Introduction
Lung cancer affects more individuals worldwide than
any other type of cancer, and is the leading cause of cancer
mortality in males and females. The global incidence of this
disease is rising by 0.5% per year, and the number of cancer
mortalities caused by lung cancer is expected to increase by up to
50% by 2020 (1).
Formation of the pulmonary circulation has been
described as a process that depends on two basic mechanisms:
vasculogenesis and angiogenesis. The distal vasculature arises by
vasculogenesis (embryonic day nine in the mouse model) (2) and the proximal vasculature arises by
angiogenesis (starts at approximately embryonic day 12); however,
this theory remains controversial (3).
Angiogenesis, the formation of new blood vessels, is
essential for tumor growth and metastasis (4). A total of six tumor vessel types have
been identified. Four vessel types (mother vessels, capillaries,
glomeruloid microvascular proliferations and vascular
malformations) develop from preexisting normal venules and
capillaries, and two vessel types (feeder arteries and draining
veins) develop from arterio-venogenesis, a parallel process that
involves the remodeling of preexisting arteries and veins (5). There are several well-known mechanisms
of blood vessel formation in normal and tumor tissues, including
sprouting angiogenesis, vasculogenesis, intussusception, vessel
co-option, vasculogenic mimicry and tumor cell-endothelial cell
transdifferentiation (6). The new
vascular network is considered ‘immature’ and varies from normal
(or mature) vascular structures. Immature vessels lack the normal
vascular network organization, are irregularly shaped, are composed
of abnormal basement membranes and pericytes, and have an increased
permeability.
Microvessel pericyte coverage index revealed
intermediate values in lung and prostate carcinomas, high values in
mammary and colon carcinomas, and low values in glioblastomas and
renal cell carcinomas (7).
Passalidou et al (8)
described a group of non-small cell lung carcinomas (NSCLC) without
morphological evidence of neoangiogenesis. In these tumors, the
vascular phenotype was that of normal vessels and there was no
neoangiogenesis. Following this, the vascular architecture in lung
adenocarcinomas (ADCs) was classified into three types of patterns:
diffuse, alveolar (nonangiogenic) and mixed (9). Kakolyris et al (10) demonstrated that there is a large
variation in the level of differentiation of tumor vasculature in
lung carcinoma subtypes. The authors suggested that capillary
maturation may be correlated with microvessel number, improving the
identification of patients who may benefit from specific
anti-angiogenic therapies.
Despite advances in treatment, the five-year
survival rate does not exceed 15% (11). In 2006, bevacizumab was approved for
first-line treatment of advanced, non-squamous NSCLC to be
administered in combination with platinium-based chemotherapy
(12). Nevertheless, the prognosis
for patients with lung cancer remains poor; thus, quantifying the
types of blood vessels may aid the improvement of lung cancer
therapy.
The aim of this study was to use double
immunostaining methods to evaluate the types and morphology of
blood vessels in various types of lung carcinomas.
Materials and methods
In our study, we included 39 biopsies from patients
with various types of lung carcinoma. Sections (5 μm) were
fixed in buffered formalin and embedded in paraffin. For
pathological diagnosis, slides were stained with haematoxylin and
eosin (H&E). For double immunostainings, CD34 (QBEnd10 clone;
dilution, 1:25) was applied for 30 min and smooth muscle actin
(SMA; clone 1A4; ready to use) for 30 min. The EnVision Doublestain
kit was used as a visualization system (DakoCytomation; Glostrup,
Denmark). All reagents were purchased from Dako Cytomation. The
entire immunohistochemical procedure was developed with a Dako
Cytomation Autostainer (Dako Cytomation). Tumor blood vessels were
quantified separately for CD34/SMA according to Gee et al
(13) as either immature,
intermediate or mature. We used the ‘hot spot’ method to evaluate
the total number of vessels, which were then separately counted in
the same field and defined as immature
(CD34+/SMA−, without lumen), intermediate
(CD34+, with perfused lumen and with negative or weak
positive reaction for SMA) or mature vessels
(CD34+/SMA+). Microscopic images were
captured and processed using Nikon Lucia G software (Nikon, Tokyo,
Japan).
This study was approved by the local research ethics
committee of ‘Victor Babes’ University of Medicine and Pharmacy
Timisoara, Romania, and informed consent was obtained from all
subjects according to the World Medical Association Declaration of
Helsinki.
Results
Pathological evaluation
Pathological evaluation of lung cancer specimens
revealed seven cases of small cell carcinomas, five cases of ADC,
one case of large cell lung carcinoma (LCLC), one case of hepatoid
carcinoma and 25 cases of squamous cell carcinoma (SCC).
Double immunostaining
Double immunostaining revealed tumor blood vessel
heterogeneity in the same and different pathological subtypes of
lung carcinoma. Vessels from peritumoral and intratumoral areas
varied in size, were irregular in shape and contained numerous
branches. The vessels were arranged among the tumor cells with
relatively uniform distribution. We identified a high level of
variability in terms of blood vessel morphology. Vessels presented
with variable dimensions, irregular and narrow lumens, and intense
branching character. The presence of cords and isolated endothelial
cells suggests the possibility of the sprouting angiogenesis
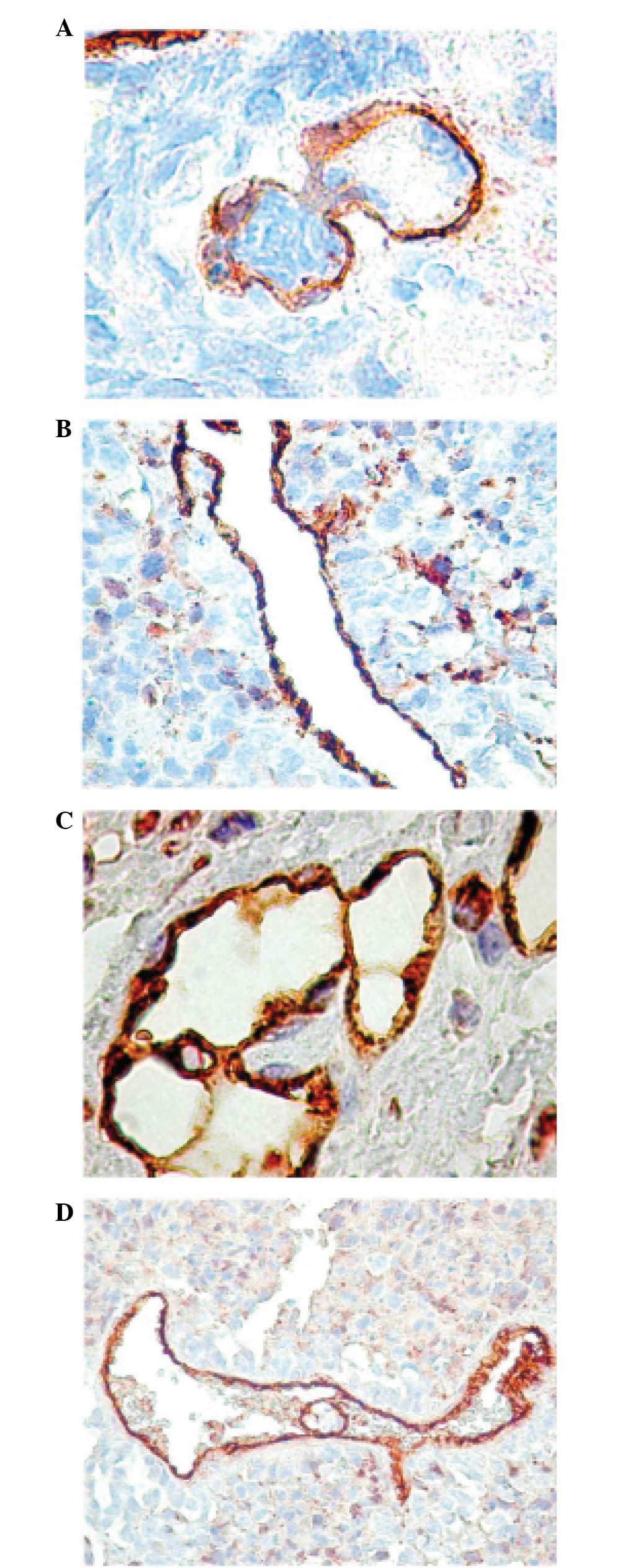
mechanism. In the tumor area, we occasionally identified vessels
with a large lumen and prominent endothelium, suggesting the
possibility of the intussusception mechanism (Fig. 1A). This process is considered to
arise from preexisting blood vessels during tumor proliferation. In
the peritumoral area, the vessels were composed of a large lumen,
relatively regular shape and were usually surrounded by
inflammatory infiltrate (Fig.
1B).
Microvascular density (MVD)
The mean MVD value of the vessels was 31.86 for
ADCs, 23.28 for small cell carcinomas, 16 for LCLCs, 23 for
hepatoid carcinomas and 23.99 for SCCs. The highest and lowest MVD
values were identified in lung ADCs and LCLCs, respectively. In all
cases, the immature and intermediate types of blood vessels were
predominant with a few differences associated with pathological
subtypes. No correlation was identified between the pathological
type and the total number of blood vessels (P=0.357). We identified
a significant correlation between the pathological type and the
number of immature (P=0.038) and mature vessels (P=0.036); however,
no correlation was identified between the pathological type and the
number of intermediate type vessels (P=0.447).
Lung ADC
The average number of the immature (16.66) and the
intermediate (14.06) vessels demonstrated almost similar values
compared to the average number of mature vessels (1.19) from lung
ADCs. These observations are supported by the significant
correlation identified between the total number of vessels and the
number of immature vessels (P=0.039) from this pathological type.
In the ADC specimens, immature vessels predominated, except in one
case where we revealed the presence of intussusceptions and a high
number of CD34 structures without SMA expression, suggesting the
formation of glomeruloid bodies (Fig.
1C).
Small cell lung carcinoma
Immature blood vessels were the predominant type
observed in small cell lung carcinoma samples. This was supported
by the correlation between immature and pathological vessels
(P=0.011). The pathological type did not correlate with
intermediate and mature types of vessels (P=0.151 and P=0.405,
respectively).
SCC
A significant correlation between the total number
of vessels and the number of immature vessels (P=0.005) was
identified in lung SCC samples. A representative correlation with
intermediate type blood vessels (P=0.018) was also revealed;
however, there was no correlation with mature type blood vessels
(P=0.512).
LCLC
A high number of SMA+ tumor cells was
observed in the tumor tissues and around the blood vessels in the
LCLC samples. Vessel-like structures containing numerous tumor
emboli were also detected in the blood vessels (Fig. 1D).
Discussion
Malignant human tumors are characterized by varying
degrees of angiogenesis and pericyte recruitment. The degree of
angiogenesis in human tumors varies and may be extremely low in
certain types of tumors. The suitability of tumors for
antiangiogenic therapies may differ between various tumor types or
within one type of tumor.
Maeda et al (14) examined the association between the
number of circulating endothelial progenitor cells (EPCs) and
intratumoral MVD, both of which may be markers for
neovascularization, as well as the various lung cancer histological
types, particularly ADC. They revealed no statistically significant
differences in the number of EPCs or the MVD value between the ADC
and SCC subtypes. Among the ADC histological subtypes, a higher
number of EPCs and a greater MVD value was identified, which was
significantly more frequent in solid ADCs compared with non-solid.
These patients may be the best candidates for antiangiogenic
therapies.
Dagnon et al (15) analyzed the distance between cancer
cells, blood vessels and the microvasculature organization in NSCLC
in comparison with SCC and ADC. This computerized morphometric
study revealed a significantly higher MVD value in ADCs compared
with SCCs, particularly close to the invading edge. We also
identified similar mean values for the number of immature,
intermediate and mature vessels in the lung SCC and ADC
subtypes.
Due to the continuous and excessive synthesis of the
vascular endothelial growth factor (VEGF) in cancer tissue, tumor
vessels remain immature and lack the tight association between
mural cells and endothelial tubes. The immature tumor vessels
display high vascular permeability; thus, the tumor tissue is
edematous, containing extravasated plasma components. In addition
to edema, the expansion of cancer tissue results in increased
interstitial pressure, causing impaired tumor blood flow (16).
Antiangiogenic therapy with bevacizumab, an
anti-VEGF antibody, predominantly targets immature blood vessels.
Zhao et al (17)
demonstrated that there are two major types of microvessels in lung
cancer vasculature, undifferentiated and differentiated. The MVD
value of undifferentiated vessels is a favorable predictor for
patients with NSCLC treated with a chemotherapy regimen and
bevacizumab, with a higher MVD value correlating with a better
treatment response. Further studies are required to verify the
predictive role of MVD in the treatment of NSCLC with bevacizumab.
We identified that immature and intermediate vessel types are
predominantly expressed in lung SCCs and ADCs, while immature
vessel types are predominantly expressed in small cell lung
carcinoma.
One of the major problems associated with
bevacizumab therapy is the exclusion of patients with brain
metastases and/or squamous histology who are receiving this
therapeutic option as they represent a significant proportion of
the advanced NSCLC patient population (18). In our study, we revealed a
significant correlation between the total number of vessels in the
lung SCCs and the number of immature and intermediate type vessels;
however, no correlation was identified with mature type vessels.
Jubb et al (19)
demonstrated that the proliferation index, VEGF expression, MVD and
the number of mature vessels were discordant between primary and
secondary cancers.
In conclusion, double immunostaining evaluation of
the types of blood vessels in lung carcinoma demonstrated a marked
heterogeneity. The highest MVD was identified in lung ADCs and the
lowest in LCLCs. The immature and intermediate types of vessels
were more common in ADCs and lung SCCs. Small cell lung carcinoma
presented a significant correlation between the pathological and
immature type of blood vessels.
References
|
1.
|
A JemalR SiegelE WardY HaoJ XuT MurrayMJ
ThunCancer statistics, 2008CA Cancer J
Clin587196200810.3322/CA.2007.0010
|
|
2.
|
DE deMelloLM ReidEmbryonic and early fetal
development of human lung vasculature and its functional
implicationsPediatr Dev
Pathol3439449200010.1007/s10024001009010890928
|
|
3.
|
MC PareraM van DoorenM van KempenR de
KrijgerF GrosveldD TibboelR RottierDistal angiogenesis: a new
concept for lung vascular morphogenesisAm J Physiol Lung Cell Mol
Physiol288L141L149200510.1152/ajplung.00148.200415377499
|
|
4.
|
J FolkmanWhat is the evidence that tumours
are angiogenesis dependent?J Natl Cancer
Inst8246199010.1093/jnci/82.1.41688381
|
|
5.
|
JA NagySH ChangSC ShihAM DvorakHF
DvorakHeterogeneity of the tumor vasculatureSemin Thromb
Hemost36321331201010.1055/s-0030-125345420490982
|
|
6.
|
P CarmelietRK JainMolecular mechanisms and
clinical applications of
angiogenesisNature473298307201110.1038/nature1014421593862
|
|
7.
|
A EberhardS KahlertV GoedeB HemmerleinKH
PlateHG AugustinHeterogeneity of angiogenesis and blood vessel
maturation in human tumors: implications for antiangiogenic tumor
therapiesCancer Res60138813932000
|
|
8.
|
E PassalidouM TrivellaN SinghM FergusonJ
HuA CesarioP GranoneAG NicholsonP GoldstrawC RatcliffeVascular
phenotype in angiogenic and non-angiogenic lung non-small cell
carcinomasBr J Cancer86244249200210.1038/sj.bjc.660001511870514
|
|
9.
|
J GuoK HigashiY UedaM OguchiT TakegamiH
TogaT SakumaH YokotaS KatsudaH TonamiI YamamotoMicrovessel density:
correlation with 18F-FDG uptake and prognostic impact in lung
adenocarcinomasJ Nucl Med47419425200616513610
|
|
10.
|
S KakolyrisA GiatromanolakiM KoukourakisL
KaklamanisCH KouroussisV BozionelouV GeorgouliasKC GatterAL
HarrisAssessement of vascular maturation in lung and breast
carcinomas using a novel basement membrane component,
LH39Anticancer Res21431143162001
|
|
11.
|
CF MountainRevisions in the International
System for Staging Lung
CancerChest11117101717199710.1378/chest.111.6.17109187198
|
|
12.
|
A SandlerR GrayMC PerryJ BrahmerJH
SchillerA DowlatiR LilenbaumDH JohnsonPaclitaxel-carboplatin alone
or with bevacizumab for non-small-cell lung cancerN Engl J
Med35525422550200610.1056/NEJMoa06188417167137
|
|
13.
|
MS GeeWN ProcopioS MakonnenMD FeldmanNM
YeildingWM LeeTumor vessel development and maturation impose limits
on the effectiveness of anti-vascular therapyAm J
Pathol162183193200310.1016/S0002-9440(10)63809-6
|
|
14.
|
R MaedaG IshiiM ItoT HishidaJ YoshidaM
NishimuraH HagaK NagaiA OchiaiNumber of circulating endothelial
progenitor cells and intratumoral microvessel density in non-small
cell lung cancer patients: differences in angiogenic status between
adenocarcinoma histologic subtypesJ Thorac
Oncol7503511201210.1097/JTO.0b013e318241780e
|
|
15.
|
K DagnonD HeudesJF BernaudinP
CallardComputerized morphometric analysis of microvasculature in
non-small cell lung carcinomaMicrovasc
Res75112118200810.1016/j.mvr.2007.04.00417560614
|
|
16.
|
P CarmelietRK JainAngiogenesis in cancer
and other diseasesNature407249257200010.1038/3502522011001068
|
|
17.
|
YY ZhaoC XueW JiangHY ZhaoY HuangK
FeenstraJH ResauCN QianL ZhangPredictive value of intratumoral
microvascular density in patients with advanced non-small cell lung
cancer receiving chemotherapy plus bevacizumabJ Thorac
Oncol77175201210.1097/JTO.0b013e31823085f422011670
|
|
18.
|
C GridelliP MaioneA RossiF De
MarinisTreatment of non-small cell lung cancer: current indications
and future
developmentsOncologist1211831193200710.1634/theoncologist.12-10-118317962612
|
|
19.
|
AM JubbA CesarioM FergusonMT CongedoKC
GatterF LococoA MulèF PezzellaVascular phenotypes in primary
non-small cell lung carcinomas and matched brain metastasesBr J
Cancer718771881201110.1038/bjc.2011.14721540863
|