. 58 6
Suppl:S3–S43. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shinji A, Sano K, Hamano H, Unno H,
Fukushima M, Nakamura N, Akamatsu T, Kawa S and Kiyosawa K:
Autoimmune pancreatitis is closely associated with gastric ulcer
presenting with abundant IgG4-bearing plasma cell infiltration.
Gastrointest Endosc. 59:506–511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rugge M, Capelle LG, Cappellesso R, Nitti
D and Kuipers EJ: Precancerous lesions in the stomach: From biology
to clinical patient management. Best Pract Res Clin Gastroenterol.
27:205–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thomopoulos KC, Melachrinou MP, Mimidis
KP, Katsakoulis EC, Margaritis VG, Vagianos CE and Nikolopoulou VN:
Gastric ulcers and risk for cancer. Is follow-up necessary for all
gastric ulcers? Int J Clin Pract. 58:675–677. 2004.PubMed/NCBI
|
|
12
|
Bustamante M, Devesa F, Borghol A, Ortuño
J and Ferrando MJ: Accuracy of the initial endoscopic diagnosis in
the discrimination of gastric ulcers: Is endoscopic follow-up study
always needed? J Clin Gastroenterol. 35:25–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mañas MD, Domper A, Albillos A, Hernández
A, Carpintero P, Lorente R, López B, De la Santa E, Olmedo J and
Rodríguez E: Endoscopic follow-up of gastric ulcer in a population
at intermediate risk for gastric cancer. Rev Esp Enferm Dig.
101:317–324. 2009.(Article in English, Spanish). PubMed/NCBI
|
|
14
|
Gielisse EA and Kuyvenhoven JP: Follow-up
endoscopy for benign-appearing gastric ulcers has no additive value
in detecting malignancy: It is time to individualise surveillance
endoscopy. Gastric Cancer. 18:803–809. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lübbers H, Mahlke R, Lankisch PG and
Stolte M: Follow-up endoscopy in gastroenterology: When is it
helpful. Dtsch Arztebl Int. 107:30–39. 2010.PubMed/NCBI
|
|
16
|
Farinati F, Cardin F, Di Mario F, Vianello
F, Battaglia G, Arslan-Pagnini C, Cannizzaro R, Sava GA, Rugge M
and Naccarato R: Early and advanced gastric cancer during follow-up
of apparently benign gastric ulcer: Significance of the presence of
epithelial dysplasia. J Surg Oncol. 36:263–267. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Todd JA, Richards CJ, Dixon A and Robinson
RJ: Gastric ulcer and malignancy-is there a need for follow-up
endoscopy? Aliment Pharmacol Ther. 19:989–991. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Allum WH, Blazeby JM, Griffin SM,
Cunningham D, Jankowski JA and Wong R; Association of Upper
Gastrointestinal Surgeons of Great Britain and Ireland, the British
Society of Gastroenterology and the British Association of Surgical
Oncology, : Guidelines for the management of oesophageal and
gastric cancer. Gut. 60:1449–1472. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eckardt VF, Giessler W, Kanzler G and
Bernhard G: Does endoscopic follow-up improve the outcome of
patients with benign gastric ulcers and gastric cancer? Cancer.
69:301–305. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hopper AN, Stephens MR, Lewis WG,
Blackshaw GR, Morgan MA, Thompson I and Allison MC: Relative value
of repeat gastric ulcer surveillance gastroscopy in diagnosing
gastric cancer. Gastric Cancer. 9:217–222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Podolsky I, Storms PR, Richardson CT,
Peterson WL and Fordtran JS: Gastric adenocarcinoma masquerading
endoscopically as benign gastric ulcer. A five-year experience. Dig
Dis Sci. 33:1057–1063. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cherian PT, Cherian S and Singh P:
Long-term follow-up of patients with gastric outlet obstruction
related to peptic ulcer disease treated with endoscopic balloon
dilatation and drug therapy. Gastrointest Endosc. 66:491–497. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Graham DY, Schwartz JT, Cain GD and
Gyorkey F: Prospective evaluation of biopsy number in the diagnosis
of esophageal and gastric carcinoma. Gastroenterology. 82:228–231.
1982.PubMed/NCBI
|
|
24
|
Lupano F and Sategna-Guidetti C:
Endoscopic follow-up of patients with gastric ulcer. A prospective
study. J Clin Gastroenterol. 8:430–434. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lv SX, Gan JH, Ma XG, Wang CC, Chen HM,
Luo EP, Huang XP, Wu SH, Qin AL, Ke-Chen, Wang XH, et al: Biopsy
from the base and edge of gastric ulcer healing or complete healing
may lead to detection of gastric cancer earlier: An 8 years
endoscopic follow-up study. Hepatogastroenterology. 59:947–950.
2012.PubMed/NCBI
|
|
26
|
ASGE Standards of Practice Committee, ;
Banerjee S, Cash BD, Dominitz JA, Baron TH, Anderson MA,
Ben-Menachem T, Fisher L, Fukami N, Harrison ME, et al: The role of
endoscopy in the management of patients with peptic ulcer disease.
Gastrointest Endosc. 71:663–668. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Esmadi M, Ahmad DS and Hammad HT:
Endoscopic surveillance for gastric ulcers. South Med J.
107:289–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ogura M, Yamaji Y, Hikiba Y, Maeda S,
Matsumura M, Okano K, Sassa R, Yoshida H, Kawabe T and Omata M:
Gastric cancer among peptic ulcer patients: Retrospective,
long-term follow-up. Dig Liver Dis. 38:811–814. 2006. View Article : Google Scholar : PubMed/NCBI
|