Introduction
Lung cancer is one of the most common malignancies
in the world. With the aggregated environmental pollution,
incidence of lung cancer gradually increases. Clinical data show
that in all stages of lung cancer, the 5-year survival rate of
patients is only 15%, and this situation has not improved in the
past 30 years (1–3). Detection of most lung cancers depends on
the discovery of ground-glass opacity (GGO) and ground-glass
nodules (GGNs) (4). CT image of GGO
appears as a lightly-enhanced cloud-like shadow circular nodule in
the style of a frosted glass (5).
Pathological basis of GGO is alveolar wall thickening, alveolar
cavity collapse, alveolar cavity gas content reduction. Besides
that, pulmonary inflammation, fibrosis, intrapulmonary lymph nodes,
and inflammatory pseudo-tumors may also appear (6).
GGN in some cases can be diagnosed as cancer. GGN is
divided into components (whether or not they contain solid
components): simple/complete GGN (pGGN) and mixed or part-solid GGN
(mGGN) (7). mGGN due to the
occurrence of atypical hyperplasia in solid hyperplasia has a
significantly higher risk of cancer than pGGN. Based on
histopathological findings, these tissues are mainly divided into
atypical adenomatoid hyperplasia (AAH), adenocarcinoma in
situ (AIS) and minimally invasive adenocarcinoma (MIA)
(8,9).
GGN cannot be diagnosed solely based on CT. Blood test is not
reliable in some cases. Therefore, most patients were diagnosed at
advanced stages (10). Therefore,
identification of novel molecular targets for the treatment of GGN
is urgently needed.
Inactivation of p53 is closely related to the
development of many human tumors. p53 inhibits the replication of
damaged DNA in normal cells and promotes the death (apoptosis) of
these cells (11). Inactivated or
altered p53 can cause abnormal cells with damaged DNA to survive
and divide and transmit mutations to daughter cells, resulting in
the occurrence of cancer. p53 is defective in most human cancers
(12). However, expression of p53
gene and protein in lung GGN nodules has not been reported.
Therefore, 60 cases of GGN lung cancer and 60 cases of GGN
non-cancer patients were included in this study. Fluorescence in
situ hybridization (FISH) and immunohistochemistry (IHC) were
used to detect the expression of p53 protein and mRNA,
respectively, and the relationship between the abnormal expression
of p53 and the survival time of lung cancer patients was further
analyzed.
Materials and methods
Clinical data
From March 2010 to March 2014, 120 patients with GGN
admitted to the Department of Respiratory Medicine in The Second
Affiliated Hospital of Zhejiang University School of Medicine
(Hangzhou, China) were selected including 60 patients with lung
cancer and 60 non-cancer patients. Those patients included 64 males
and 56 females, with an average age of 57±20.6 years. Simple pGGN
was observed in 36 cases (12 cases in the upper right lobe, 13
cases in the left upper lobe, 10 cases in the left lower lobe and
right lower lobe, and 1 case in the right middle lobe). Mixed mGGN
was observed in 66 cases (19 cases in the upper right lobe, 16
cases in the left upper lobe, 14 cases in the left lower lobe, 14
cases in the right lower lobe and 3 cases in the right middle
lobe). There were 75 cases with single GGN and 35 cases with
multiple GGN. There was no significant difference in age, sex,
location of lesions, and general GGN types between the two groups.
CT image data and biopsy/surgical specimens were collected. All
patients were followed up for 3 years after biopsy/surgery. The
study was approved by the Ethics Committee of the Second Affiliated
Hospital of Zhejiang University School of Medicine, and each
participant signed an informed consent.
H&E histopathology and IHC
methods
H&E staining
Tissues of the biopsy or surgical specimens were
fixed overnight in an appropriate amount of 4% paraformaldehyde
(Google-BIO, Wuhan, China). After dehydration and embedding,
paraffin sections were performed at a thickness of 0.4 µm. Sections
were kept in an oven at 65°C for 4 h. After dehydration by passing
a graded series of ethanol concentrations, sections were subjected
to H&E staining, and rehydration by passing a graded series of
ethanol concentrations. Sections were finally sealed with neutral
gum. Tissue morphology was observed and photographed under a
microscope (DM-5000B; Leica Microsystems GmbH, Wetzlar,
Germany).
IHC
Streptomycin-biotin-peroxidase (SP) staining method
was used. Tissue sections were routinely dewaxed, rehydrated,
blocked with H2O2, digested with trypsin, and
incubated with normal sheep serum at room temperature. Sections
were incubated with mouse anti-human p53 primary monoclonal
antibody (dilution, 1:200; cat. no. ab1101; Abcam, Cambridge, MA,
USA) diluted in PBST overnight at 4°C, followed by incubation with
biotinylated goat anti-mouse secondary polyclonal antibody
(dilution, 1:900; cat. no. ab6788; Abcam) at room temperature for
30 min. After incubation with SP complex at room temperature for 20
min (dilution, 1:200), color development with DAB was performed.
Hematoxylin staining was performed and sections were sealed. Brown
nucleus indicated positive signals.
Analysis of the results
Sections were analyzed by two experienced
pathologists using a double-blind method. Ten visual fields were
selected under a 400-fold microscope (Olympus Corporation, Tokyo,
Japan), and 100 cells of each field were counted. Cells with brown
nucleus were counted to calculate the percentage of tumor cells.
Percentage >6% was positive and ≤5% was negative.
Detection of p53 gene expression in GGN tissue by
FISH
FISH method
Probe preparation: GLP p53 DNA (PathVysion™ p53 DNA
Probe kit) was purchased from Vysis, Inc. (cat. no. FG0011; Vysis,
Inc.: Abbott Laboratories, Downers Grove, IL, USA). This probe
(green fluorescent marker) targets p53 on chromosome 17p13.1. Probe
mixture (7 µl of hybridization buffer, 2 µl of probe, and 1 µl of
deionized water) was mixed. The mixture was vortexed and stored in
the dark (2). Hybridization: probe
mixture was added to the surface of specimen, and the specimen was
covered with a cover glass. After denaturing at 83°C in the dark
for 5 min, hybridization was performed at 42°C for 16 h in the
dark. The next day, sections were washed with 2X SSC solution (pH
7.2) at 46°C for 5 min, followed by incubation in 70% ethanol for 3
min. After air dry in the dark, 15 µl of DAPI counterstain was
added to the surface of each slide. After incubation in the dark
for 20 min, the results were observed under a fluorescence
microscope.
Analysis of results
Olympus BX51 fluorescence microscope (Olympus
Corporation) (DAPI/TRITC/FITC) was used to observe the results.
Appearance of 0 or 1 green fluorescence (FITC) dot in the nucleus
indicated p53 absence. Appearance of ≥2 green fluorescence (FITC)
dots in nucleus was not counted. a total of 200 interphase cells
were selected from each slide and the percentage of p53-deficient
cells was calculated. Absence of >20% was defined as
deletion.
Statistical analysis
Statistical data were analyzed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). Enumeration data were
expressed as percentage (%), and comparison between the two groups
was performed using the Chi-square test. The Kaplan-Meier method
was used to plot the survival curve, and survival curves were
compared using the log-rank test. P<0.05 was considered to be
statistically significant.
Results
CT signs and pathological data of
patients with GGN
There was no significant difference between the two
groups in terms of age, sex, site of lesions, and general GGN data.
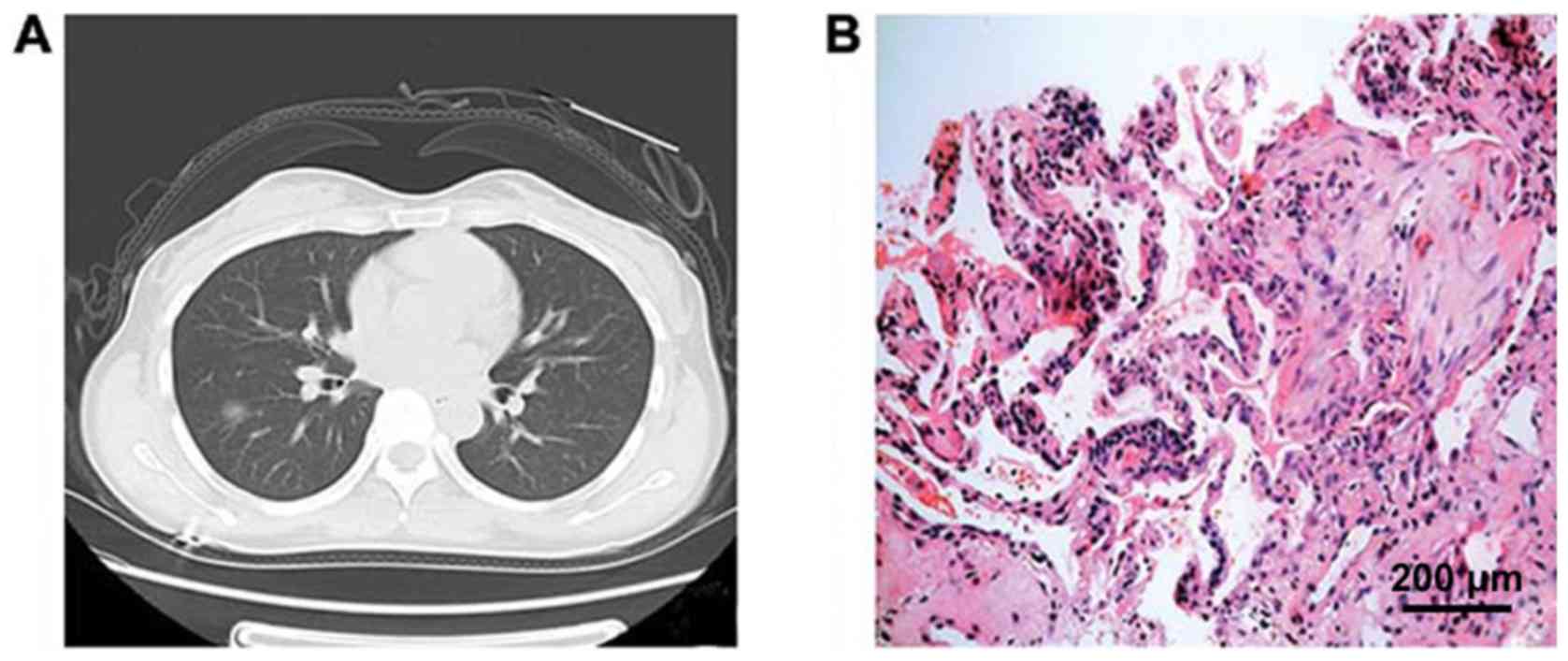
CT image of GGN patients showed an increase in local density of
lungs, a focal cloudiness shade, and the shadow veins and bronchial
texture were clearly discernible. Pathological studies showed that
ground-glass changes in lungs were caused by filling of alveolar
space with exudate, a small amount of lymphocytes, neutrophils,
macrophages or amorphous substances, accompanied by alveolar wall
thickening and other causes (Figs. 1
and 2).
Detection of p53 protein expression
and gene absence in GGN tissues by IHC and FISH
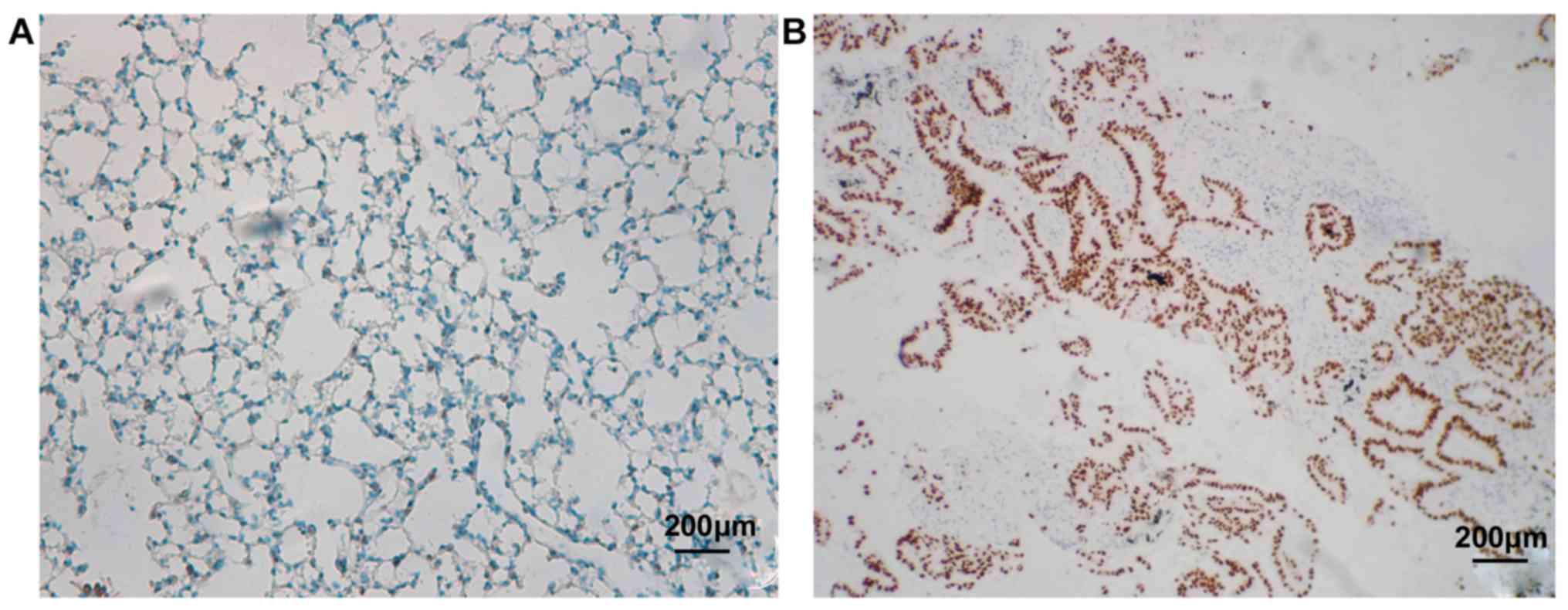
p53 is located in the nucleus and positive cells are
brown. As shown in Fig. 3 and
Table I, 8 out of 70 cases of
non-cancer GGN patients enrolled in this study were p53 positive,
and 52 cases were negative, and the positive rate was 13.33%.
However, in 60 patients with lung cancer, GGN tissues of 39
patients showed p53 positive and 21 patients were negative, and the
positive rate was 65.0%. There was a statistically significant
difference between the two groups (P<0.05).
 | Table I.Relationship of p53 gene deletion and
abnormal protein expression with the occurrence of lung cancer. |
Table I.
Relationship of p53 gene deletion and
abnormal protein expression with the occurrence of lung cancer.
|
|
| p53 protein abnormal
expression | p53 gene
deletion |
|---|
|
|
|
|
|
|---|
| Groups | Cases | p53 protein
expression | Expression percentage
(%) | p53 absence | Absence rate (%) |
|---|
| Non-cancer | 60 | 8 | 13.33 | 6 | 10 |
| Cancer | 60 | 39 | 65.0a | 34 | 56.67b |
The standard for deletion of p53 absence by FISH was
cells with 0 or 1 green fluorescence (FITC) dot appeared in intact
nuclei. As shown in Fig. 4 and
Table I, the number of p53 absence in
70 non-cancer GGN patients was 6 and the absence rate was 10.0%.
The number of p53 deletions in 70 patients with non-cancer GGN was
34 and the absence rate was 56.67%. There was a statistically
significant difference between the two groups (P<0.05, Fig. 5).
Follow-up results
Median follow-up time for all patients was 961 days
(25–1,121 days). No deaths occurred in the GGN non-cancer group
(n=60), and 43 cases died in the GGN lung cancer group. The median
survival time of patients with p53-positive expression was 16.8
months, and the 3-year survival rate was 31.5%. The median survival
time of p53-negative patients was 19.8 months, and the 3-year
survival rate was 49.6%. There was a statistically significant
difference between the two groups (P<0.05).
Discussion
GGN cannot be diagnosed solely based on CT. Blood
test is also not reliable in some cases. Therefore, most patients
were diagnosed at advanced stages (13). According to the 2016 edition of the
‘Guidelines for the Classification, Diagnosis and Treatment of Lung
Nodules in China,’ solid nodules >1 cm in diameter are defined
as high-risk nodules and require further examination (thin nodule
three-dimensional reconstruction CT scan, thin-layer
contrast-enhanced CT scan, needle biopsy) to confirm the nature of
lesion. CT scan should be performed 3 months later. If the nodule
does not shrink or grow after 3 months, the possibility of
malignancy should be considered. If the nodule shrinks, CT should
be performed 6, 12, and 24 months later. If no change is observed,
a long-term annual CT review is recommended and follow-up period
should be ≥3 years (14).
From focal GGN to early stage lung cancer, the
growth of GGN is inert, and this progress is relatively slow.
Therefore, the follow-up time for GGN is usually at least 3 years.
At initial stage (<8 mm), GGN in CT images was often
characterized as pure and round lesions with low density and clear
boundaries. At this stage, most GGNs were pGGN, and after surgery,
they were mostly confirmed as AAH (precancerous lesion) or AIS (not
invading the surrounding vascular interstitial, non-metastatic,
5-year survival rate of 100%). In extreme cases, it may also be MIA
(which invades surrounding vascular stroma <5 mm, does not
metastasize, and has a 5-year survival rate of 100% after
resection) (15). In general, AAH has
no obvious clinical symptoms and signs, and many AAHs are found in
surgically resected lung specimens. In 2004 edition of World Health
Organization's histological classification of lung cancer, AAH was
considered to be a precancerous lesion of bronchioloalveolar
carcinoma (BAC). The incidence of AAH in surgically resected
specimens was 9.3–21.4%. The incidence of AAH in resected lung
specimens for other reasons was 4.4–9.6%. However, 20% of pure GGN
lesions grow or become mixed GGN during follow-up, whereas 40% of
mixed GGN grow during follow-up (16). As the GGN gradually grows, the
percentage of solid components of pure GGN increases and mGGNs is
formed. There are even malignant changes such as lobed leaves,
burrs, vacuoles, pleural depressions, and vascular intensiveness,
which in turn lead to the occurrence of invasive adenocarcinomas
that can invade blood vessels, intrapulmonary or systemic
metastases (3). Therefore, to explore
the molecular mechanism of GGN carcinogenesis is an important issue
to be solved in the diagnosis of lung cancer.
Studies have shown that the activation of oncogenes
and inactivation of tumor suppressor genes are major events in
tumorigenesis. p53 is a ubiquitous tumor suppressor gene located on
human chromosome 17q13.1 encoding p53 protein, which regulates cell
cycle (17). When the intracellular
DNA is destroyed, wild-type p53 is activated to induce cell cycle
arrest in G1-M phase, hereby inhibiting cell proliferation.
Half-life of the mutant p53 gene is significantly prolonged and
T1/2 often reaches 20–40 h, and this abnormal protein expression
can be detected in a variety of human tumors (18). With the advantages of high
sensitivity, specificity, and intuitiveness, FISH can be used to
observe chromosome structure and abnormalities in biological
specimens. With tumor suppressor gene DNA as probe, FISH technology
can be used to perform interphase nucleus analysis to observe the
loss of gene expression in tumor cells, so as to provide molecular
genetic basis for studies on the progression of different stages of
tumors.
In this study, we used the FISH technique to
hybridize the GLP p53 DNA probe to the short arm of chromosome 17
(17p13.1), and we also used IHC techniques to detect deletions and
abnormal expression of tumor suppressor p53 gene in the GGN of
non-lung cancer and lung cancer patients. In the past, p53 gene
inactivation was reported to be induced by point mutations, which
can only explain a small part of lung cancer cases in which p53
gene is inactivated (19). In the
current experiment, the standard for FISH detection of p53 gene
deletion was the appearance of 0 or 1 green fluorescence (FITC) dot
in intact nuclei. In the GGN cells of non-cancer patients, p53
absence was observed in 6 cases and the absence rate was 10.0%
(6/60). In the GGN cells of cancer patients, 53 absence was
observed in 34 cases and the absence rate was 56.67% (34/60), which
was significantly higher than that of the non-cancer GGN group
(P<0.05). IHC results suggest that the number of p53-positive
cases in the non-tumor patients GGN group is 8, and the positive
rate is 13.33% (8/60). The number of p53-positive cases in the GGN
tissues of lung cancer patients was 39, and the positive rate was
65.0% (39/60). The difference between the two groups was
statistically significant (P<0.05). However, we detected p53
protein expression in lung cancer tissues both with and without p53
gene deletion.
According to the findings reported by Olivier et
al (20), we believe that p53
abnormalities are common in lung cancer tissues and tumor cells
contain loss of specific sites of p53 gene in the short arm of
chromosome 17 and are accompanied by genetic mutations in the
residual p53 allele. Deletion of the p53 gene may also happen, but
no gene mutations have yet occurred. Furthermore, our 3-year
follow-up data showed that no deaths occurred in the GGN non-cancer
group (n=60), whereas 43 cases died in the GGN lung cancer group.
The median survival time of patients with p53-positive group was
16.8 months, and the 3-year survival rate was 31.5%. The median
survival time of p53-negative patients was 19.8 months, and the
3-year survival rate was 49.6%.
In summary, FISH and IHC were used to investigate
the correlation between p53 gene abnormalities and survival of lung
cancer patients. We found that p53 gene deletion and protein
overexpression in lung cancer GGN tissues were significantly
associated with poor prognosis. It is suggested that p53 plays an
important role in the process of transformation of GGN to lung
cancer. Therefore, detecting the deletion of p53 gene as well as
the expression of p53 protein in GGN tissue will benefit the
diagnosis and prognosis of lung cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZT, WC, DY and ZZ were devoted to interpreting the
general data. ZT, WC and DY were responsible for IHC. ZT, WC, DY,
LZ and FW interpreted the FISH results. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Second Affiliated Hospital of Zhejiang University School of
Medicine (Hangzhou, China). Signed informed consents were obtained
from the patients or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Grunnet M and Sorensen JB:
Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung
Cancer. 76:138–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aberle DR, Adams AM, Berg CD, Black WC,
Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM and Sicks
JD: National Lung Screening Trial Research Team: Reduced
lung-cancer mortality with low-dose computed tomographic screening.
N Engl J Med. 365:395–409. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wood DE: National Comprehensive Cancer
Network (NCCN) clinical practice guidelines for lung cancer
screening. Thorac Surg Clin. 25:185–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suzuki K, Asamura H, Kusumoto M, Kondo H
and Tsuchiya R: ‘Early’ peripheral lung cancer: Prognostic
significance of ground glass opacity on thin-section computed
tomographic scan. Ann Thorac Surg. 74:1635–1639. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li F, Sone S, Abe H, Macmahon H and Doi K:
Malignant versus benign nodules at CT screening for lung cancer:
Comparison of thin-section CT findings. Radiology. 233:793–798.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aoki T, Tomoda Y, Watanabe H, Nakata H,
Kasai T, Hashimoto H, Kodate M, Osaki T and Yasumoto K: Peripheral
lung adenocarcinoma: Correlation of thin-section CT findings with
histologic prognostic factors and survival. Radiology. 220:803–809.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hattori A, Suzuki K, Matsunaga T, Fukui M,
Tsushima Y, Takamochi K and Oh S: Tumour standardized uptake value
on positron emission tomography is a novel predictor of
adenocarcinoma in situ for c-stage IA lung cancer patients with a
part-solid nodule on thin-section computed tomography scan.
Interact Cardiovasc Thorac Surg. 18:329–334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Detterbeck FC, Marom EM, Arenberg DA,
Franklin WA, Nicholson AG, Travis WD, Girard N, Mazzone PJ,
Donington JS, Tanoue LT, et al: The IASLC Lung Cancer Staging
Project: Background data and proposals for the application of TNM
staging rules to lung cancer presenting as multiple nodules with
ground glass or lepidic features or a pneumonic type of involvement
in the forthcoming eighth edition of the TNM classification. J
Thorac Oncol. 11:666–680. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee HY, Choi YL, Lee KS, Han J, Zo JI,
Shim YM and Moon JW: Pure ground-glass opacity neoplastic lung
nodules: Histopathology, imaging, and management. AJR Am J
Roentgenol. 202:W224–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goo JM, Park CM and Lee HJ: Ground-glass
nodules on chest CT as imaging biomarkers in the management of lung
adenocarcinoma. AJR Am J Roentgenol. 196:533–543. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Harris CC: p53 tumor suppressor gene: At
the crossroads of molecular carcinogenesis, molecular epidemiology,
and cancer risk assessment. Environ Health Perspect. 104 Suppl
3:435–439. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kakeji Y, Korenaga D, Tsujitani S, Baba H,
Anai H, Maehara Y and Sugimachi K: Gastric cancer with p53
overexpression has high potential for metastasising to lymph nodes.
Br J Cancer. 67:589–593. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tao Y, Lu L, Dewan M, Chen AY, Corso J,
Xuan J, Salganicoff M and Krishnan A: Multi-level ground glass
nodule detection and segmentation in CT lung images. Med Image
Comput Comput Assist Interv. 12:715–723. 2009.PubMed/NCBI
|
|
14
|
Zhou Q, Fan Y, Wang Y, Qiao Y, Wang G,
Huang Y, Wang X, Wu N, Zhang G, Zheng X, et al: China national
guideline of classification, diagnosis and treatment for lung
nodules (2016 version). Zhongguo Fei Ai Za Zhi. 19:793–798.
2016.(In Chinese). PubMed/NCBI
|
|
15
|
Wu C, Zhao C, Yang Y, He Y, Hou L, Li X,
Gao G, Shi J, Ren S, Chu H, et al: High discrepancy of driver
mutations in patients with NSCLC and synchronous multiple lung
ground-glass nodules. J Thorac Oncol. 10:778–783. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Qiang JW, Ye JD, Ye XD and Zhang
J: High resolution CT in differentiating minimally invasive
component in early lung adenocarcinoma. Lung Cancer. 84:236–241.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Muller PA and Vousden KH: Mutant p53 in
cancer: New functions and therapeutic opportunities. Cancer Cell.
25:304–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lane DP, Cheok CF and Lain S: p53-based
cancer therapy. Cold Spring Harb Perspect Biol. 2:a0012222010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jolly KW, Malkin D, Douglass EC, Brown TF,
Sinclair AE and Look AT: Splice-site mutation of the p53 gene in a
family with hereditary breast-ovarian cancer. Oncogene. 9:97–102.
1994.PubMed/NCBI
|
|
20
|
Olivier M, Hollstein M and Hainaut P: TP53
mutations in human cancers: Origins, consequences, and clinical
use. Cold Spring Harb Perspect Biol. 2:a0010082010. View Article : Google Scholar : PubMed/NCBI
|