Introduction
Acute promyelocytic leukemia (APL) is defined as a
M3 subtype, according to the French-American-British
classification, and is characterized by the translocation
t(15;17)(q22;q21) (1,2). The promyelocytic leukemia/retinoic acid
receptor α (PML/RARα) oncoprotein is formed following the specific
chromosomal translocation t(15;17)(q22;q21), and is considered to
be responsible for the arrest of granulopoiesis by directly
inhibiting the transcription of retinoic acid target genes
(3,4).
All-trans retinoic acid (ATRA) is the first drug to cause disease
regression in patients with APL, and interacts with the
ligand-binding domain present on the RARα moiety of the chimeric
oncoprotein. ATRA causes transcriptional activation, as well as
proteolytic degradation, resulting in the granulocyte
differentiation of APL cells (5).
Previous studies indicated that patients with
additional chromosome abnormalities (ACA) and gene mutations, such
as FMS-related tyrosine kinase 3 internal tandem duplication
(FLT3-ITD) and PML/RARα mutations, do not respond to conventional
treatment regimens, such as ATRA (6,7);
therefore, molecular and cytogenetic characterization of variant
translocations and mutations of key genes are important for
understanding the pathogenesis of the disease and predicting the
response to the ATRA treatment. In the present study, the case of a
patient with APL with FLT3-ITD harboring the novel three-way
variant translocation t(6;17;15)(p21;q21;q22) and ACA add(7)(q32)
was described.
Case report
A 19-year-old female was referred to the First
Affiliated Hospital of Nanchang University (Nanchang, China) with a
1-month history of the common cold and leukocytopenia in August
2012. At admission, peripheral blood examination demonstrated a
white blood cell count of 60.19×109/l (normal range,
4–10×109/l), with 67% immature white blood cells (normal
range, <0.01%), a hemoglobin concentration of 89 g/l (normal
range, 110–150 g/l) and a platelet count of 33×109/l
(normal range, 100–300×109/l). This patient had
disseminated intravascular coagulation (DIC) with 1.10 g/l (normal
range, 1.8–3.5 g/l) fibrinogen and 54.2 mg/l (normal range,
0.01–0.55 mg/l) fibrinogen degradation product (D-dimer). A bone
marrow smear was performed and the procedure indicated hyperplasia;
it contained 84% abnormal promyelocytes, numerous cytoplasmic
azurophilic granules, occasional Auer rods and a number of them
were faggot cells (Fig. 1).
Immunophenotyping demonstrated that the leukemic cells were
positive for clusters of differentiation (CD)9, CD13, CD15, CD33,
CD38, CD64, CD117, CD123 and myeloperoxidase, but negative for
CD34, human leukocyte antigen-antigen D related, CD2, and CD56.
Chromosome analysis using GTG-banding indicated a karyotype of 46,
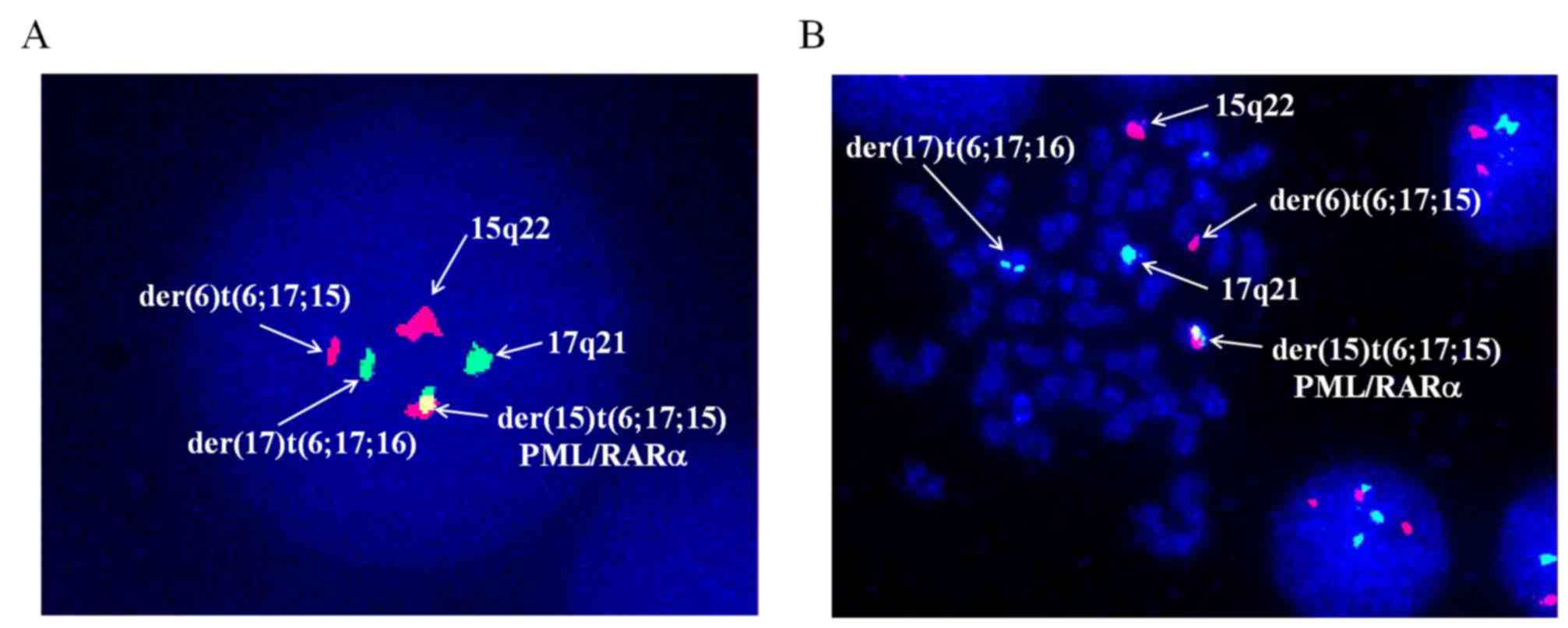
XX, t(6;17;15)(p21;q21;q22), add(7)(q32) [17]/46, XX [3] (Fig. 2). Dual-color fluorescence in
situ hybridization was conducted to detect PML/RARα fusion by a
specific probe of PML and RARα. The results demonstrated the novel
complex variant translocation t(6;17;15) (Fig. 3). Total RNA of the bone marrow were
extracted by TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and reverse transcribed to
complementary DNA (cDNA) with a QuantScript RT kit (cat. no. KR103;
Tiangen Biotech Co., Ltd., Beijing, China), according to the
manufacturer's protocols. The following polymerase chain reaction
(PCR) with the specific primers (forward,
5′-CCGTCATAGGAAGTGAGGTCT-3′, and reverse,
5′-GGCTGGGCACTATCTCTTCA-3′) indicated long and short PML/RARα
transcripts, demonstrating the L-type PML/RARα (data not shown) in
the patient. Further molecular studies indicated the presence of an
FLT3-ITD mutation. The genomic DNA of bone marrow extracted with a
TIANamp Blood DNA kit (Tiangen Biotech Co., Ltd.), and PCR were
detected at pre-denatured at 95°C for 5 min, followed by 30 cycles
of denaturing at 95°C for 10 sec and annealing and elongation at
55°C for 20 sec and elongation at 72°C for 20 sec using the ABI2720
Thermal Cycler (Applied Biosystems; Thermo Fisher Scientific, Inc.)
with specific primers: Forward, 5′-GCAATTTAGGTATGAAAGCCAGC-3′, and
reverse, 5′-CTTTCAGCATTTTGACGGCAACC-3′. The PCR products were
separated by 3% agarose gel electrophoresis and analyzed using a
gel imager (Peiqing Science & Technology Inc.) (Fig. 4). According to MICM classification,
the patient was diagnosed with high risk APL (8).
The patient was then treated with ATRA combined with
arsenic trioxide (ATO). Subsequently, differentiation of APL cells
was morphologically observed and DIC improved immediately.
Re-examination of the bone marrow smear with Wright-Giemsa staining
[10 µl bone marrow sample was spread on a slide to produce a smear
and was dried at room temperature for 1 h. Each smear was stained
in Wright-Giemsa Stain for 10 min at room temperature, and then
they were rinsed with water. Following air-drying, the smear was
inspected under a light microscope (Olympus BX51; Olympus
Corporation, Tokyo, Japan; ×1,000 magnification)] and
immunophenotyping [100 µl bone marrow sample incubated with
antibodies CD9, CD33, CD13, CD117 and CD34 for 15 min in dark room,
centrifuged at 200 × g at room temperature for 5 min following
adding 200 µl OptiLyse C Lysing solution (Beckman Coulter, Inc.,
Brea, CA, USA) for 5 min, and then detected using CYTOMICS FC500
(Beckman Coulter, Inc.)] after 1 month revealed complete remission.
The patient received several courses of consolidation therapy and
the development of illness was monitored by detecting the PML/RARα
chimeric transcript with reverse transcription-quantitative PCR
(RT-qPCR). [For RT-qPCR, patient bone marrow RNA was reverse
transcribed to cDNA as aforementioned and PML/RARα transcript was
detected with primers: Forward, 5′-GCAATTTAGGTATGAAAGCCAGC-3′, and
reverse, 5′-CTTTCAGCATTTTGACGGCAACC-3′; and fluorescein amidite
(FAM)-labeled probe (Sangon Biotech Co., Ltd., Shanghai, China):
5′-CTGCTCTGGGTCTCAATGGCTGCCTCC-3′; ABL was used as the reference
gene and detected with the primers: Forward,
5′-TCCATCTCGCTGAGATACGAAG-3′, and reverse,
5′-ATGATGAACCAACTCGGCCA-3′; and VIC-labeled probe
5′CAACACTGCTTCTGATGGCAAGCTCTACG3′. RT-qPCR were detected at 50°C
for 2 min and pre-denatured at 95°C for 3 min, followed by 40
cycles of denaturing at 95°C for 5 sec and annealing and elongation
at 58°C for 30 sec using the ABI 7500 Real-time PCR system and the
data were collected and analyzed with ABI 7500 software v2.3
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The relative
quantitation of PML/RARa=(copiesPML/RARa/copiesABL) ×100% (9)] (Fig. 5).
The patient had no recurrences within 5 years of treatment.
Discussion
To the best of our knowledge, the present case
report is the first to describe an APL case containing a FLT3-ITD
mutation with the novel three-way variant translocation
t(6;17;15)(p21;q22;q21). The fusion gene PML/RARα is observed in
the majority of APL cases (10),
whilst only a limited numbers of patients harbored complex variant
translocations (11). Although no
research has indicated an association between chromosome 6 and
PML//RARα, genes located on chromosome 6, such as tumor protein p53
pathway corepressor 1, NOTCH4 and B-cell lymphoma-2 antagonist/kill
1, are involved in the genesis and development of leukemia
(12–15). FLT3 encodes a tyrosine kinase III
receptor involved in hematopoietic malignancy types, and is
associated with a poor prognosis and high rate of relapse (16–21);
therefore, complex fusion and FLT3-ITD positive mutations are
considered adverse prognostic factors. Conversely, in the present
report, a patient with a poor prognosis successfully entered
remission.
In this case, the age of onset was 20 years, which
may have contributed to the patient's good prognosis. A recent
study investigated the characteristics and outcomes of patients
with acute myeloid leukemia treated in the Department of Leukemia
of the MD Anderson Cancer Center (Houston, TX, USA) from 1965 to
2009 and determined that younger patients (<65 years old) have
improved outcomes, compared with older patients (≥65 years old)
(22). The patient received ATO
during their hospitalization. Research conducted in 2011 indicated
that ATRA combined with anthracycline chemotherapy was unable to
ameliorate the progression of the patient with an FLT3-ITD positive
mutation (23); however, previous
studies have indicated that ATO may abrogate the unfavorable impact
of FLT-ITD (6,24), accounting for an improved outcome for
the patient. Additionally, disease compliance is critical for
prognosis, which has been demonstrated in other diseases, including
osteosarcoma, breast cancer and tumor associated neutrophils
(25,26). Upon discharge, the patient revisited
the hospital once a month for the first three months and every
three months for the following nine months for follow-up.
Subsequently, they revisited the hospital once a year until April
2018. The treatment of leukemia is multi-factorial and involves
complex mechanisms, and it is considered that the effect of
multiple factors is synergistic.
In conclusion, a patient with high-risk APL
containing FLT3-ITD with the novel three-way variant translocation
t(6;17;15)(p21;q21;q22) had favorable outcomes. The present case
provides support for the hypothesis that treatment with ATRA and
ATO results in an ameliorative effect for the outcome of a patient
with an FLT-ITD mutation who did not respond to conventional
treatments.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of China (grant no. 81760539) and the Science
and Technology Plan Project of Jiangxi Provincial Health Planning
Commission (grant no. 20171045).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZLZ conceived and designed the study. YLZ, MJ and
JHW performed the experiments. YLZ and ZLZ wrote the paper. SYL,
SQL and LGW interpreted the data and provided final approval of the
version to be published. All authors read and approved the
manuscript.
Ethics approval and consent to
participate
The patient provided written informed consent for
the publication of this study and the study was approved by the
Medical Ethics Committee of the First Affiliated Hospital of
Nanchang University (Nanchang, China).
Patient consent for publication
The patient provided written informed consent for
the publication of this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cripe LD: Adult acute leukemia. Curr Probl
Cancer. 21:1–64. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bloomfield CD and Brunning RD: The revised
French- American-British classification of acute myeloid leukemia:
Is new better? Ann Intern Med. 103:614–616. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grignani F, Ferrucci PF, Testa U, Talamo
G, Fagioli M, Alcalay M, Mencarelli A, Grignani F, Peschle C,
Nicoletti I, et al: The acute promyelocytic leukemia-specific
PML-RAR alpha fusion protein inhibits differentiation and promotes
survival of myeloid precursor cells. Cell. 74:423–431. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen Z, Tong JH, Dong S, Zhu J, Wang ZY
and Chen SJ: Retinoic acid regulatory pathways, chromosomal
translocations, and acute promyelocytic leukemia. Genes Chromosomes
Cancer. 15:147–156. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Masetti R, Vendemini F, Zama D, Biagi C,
Gasperini P and Pession A: All-trans retinoic acid in the treatment
of pediatric acute promyelocytic leukemia. Expert Rev Anticancer
Ther. 12:1191–1204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cicconi L, Divona M, Ciardi C, Ottone T,
Ferrantini A, Lavorgna S, Alfonso V, Paoloni F, Piciocchi A,
Avvisati G, et al: PML-RARalpha kinetics and impact of FLT3-ITD
mutations in newly diagnosed acute promyelocytic leukaemia treated
with ATRA and ATO or ATRA and chemotherapy. Leukemia. 30:1987–1992.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gallagher RE, Moser BK, Racevskis J, Poiré
X, Bloomfield CD, Carroll AJ, Ketterling RP, Roulston D,
Schachter-Tokarz E, Zhou DC, et al: Treatment-influenced
associations of PML-RARalpha mutations, FLT3 mutations, and
additional chromosome abnormalities in relapsed acute promyelocytic
leukemia. Blood. 120:2098–2108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Z-Y and Chen Z: Acute promyelocytic
leukemia: From highly fatal to highly curable. Blood.
111:2505–2515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Caprodossi S, Pedinotti M, Amantini C,
Santoni G, Minucci S, Pelicci PG and Fanelli M: Differentiation
response of acute promyelocytic leukemia cells and PML/RARa
leukemogenic activity studies by real-time RT-PCR. Mol Biotechnol.
30:231–238. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiao R and Zhang R: Combined analysis on
morphology, immunology, cytogenetics and molecular biology (MICM)
classification of 55 patients with acute promyelocytic leukemia.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 12:147–150. 2004.(In Chinese).
PubMed/NCBI
|
|
11
|
Adams J and Nassiri M: Acute Promyelocytic
Leukemia: A review and discussion of variant translocations. Arch
Pathol Lab Med. 139:1308–1313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choudhury AR, Ju Z, Djojosubroto MW,
Schienke A, Lechel A, Schaetzlein S, Jiang H, Stepczynska A, Wang
C, Buer J, et al: Cdkn1a deletion improves stem cell function and
lifespan of mice with dysfunctional telomeres without accelerating
cancer formation. Nat Genet. 39:99–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye Q, Shieh JH, Morrone G and Moore MAS:
Expression of constitutively active Notch4 (Int-3) modulates
myeloid proliferation and differentiation and promotes expansion of
hematopoietic progenitors. Leukemia. 18:777–787. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shirley CM, Chu SH, Yang Y, Wright GW,
Staudt LM and Small D: Genetic and pharmacologic Notch4 inhibition
synergizes with FLT3 tyrosine kinase inhibition in vitro to more
effectively eliminate FLT3/ITD-positive leukemia cells. Blood.
124:3583. 2014.PubMed/NCBI
|
|
15
|
Pancewicz J and Nicot C: Current views on
the role of Notch signaling and the pathogenesis of human leukemia.
BMC Cancer. 11:5022011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gilliland DG and Griffin JD: The roles of
FLT3 in hematopoiesis and leukemia. Blood. 100:1532–1542. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Callens C, Chevret S, Cayuela JM, Cassinat
B, Raffoux E, de Botton S, Thomas X, Guerci A, Fegueux N, Pigneux
A, et al: Prognostic implication of FLT3 and Ras gene mutations in
patients with acute promyelocytic leukemia (APL): A retrospective
study from the European APL Group. Leukemia. 19:1153–1160. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barragan E, Montesinos P, Camos M,
González M, Calasanz MJ, Román-Gómez J, Gómez-Casares MT, Ayala R,
López J, Fuster Ó, et al: Prognostic value of FLT3 mutations in
patients with acute promyelocytic leukemia treated with all-trans
retinoic acid and anthracycline monochemotherapy. Haematologica.
96:1470–1477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chillon MC, Santamaria C, Garcia-Sanz R,
Balanzategui A, Sarasquete ME, Alcoceba M, Marín L, Caballero MD,
Vidriales MB, Ramos F, et al: Long FLT3 internal tandem
duplications and reduced PML-RARalpha expression at diagnosis
characterize a high-risk subgroup of acute promyelocytic leukemia
patients. Haematologica. 95:745–751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gale RE, Hills R, Pizzey AR, Kottaridis
PD, Swirsky D, Gilkes AF, Nugent E, Mills KI, Wheatley K, Solomon
E, et al: Relationship between FLT3 mutation status, biologic
characteristics, and response to targeted therapy in acute
promyelocytic leukemia. Blood. 106:3768–3776. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fleischmann M, Schnetzke U, Schrenk KG,
Schmidt V, Sayer HG, Hilgendorf I, Hochhaus A and Scholl S: Outcome
of FLT3-ITD-positive acute myeloid leukemia: Impact of allogeneic
stem cell transplantation and tyrosine kinase inhibitor treatment.
J Cancer Res Clin Oncol. 143:337–345. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pemmaraju N, Kantarjian H, Ravandi F,
Nogueras-Gonzalez GM, Huang X, O'Brien S, Wierda W, Garcia-Manero
G, Thomas D, Pierce S, et al: Patient characteristics and outcomes
in adolescents and young adults (AYA) with acute myeloid leukemia
(AML). Clin Lymphoma Myeloma Leuk. 16(213–222): e2122016.
|
|
23
|
Hong SD, Kim YK, Kim HN, Lee SR, Ahn JS,
Yang DH, Lee JJ, Lee IK, Shin MG and Kim HJ: Treatment outcome of
all-trans retinoic acid/anthracycline combination chemotherapy and
the prognostic impact of FLT3/ITD mutation in acute promyelocytic
leukemia patients. Korean J Hematol. 46:24–30. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lou Y, Ma Y, Suo S, Ni W, Wang Y, Pan H,
Tong H, Qian W, Meng H, Mai W, et al: Prognostic factors of
patients with newly diagnosed acute promyelocytic leukemia treated
with arsenic trioxide-based frontline therapy. Leuk Res.
39:938–944. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Faisham WI, Saad Mat AZ, Alsaigh LN, Azman
Nor MZ, Imran Kamarul M, Biswal BM, Bhavaraju VM, Salzihan MS,
Hasnan J, Ezane AM, et al: Prognostic factors and survival rate of
osteosarcoma: A single-institution study. Asia Pac J Clin Onco.
13:e104–e110. 2017. View Article : Google Scholar
|
|
26
|
Seifert J, Selle A, Flieger C and Günther
KP: Compliance as a prognostic factor in the treatment of
idiopathic scoliosis. Orthopäde. 38:151–158. 2009. View Article : Google Scholar
|