Introduction
Ovarian cancer accounts for 3% of all cases of
cancer in females; however, it has the highest mortality of all
gynecological cancers (1). Most
patients have advanced stage disease at presentation due to a
paucity and incidious onset of symptoms. Initial treatment consists
of cytoreductive surgery and adjuvant platinum-based cytotoxic
chemotherapy (1,2). Despite high response after initial
treatment, 20–30% of patients with early-stage disease (stage
IA–IIA) and up to 75% of patients with advanced disease (IIB–IV)
present with recurrence within two years (2,3).
Imaging examinations, including ultrasound (US),
computed tomography (CT) and magnetic resonance imaging (MRI),
should be performed during patient follow-up when there is clinical
suspicion of ovarian cancer recurrence or CA-125 elevation. A
systematic review and meta-analysis by Gu et al(4) evaluated CA-125 levels, positron
emission tomography (PET) alone, PET/CT, CT and MRI in diagnosing
recurrent ovarian carcinoma. CA-125 levels had the highest pooled
specificity (93%), while PET/CT had the highest pooled sensitivity
(91%). CT (sensitivity, 79%; specificity, 84%) and MRI
(sensitivity, 75%; specificity, 78%) had a similar diagnostic
performance.
While the level of CA-125 has been shown to be a
sensitive marker for tumor recurrence and levels may rise 3 to 6
months before there is clinically apparent disease, it does not
provide information concerning the size and distribution of the
lesions (3–5). Levels may also increase in a number of
benign conditions, not being a specific marker for ovarian cancer,
and a number of patients with relapse of disease present with
normal CA-125 levels (4,5). CT has low sensitivity for detecting
disease recurrence, probably due to its inability to detect small
peritoneal implants and normal-sized lymph node metastases
(4,6). Pelvic MRI is useful for the evaluation
of local recurrence of disease, however, it has low specificity due
to post-surgical anatomical alterations (4,7).
2-deoxy-2-(18F)-fluoro-D-glucose
(18FDG) PET/CT may play an important role in ovarian
cancer recurrence, as the metabolic tracer is able to increase
lesion detection, the fusion of metabolic and anatomical imaging
aids the determination of the exact location of disease and it is
capable of surveying the whole body. Several studies have examined
the performance of PET/CT scanning in patients with recurrent
ovarian cancer (4,8–14).
An Australian prospective, multi-center cohort study
of 90 females (14) assessed the
impact of 18FDG PET/CT in the management of patients
with suspected recurrent ovarian cancer and evaluated information
provided by 18FDG PET/CT in this clinical context.
PET/CT detected 168 additional and unsuspected sites of disease in
61 patients (67.8%). The management was changed in 53 patients
(58.9%) based on PET/CT scan findings. PET/CT was superior to
abdominal and pelvic CT in the detection of nodal, peritoneal and
subcapsular liver disease and it also allowed the identification of
patients whose disease was likely to progress within 12 months. The
authors suggested that PET/CT should be the preferred imaging
modality in patients with suspected ovarian carcinoma
recurrence.
The aim of the present study was to evaluate the use
of 18FDG PET/CT in patients with suspicion of ovarian
cancer recurrence and describe the distribution of metastasis.
Patients and methods
Patients
A total of 45 female patients (age range, 39–84
years; mean age ± SD, 59.5±10.0) with suspicion of ovarian cancer
recurrence were included in this retrospective study. The patients
underwent a PET/CT scan at PET/CT Campinas, private clinic,
Campinas, São Paulo, Brazil, between November 2006 and November
2010. Indications for PET/CT were clinical suspicion of relapse of
disease, elevated CA-125 or abnormal or equivocal findings on
abdominal/pelvic US, CT or MRI. All patients had undergone surgery
and all but one had received adjuvant chemotherapy at the time of
diagnosis. A total of 18 patients had already had relapse of
disease during their previous follow-up and PET/CT was performed
for the suspicion of new progression of disease. The study was
approved by the ethics committee of the Medical Sciences Faculty,
State University of Campinas, Unicamp, São Paulo, Brazil. Detailed
patient and tumor characteristics are shown in Table I.
 | Table I.Patient and tumor characteristics
(n=45). |
Table I.
Patient and tumor characteristics
(n=45).
| Characteristic | Value |
|---|
| Age (years) | |
| Mean ± SD | 59.5±10.0 |
| Range | 39–84 |
| Histological type
(n) | |
| Serous
adenocarcinoma | 29 |
| Endometrioid
adenocarcinoma | 9 |
| Mixed type
adenocarcinoma | 1 |
| Clear cell
adenocarcinoma | 1 |
| Adenocarcinoma
(NOS) | 4 |
| Sertoli cell
tumor | 1 |
| FIGO stage at
diagnosis (n) | |
| I | 2 |
| II | 3 |
| III | 34 |
| IV | 6 |
18FDG PET/CT imaging
All patients fasted for at least 6 h, maintaining
their blood glucose levels <150 mg/dl, before the injection of
∼12 mCi (mean ± SD, 13.0±2.3 mCi) of 18FDG. The patients
rested in a supine position for 40 to 60 min after the injection
and were then positioned for PET/CT imaging. All PET/CT scans were
performed on a combined 16-slice CT/BGO PET scanner (Discovery STE,
GE Medical Systems Inc., Milwaukee, WI, USA). The patients received
oral contrast (Urografina® 291, Schering-Plough
Corporation; Kenilworth, New Jersey, USA; 25 ml diluted in 1 liter
of water), two glasses before the 18FDG injection and
two glasses immediately before the imaging. A contrast-enhanced CT
was acquired from the top of the head to mid-thigh, without any
specific breath-holding instructions. Intravenous contrast (100 ml,
Optiray, Mallinckrodt; St.Louis, MO, USA) was injected, unless the
patient was allergic to iodine. The parameters of the CT scan were
140 kV, 150–250 mAs, slice thickness of 3.75 mm. The CT was
followed by PET scanning, covering the same transverse field of
view during normal breathing. The imaging was acquired with 6 to 8
bed positions on a 2D mode for 5 min per bed position (n=20). In
August 2008, the protocol of the institution changed, therefore,
the scans of 25 patients were acquired on a 3D mode for 3 min per
bed position. PET images were reconstructed iteratively using the
contrast-enhanced CT data for attenuation correction. Coregistered
images were displayed on a workstation, using dedicated software
which allowed the viewing of PET, CT and fusion images on
transaxial, sagittal and coronal displays.
18FDG PET/CT analysis
All 18FDG PET/CT scans were interpreted
by an experienced radiologist in conjunction with an experienced
nuclear medicine physician, who were both aware of the suspicion of
ovarian carcinoma recurrence and the laboratory and imaging
findings of the patients. The 18FDG PET portion and the
CT portion of PET/CT were jointly interpreted using a dedicated
image fusion workstation. All areas of increased 18FDG
uptake that corresponded to a CT abnormality were interpreted as
positive for recurrent disease. Semi-quantitative analysis was also
performed to derive a standardized uptake value (SUV). All PET/CT
reports and images were reviewed by an experienced nuclear
physician for consistency of the data.
The results of 18FDG PET/CT were
correlated with patient follow-up information for at least 6 months
after the examination (mean ± SD, 21.0±12.0). The diagnosis of
recurrence was confirmed with surgery (n=15) or clinically (n=30),
by persistent elevation of CA-125 levels with abnormal findings on
further imaging and treatment response following chemotherapy.
Statistical analysis
For the comparison of the SUVs from different tumor
types the ANOVA test was used, and P<0.05 was considered to
indicate a statistically significant difference.
Results
A total of 42 patients were diagnosed with
recurrence of ovarian cancer after surgery or during clinical
follow-up. Three patients remained free of disease during clinical
follow-up. CA-125 levels were raised in a total of 34 patients, 14
patients had clinical suspicion of recurrence and 23 presented with
alterations on US, CT or MRI. There were 11 patients with raised
CA-125 levels and normal imaging examinations. The characteristics
of the patients according to the PET/CT findings are shown in
Table II.
 | Table II.Characteristics of patients according
to PET/CT findings. |
Table II.
Characteristics of patients according
to PET/CT findings.
| PET/CT
|
|---|
| Characteristic | Positive | Negative |
|---|
| Recurrent
disease | | |
| Yes | 42 | - |
| No | - | 3 |
| CA-125 (U/ml) | | |
| >35 | 34 | - |
| ≤35 | 8 | 3 |
| Clinical
symptoms | | |
| Positive | 14 | - |
| Negative | 18 | 1 |
| No data | 10 | 2 |
| US | | |
| Positive | 4 | - |
| Negative | 6 | 1 |
| No data | 32 | 2 |
| MRI | | |
| Positive | 4 | - |
| Negative | 5 | - |
| No data | 33 | 3 |
| CT | | |
| Positive | 13 | 2 |
| Negative | 10 | 1 |
| No data | 19 | - |
| Surgery after
PET/CT | | |
| Positive | 15 | - |
| Negative | - | - |
| Not performed | 27 | 3 |
18FDG PET/CT scan was positive in all 42
patients who were confirmed to have recurrence of disease.
18FDG PET/CT scan was negative in 3 patients, all free
from disease during follow-up, with normal CA-125 levels and no
evidence of disease on imaging examinations. One of the patients
without ovarian cancer recurrence presented with focal abnormal
uptake in the right thyroid lobe (SUV, 17.0) and a new primary
tumor was diagnosed following surgery.
There were 11 patients with elevated CA-125 levels
and normal conventional imaging, all with positive PET/CT findings.
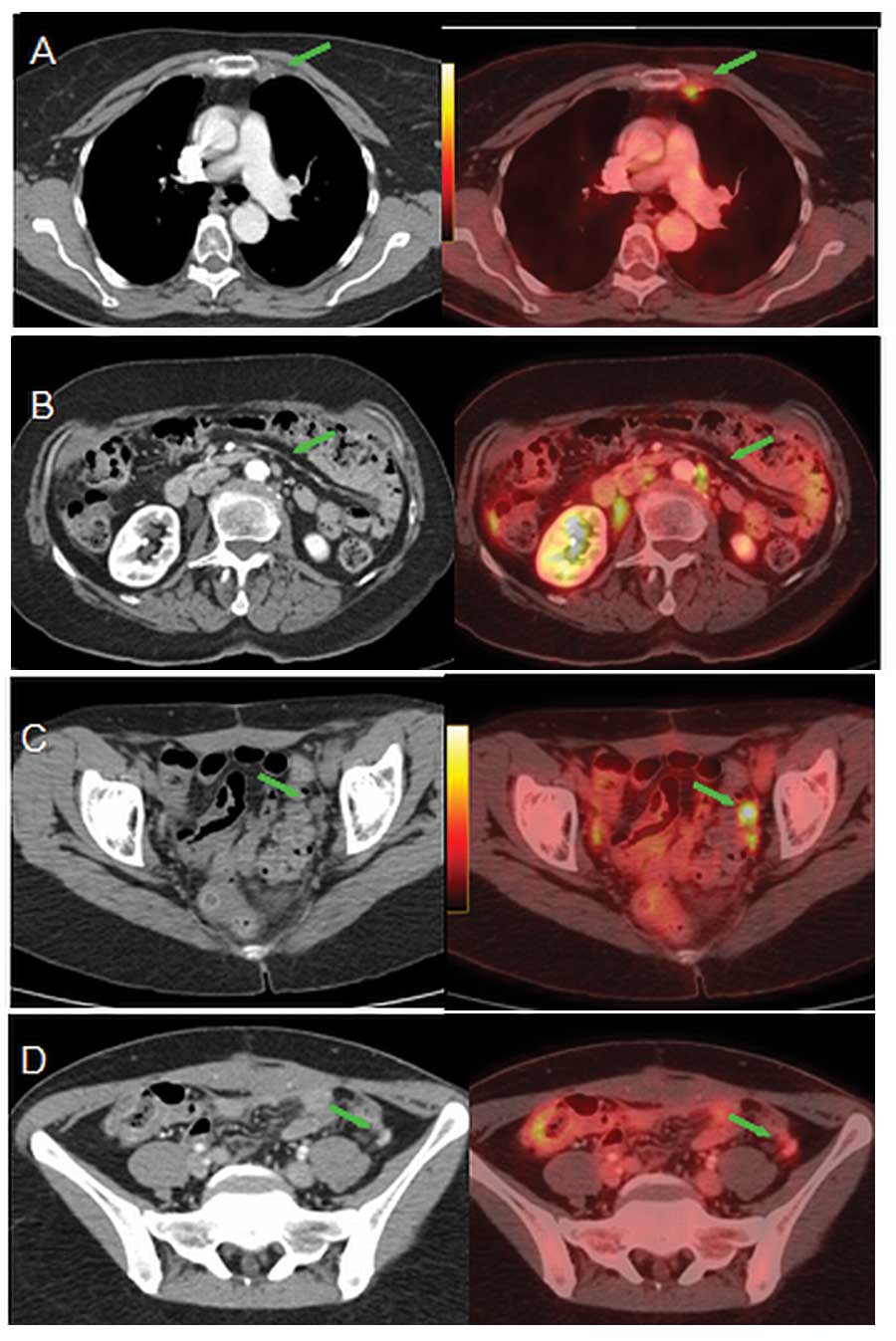
However, of the 11 patients with normal CA-125 levels, eight
presented with a positive PET/CT scan (Fig. 1). Four of those patients had
localized pelvic or abdominal disease (two of which were amenable
to surgical resection) and 4 had supra-diaphragmatic lymph node
metastasis. Patients with elevated CA-125 levels tended to have a
more disseminated disease.
Overall, lymph nodes were the most frequent site of
relapse of disease (Table III),
being localized to the pelvic/abdominal region in 30 patients
(66.7%) and the thoracic region in 16 (35.6%). Six patients had
internal mammary metastasis, with surgical resection in two
patients. Peritoneal implants were found in 27 patients (60%).
Distant sites of metastasis included the liver (n=6), spleen (n=2),
pleura (n=2), lung (n=2) and bone (n=2). However, with regard to
the subgroup of 11 patients with elevated CA-125 levels and normal
conventional imaging, peritoneal implants were the most frequent
site of relapse of disease (n=9), followed by lymph nodes, most
being in the pelvic/abdominal region (n=7).
 | Table III.Distribution of metastases found on
PET/CT. |
Table III.
Distribution of metastases found on
PET/CT.
| | SUV (mean ± SD)
|
|---|
| Localization | Number of
patients | Normal CA-125
(n=8) | Elevated CA-125
(n=34) |
|---|
| Pelvic and abdominal
lymph nodes | 30 | 6.1±4.0 | 6.9±4.6 |
| Thoracic lymph
nodes | 16 | 11.3±4.3 | 4.8±3.0 |
| Peritoneal
implants | 27 | 7.5±3.1 | 6.8±3.8 |
| Liver | 5 | - | 7.6±4.0 |
| Spleen | 2 | - | 9.0±5.3 |
| Pleura | 2 | - | 4.4±4.9 |
| Lung | 2 | - | 1.7±0.1 |
| Bone | 2 | - | 2.8±0.8 |
| Thoracic wall
implants | 1 | 5.7±0.0 | - |
PET/CT found unsuspected lesions in 20 out of 45
patients (44.4%), most being supra-diaphragmatic lymph node
metastases or normal sized abdominal lymph nodes with abnormal
18FDG uptake (Fig. 2).
There was no statistical difference when comparing the SUVs of
lesions from different tumor types (P=0.6683; Table IV).
 | Table IV.SUV of the lesions among different
cancer types (P=0.6683). |
Table IV.
SUV of the lesions among different
cancer types (P=0.6683).
| Histological type
of tumor | Number of
lesions | SUV (mean ±
SD) |
|---|
| Serous
adenocarcinoma | 133 | 6.8±4.3 |
| Endometrioid
adenocarcinoma | 29 | 6.1±3.7 |
| Mixed type
adenocarcinoma | 3 | 3.9±0.2 |
| Clear cell
adenocarcinoma | 3 | 4.5±1.7 |
| Adenocarcinoma
(NOS) | 15 | 5.2±2.9 |
| Sertoli cell
tumor | 3 | 7.5±4.0 |
A total of 12 patients (26%) died during follow-up
(mean ± SD, 15.3±7.9 months after examination; range, 6–35 months).
Five of these patients had disseminated abdominal and
supra-diaphragmatic disease, while 7 had disease limited to the
pelvic/abdominal region.
Discussion
PET/CT correctly diagnosed patients with suspected
ovarian cancer recurrence. All patients with elevated CA-125 levels
and normal conventional imaging had positive PET/CT scan. However,
most patients with normal CA-125 levels in this series presented
with positive PET/CT scan. Lymph nodes were the most frequent site
of relapse of disease, most being in the pelvic/abdominal region
and others in the thoracic region. Peritoneal implants were found
in more than half of patients. Distant sites of metastasis included
the liver, spleen, pleura, lung and bone. PET/CT detected
unsuspected lesions in almost half of the patients, most being
supra-diaphragmatic lymph node metastasis.
Our population had a high pre-test probability of
disseminated disease, given that 18/45 patients (40%) had
previously had relapse of ovarian cancer and the referral to PET/CT
was to evaluate the progression of the disease and to restage. The
advantage of PET/CT in this clinical setting was ability to
evaluate the whole body that may aid the correct selection of
patients who are amenable to surgical resection.
Most of our findings are in accordance with those
previously described in the literature, with the exception of the
high prevalence of supra-diaphragmatic lymph node metastases
(7–14). Iagaru et al(9) retrospectively evaluated 43 patients
with ovarian carcinoma and described the distribution of
extra-pelvic metastases in 19 patients, 5 of which (11.6%) had
supra-diaphragmatic lymph node involvement. A prospective,
multi-center Australian study (14)
showed that 14 out of 90 patients (15%) presented with disease
above the diaphragm.
In the Australian study (14), PET/CT findings changed the
management plan with medium to high impact in 58% of patients, due
to its ability to detect unsuspected lesions and the advantage of
the whole body evaluation.
The change in management based on PET/CT was also
previously described by Simcock et al(8). The authors prospectively evaluated 56
females with ovarian cancer who underwent PET/CT scan for suspicion
of recurrence or surveillance with no evidence of diseacse. PET/CT
altered the apparent disease distribution in 40 scans (61%), with a
high impact on management plan in 32 patients (57%).
Numerous clinicians routinely measure the level of
CA-125 since it is often the first evidence of ovarian cancer
recurrence and may rise 3 to 6 months before clinical evidence of
disease (3–5). However, without localized disease,
there is no rationale to initiate treatment based on a laboratory
test alone and this can cause considerable patient anxiety.
Therefore, it is important to have an accurate method that is able
to both diagnose and localize the recurrence of disease and aid the
selection of patients amenable to surgical resection.
Recent meta-analyses evaluated CT, MRI, PET and
PET/CT for the detection of metastatic lymph nodes in patients with
ovarian cancer (15). PET and
PET/CT were a more accurate modality for lymph node metastasis
detection, with a global pooled sensitivity of 73.2% and a
specificity of 96.7%. However, the greater specificity of PET or
PET/CT compared with those of CT or MRI was statistically
insignificant. CT and MRI showed similar diagnostic performance,
with pooled sensitivity of 42.6 and 54.7% and pooled specificity of
95.0 and 88.3%, respectively.
PET/contrast-enhanced CT in the same study (15) showed a sensitivity of 84.4% and
specificity of 97.4%, which was better than non-contrast-enhanced
PET/CT. Certain authors believe that the CT portion of the study
should be performed without contrast (8). Those who defend the use of contrast
usually perform a non-contrast-enhanced CT before the diagnostic
contrast-enhanced CT, for the purposes of attenuation correction of
the PET images. Yau et al(16) showed that application of intravenous
contrast does not interfere with the diagnostic value of PET/CT
when contrast-enhanced CT is used for attenuation correction
purposes.
The PET/CT evaluation of pelvic and abdominal
regions may be challenging due to urinary excretion and bladder
concentration of 18FDG. Contrast material may aid the
distinguishing of vessels and urethers from small nodal disease,
which can result in better sensitivity of the PET/CT scan. This may
be of particular importance in patients with ovarian cancer, since
most metastases involve the pelvic and abdominal lymph nodes or
implants. Certain authors (17)
suggest the use of diuretics and dual time imaging as another way
of improving the sensitivity of the examination for detection of
pelvic lesions.
A limitation of our study is that there was no
pathological confirmation of all the sites of abnormal
18FDG uptake. However, the confirmation of all the sites
would not have been ethical solely for the purpose of validation of
PET/CT findings. Accurate surgical assessment of pelvic and
retroperitoneal lymph nodes is difficult, and surgery appears to be
an unreliable gold standard, with disease recurrence in a third of
females with negative surgical findings (18). We agree with Simcock et
al(8) and believe that the
course of disease and clinical outcomes may more accurately
validate PET/CT data.
Another limitation was that we did not have data
concerning the treatment plans prior to the PET/CT, therefore, it
was not possible to evaluate the change in management in our study.
However, PET/CT revealed unsuspected lesions in 44.4% of our
patients, which is in accordance with previously published
data.
There is no evidence that PET/CT improves the
overall survival of patients diagnosed with ovarian cancer
recurrence. However, the whole body examination shows the extent of
the disease. This may aid the correct restaging of patients
considered for further treatment.
In conclusion, 18FDG PET/CT was an
accurate and useful tool for diagnosing ovarian cancer recurrence.
The advantage of a whole body scan and metabolic imaging is that it
may aid the detection of additional sites of disease.
Supra-diaphragmatic disease in this series of patients with
suspicion of ovarian cancer recurrence was more frequent than
previously described.
References
|
1.
|
Bertone-Johnson ER: Epidemiology of
ovarian cancer: a status report. Lancet. 365:101–102. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Cannistra SA: Cancer of the ovary. N Engl
J Med. 351:2519–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Gadducci A, Cosio S, Zola P, et al:
Surveillance procedures for patients treated for epithelial ovarian
cancer: a review of the literature. Int J Gynecol Cancer. 17:21–31.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Gu P, Pan LL, Wu SQ, et al: CA 125, PET
alone, PET-CT, CT and MRI in diagnosing recurrent ovarian
carcinoma: a systematic review and meta-analysis. Eur J Radiol.
71:164–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Goonewardene TI, Hall MR and Rustin GJ:
Management of asymptomatic patients on follow-up for ovarian cancer
with rising CA-125 concentrations. Lancet Oncol. 8:813–821. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Togashi K: Ovarian cancer: the clinical
role of US, CT, and MRI. Eur Radiol. 13(Suppl 4): L87–L104. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Low RN, Duggan B, Barone RM, et al:
Treated ovarian cancer: MR imaging, laparotomy reassessment, and
serum CA-125 values compared with clinical outcome at 1 year.
Radiology. 235:918–926. 2005.PubMed/NCBI
|
|
8.
|
Simcock B, Neesham D, Quinn M, et al: The
impact of PET/CT in the management of recurrent ovarian cancer.
Gynecol Oncol. 103:271–276. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Iagaru A, Mittra ES, McDougall R, et al:
18F-FDG PET/CT evaluation of patients with ovarian
carcinoma. Nucl Med Commun. 29:1046–1051. 2008. View Article : Google Scholar
|
|
10.
|
Bilici A, Ustaalioglu BB, Seker M, et al:
Clinical value of FDG PET/CT in the diagnosis of suspected
recurrent ovarian cancer: is there an impact of FDG PET/CT on
patient management? Eur J Nucl Med Mol Imaging. 37:1259–1269. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Soussan M, Wartski M, Cherel P, et al:
Impact of FDG PET/CT imaging on the decision making in the biologic
suspicion of ovarian carcinoma recurrence. Gynecol Oncol.
108:160–165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Nanni C, Rubello D, Farsad M, et al:
(18)F-FDG PET/CT in the evaluation of recurrent ovarian cancer: a
prospective study on forty-one patients. Eur J Surg Oncol.
31:792–797. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Pan HS, Lee SL, Huang LW and Chen YK:
Combined positron emission tomography-computed tomography and tumor
markers for detecting recurrent ovarian cancer. Arch Gynecol
Obstet. 283:335–341. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Fulham MJ, Carter J, Baldey A, et al: The
impact of PET-CT in suspected recurrent ovarian cancer: A
prospective multi-centre study as part of the Australian PET Data
Collection Project. Gynecol Oncol. 112:462–468. 2009. View Article : Google Scholar
|
|
15.
|
Yuan Y, Gu ZX, Tao XF and Liu SY: Computer
tomography, magnetic resonance imaging, and positron emission
tomography or positron emission tomography/computer tomography for
detection of metastatic lymph nodes in patients with ovarian
cancer: A meta-analysis. Eur J Radiol. 81:1002–1006. 2012.
View Article : Google Scholar
|
|
16.
|
Yau YY, Chan WS, Tam YM, et al:
Application of intravenous contrast in PET/CT: does it really
introduce significant attenuation correction error? J Nucl Med.
46:283–291. 2005.PubMed/NCBI
|
|
17.
|
Anjos DA, Etchebehere EC, Ramos CD, et al:
18F-FDG PET/CT delayed images after diuretic for
restaging invasive bladder cancer. J Nucl Med. 48:764–770. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Friedman JB and Weiss NS: Second thoughts
about second-look laparotomy in advanced ovarian cancer. N Engl J
Med. 322:1079–1082. 1990. View Article : Google Scholar : PubMed/NCBI
|