Introduction
Thyroid cancer is the most common malignancy of the
endocrine system and accounts for ∼1% of all newly diagnosed cancer
cases in the USA (1,2). The most frequent type is papillary
thyroid cancer (PTC), which constitutes >80% of all cases. It
has also been reported that the thyroid cancer incidence rate has
significantly increased among males and females in numerous
countries, including the USA (1),
Southeast England (3), Italy
(4), Lithuania (5), Canada (6) and China (7), and enhanced medical scrutiny of small
tumors cannot explain this finding (1,2).
Obesity is now becoming an epidemic worldwide
(8), including in China (9,10). It
has been considered as a risk factor for several types of cancer,
including colorectal cancer, postmenopausal breast cancer, kidney
cancer and endometrial cancer (11). For thyroid cancer, a meta-analysis
(12) found that a
5-kg/m2 increase in body mass index (BMI) was strongly
associated with thyroid cancer in males (1.33, P= 0.02) and females
(1.14, P=0.001), although the association is weaker in females.
One of the important mediators between obesity and
increased cancer risk is leptin (11,13),
which is an adipokine whose major functions are regulating appetite
and energy homeostasis (14).
Leptin serum levels are closely correlated with adiposity in
humans. It exerts its effects through binding to its receptors
(OBRs), which are located in several tissues throughout the body.
Among the receptors, only the long form (OBRb) was considered to
have full potential to transduce signals. The signaling pathways
activated by OBRb include the classic cytokine Janus kinase
2/signal transducer and activator of transcription 3 (JAK2/STAT3)
pathway; the Ras/extracellular signal-regulated kinases 1/2
(Ras/ERK1/2) signaling cascade; and the phosphoinositide 3
kinase/protein kinase B (PI3K/Akt) growth/anti-apoptotic pathway
(15).
Leptin and OBRs have been reported to be
overexpressed in numerous types of cancer and cancer cell lines
(16). Moreover, their expression
intensity is associated with cancer progression and/or prognosis in
several common types of cancer, including glioblastoma (17), breast cancer (18), prostate cancer (19), ovarian cancer (20) and colorectal cancer (21,22),
as revealed by immunohistochemical studies.
Leptin and OBR expression has also been studied in
thyroid cancers. In a study on a large cohort of Saudi PTC patients
(23), OBRs and leptin were found
to be expressed in 80.1% (410/512) and 49.1% (252/513) of PTC
patients, respectively, and OBR expression was strongly associated
with age, gender, extrathyroidal extension, tumor stage, tumor
size, node metastasis and histological type. However, in another
study of Chinese PTC patients in Taiwan (24), different results were obtained. OBRs
and leptin were detected in 51.0% (25 of 49) and 36.7% (18 of 49)
of cases, respectively, and neither were associated with age,
gender, extrathyroidal extension, multifocality, thyroiditis or
BMI. The exception was tumor size, which was shown to be associated
with OBR and leptin expression. The study also revealed that PTC
tumors with both leptin and OBR expression were more likely to
develop lymph node metastasis compared with tumors with neither
leptin nor OBR expression. However, reasons for the differences
between the results of the two studies are unknown.
The aim of the present study was to detect the
expression of leptin and OBRs in a group of Chinese mainland PTC
patients, and to determine whether their expression correlated with
patient and tumor characteristics.
Patients and methods
Patients
This study included 76 patients who underwent
thyroidectomy in Jining First People’s Hospital between 2010 and
2011. Clinical and histopathological data, including tumor size,
multifocality, lymph node metastasis, age and weight, were reviewed
for all patients. Hematoxylin and eosin (HE)-stained slides for
each patient were reviewed to confirm the diagnosis of PTC. All
patients were euthyroid prior to surgery. The ethics committee of
the Affiliated Hospital of Jining Medical College censored and
approved the study. Written informed consent was obtained from the
patients.
Immunohistochemistry (IHC)
For immunohistochemical staining, 5-mm sections of
formalin-fixed, paraffin-embedded tissue blocks were dewaxed in
xylene and rehydrated through graded alcohol and phosphate-buffered
saline (PBS). Antigen retrieval was conducted by boiling slides in
10 mM sodium citrate buffer (pH 6.0) for 10 min. The sections were
then treated with 0.3% hydrogen peroxide at room temperature for 10
min to quench endogenous peroxidase activity. After rinsing in PBS,
the sections were blocked with 10% normal rabbit serum (for OBRs)
or goat serum (for leptin) at 37°C for 1 h. Then the sections were
incubated overnight at 4°C in humid chambers with primary antibody.
The primary polyclonal rabbit anti-leptin antibody (A-20, Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was diluted at 1:200
and the primary polyclonal goat anti-OBR antibody (M-18, for all
forms of OBRs; Santa Cruz Biotechnology, Inc.) was diluted at
1:200. Subsequently, the sections were incubated with a
polyperoxidase-conjugated anti-rabbit or anti-goat secondary
antibody (Gold Bridge, Beijing, China) for 30 min. Following a PBS
wash, DAB substrate (Gold Bridge) was added to the sections for 30
min. Finally, the slides were counterstained with hematoxylin,
dehydrated after a standard procedure and sealed with coverslips.
Sections that had been incubated with PBS instead of primary
antibody were used as a negative control.
Evaluation of immunostaining
The expression levels of leptin and OBRs were
evaluated semiquantitatively by two experienced pathologists (X.W.
andW.C.). Scoring was based on staining intensity and staining
extent. Staining intensity was scored as 0 (negative), 1 (weak), 2
(moderate) or 3 (strong). Staining extent was scored as 0 (0%), 1
(1–25%), 2 (26–50%) or 3 (51–100%) according to the percentage of
positively stained cells. Multiplied scores of intensity and extent
were used as the final staining score. Patients were sorted into 2
groups; positive expression was defined by final staining scores of
6 and 9, whereas the remaining cases (final scores 0–4) were
classified as negative expression.
Statistical analysis
The correlation between the expression of
OBRs/leptin and clinicopathological features was analyzed.
Continuous variables are expressed as the mean ± standard deviation
(SD). Data were evaluated for significant differences by the
2-tailed Student’s t-test or the χ2 test using the
Statistical Package for Social Sciences, version 13.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate
statistically significant differences.
Results
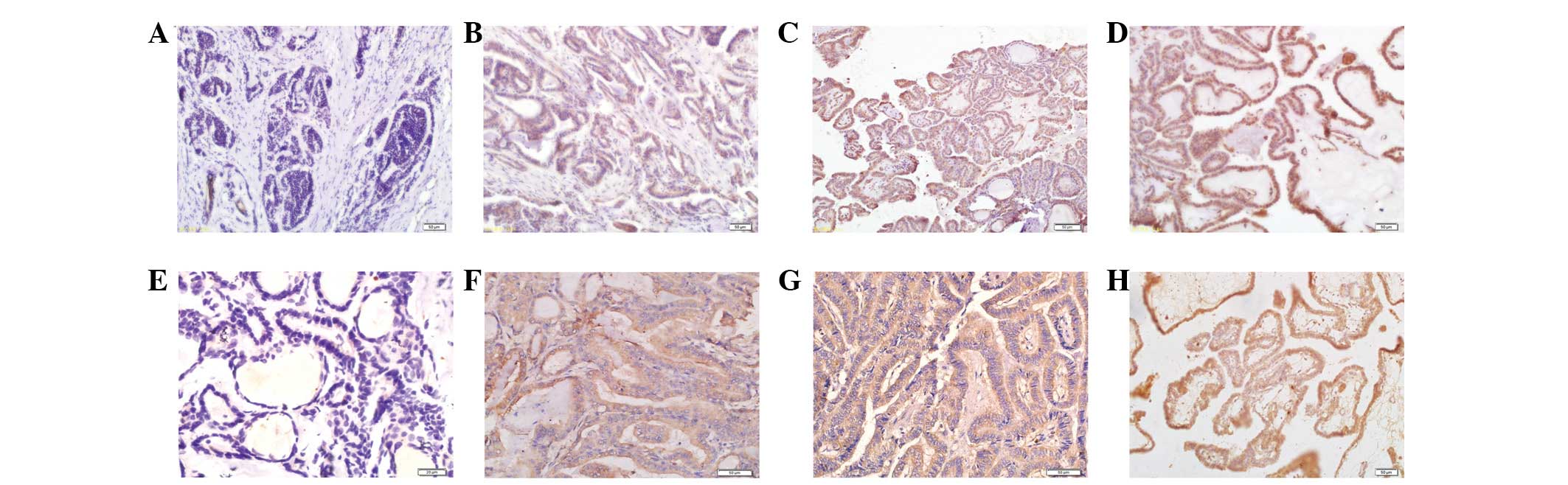
Immunohistochemical detection of OBRs and
leptin
Expression levels of OBRs and leptin were determined
by IHC in 76 PTC samples. OBR expression was observed in tumor cell
membrane and/or cytoplasm with a positive rate of 73.7% (56/76),
while leptin was expressed in tumor cell cytoplasm in 55 of 76
cases (72.4%; Fig. 1). In 57.9%
(44/76) of cases, both leptin and OBRs were expressed. The
expression levels of OBRs and leptin in PTC samples were
significantly associated with each other, as shown in Table I (P<0.05).
 | Table ICorrelation between OBR and leptin
expression. |
Table I
Correlation between OBR and leptin
expression.
| OBR expression | Leptin expression
| | |
|---|
| Positive | Negative | Total | P-value |
|---|
| Positive | 44 | 12 | 56 | |
| Negative | 11 | 9 | 20 | |
| Total | 55 | 21 | 76 | P<0.05 |
Association of expression of leptin and
OBRs with clinicopathological parameters
Our study on the association of OBR and leptin
expression with clinicopathological data demonstrated that PTC
samples with positive staining for OBRs (P=0.002) or leptin
(P=0.016) were associated with a larger tumor size. Neither leptin
nor OBR expression was associated with other parameters, including
age, body weight, postmenopausal state, multifocality or lymph node
metastasis (Table II).
 | Table IIExpression of leptin and OBRs relative
to the clinicopathological characteristics of PTC. |
Table II
Expression of leptin and OBRs relative
to the clinicopathological characteristics of PTC.
| Leptin expression
| OBR expression
|
|---|
| Characteristic | Positive | Negative | P-value | Positive | Negative | P-value |
|---|
| No. of patients | 55 | 21 | | 56 | 20 | |
| Age (years), mean ±
SD | 46.0±13.3 | 53.4±12.3 | 0.878 | 47.7±14.7 | 48.9±8.9 | 0.736 |
| Body weight (kg),
mean ± SD | 66.5±10.8 | 68.4±11.9 | 0.427 | 67.2±12 | 66.2±8.5 | 0.703 |
| No. of postmenopausal
patients (56 female patients) (%) | 14 (33.3) | 6 (42.9) | 0.520 | 15 (35.7) | 5 (35.7) | 1.000 |
| Lymph node metastasis
(%) | 17 (30.9) | 9 (42.9) | 0.326 | 20 (35.7) | 6 (30) | 0.644 |
| Tumor size (mm), mean
± SD | 22.2±10.3 | 17.1±12.8 | 0.016 | 23.7±10.1 | 15.1±10.3 | 0.002 |
| Multifocality
(%) | 31 (56.4) | 14 (66.7) | 0.414 | 32 (57.1) | 13 (65.0) | 0.539 |
Discussion
Our study showed that 73.9% (56/76) and 72.4%
(55/76) of PTC samples expressed OBRs and leptin, respectively.
These data are different from those of Cheng et al(24) who revealed that OBRs and leptin are
expressed in 51.0% (25/49) and 36.7% (18/49) of PTC cases,
respectively, and Uddin et al(23) whose results showed that OBRs and
leptin are expressed in 80.1% (410/512) and 49.1% (252/513) of PTC
cases, respectively. Although the positive rates are different, all
three studies indicated that a proportion of PTC tumors express
leptin and OBRs. However, the reasons for differences between the
data requires further study, particularly the difference between
the data of this study and those of Cheng et al, due to the
similar ethnicity of the PTC patients and also since the same
antibodies were used.
In this study, 57.9% of PTC cases expressed both
leptin and OBRs, and it was also observed that the expression
levels of leptin and OBRs were significantly associated with each
other. Similar results were obtained by Cheng et al(24) in PTC and in other cancer types,
including endometrial cancer (25),
colorectal cancer (26) and breast
cancer (18,27). This suggests that leptin and OBR
expression may be induced by some of the same mechanisms, or that
the expression of one molecule may be induced by the other. Indeed,
leptin and OBR expression is correlated with HIF-1α in endometrial
(25) and colorectal cancer
(26), which suggests that HIF-1α
may induce both. More direct evidence from in vitro cell
line studies indicated that IGF-1, insulin and estradiol induced
the expression of leptin and OBR mRNA in the MCF-7 breast cancer
cell line, while in MDA-MB-231 cells, leptin and OBR mRNA
expression was induced by insulin or hypoxia (28). There is also evidence demonstrating
that leptin enhanced the expression of OBRs, for example in ZR-75-1
breast cancer cells (29). Whether
or not these mechanisms exist in PTC cells is largely unknown.
However, insulin was previously shown to upregulate OBR expression
in a time- and dose-dependent manner, while the hypoxia-mimicking
agent cobalt chloride had no effect on OBR expression in PTC cell
lines (30). The effect of insulin
on leptin expression in thyroid cancer has not been studied.
In the present study, leptin and OBR expression
levels were found to be associated with PTC tumor size, which is
similar to the observations of two previous studies (23,24).
This result is to be expected, considering that leptin, through
OBRs, has been shown to promote proliferation and inhibit apoptosis
in numerous types of cancer (16)
and PTC cell lines (23,31,32).
We have identified several weaknesses in this study.
Firstly, the small number of patients enrolled means there is a
higher chance of producing imprecise results, compared with a
larger group of patients. The second and more important point is
the lack of study of the survival rate or recurrence in these
patients due to the relatively benign nature of PTC. However, Uddin
et al(23) showed that
patients with overexpression of OBRs had a reduced disease-free
survival rate of 68.9% at 5 years, compared with 79.3% with reduced
OBR expression.
In summary, our observations have added to the
evidence that leptin and OBRs are expressed in PTC and their
expression is associated with each other and with PTC tumor size.
Further study is required to determine the potential prognostic and
therapeutic implications of the leptin/OBR system.
Acknowledgements
This study was supported by the
Natural Science Foundation of Shandong province (ZR2009CM070),
Jining Scientific and Technological Project (2011–31) and Youth
Foundation of Jining Medical University (JYQ2011KM006).
References
|
1
|
Simard EP, Ward EM, Siegel R and Jemal A:
Cancers with increasing incidence trends in the United States: 1999
through 2008. CA Cancer J Clin. Jan 4–2012.(Epub ahead of
print).
|
|
2
|
Chen AY, Jemal A and Ward EM: Increasing
incidence of differentiated thyroid cancer in the United States,
1988–2005. Cancer. 115:3801–3807. 2009.
|
|
3
|
Olaleye O, Ekrikpo U, Moorthy R, et al:
Increasing incidence of differentiated thyroid cancer in South East
England: 1987–2006. Eur Arch Otorhinolaryngol. 268:899–906.
2011.PubMed/NCBI
|
|
4
|
Capezzone M, Morabito E, Bellitti P,
Giannasio P, De Sanctis D and Bruno R: Increasing incidence of
thyroid cancer in Basilicata: an Italian study. J Endocrinol
Invest. 30:507–512. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smailyte G, Miseikyte-Kaubriene E and
Kurtinaitis J: Increasing thyroid cancer incidence in Lithuania in
1978–2003. BMC Cancer. 6:2842006.PubMed/NCBI
|
|
6
|
Liu S, Semenciw R, Ugnat AM and Mao Y:
Increasing thyroid cancer incidence in Canada, 1970–1996: time
trends and age-period-cohort effects. Br J Cancer. 85:1335–1339.
2001.
|
|
7
|
Xiang J, Wu Y, Li DS, et al: New clinical
features of thyroid cancer in eastern China. J Visc Surg.
147:e53–e56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Finucane MM, Stevens GA, Cowan MJ, et al:
National, regional, and global trends in body-mass index since
1980: systematic analysis of health examination surveys and
epidemiological studies with 960 country-years and 9.1 million
participants. Lancet. 377:557–567. 2011.
|
|
9
|
Wang Y, Mi J, Shan XY, Wang QJ and Ge KY:
Is China facing an obesity epidemic and the consequences? The
trends in obesity and chronic disease in China. Int J Obes (Lond).
31:177–188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen CM: Overview of obesity in Mainland
China. Obes Rev. 9(Suppl 1): 14–21. 2008. View Article : Google Scholar
|
|
11
|
Khandekar MJ, Cohen P and Spiegelman BM:
Molecular mechanisms of cancer development in obesity. Nat Rev
Cancer. 11:886–895. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Renehan AG, Tyson M, Egger M, Heller RF
and Zwahlen M: Body-mass index and incidence of cancer: a
systematic review and meta-analysis of prospective observational
studies. Lancet. 371:569–578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roberts DL, Dive C and Renehan AG:
Biological mechanisms linking obesity and cancer risk: new
perspectives. Annu Rev Med. 61:301–316. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Proenca R, Maffei M, Barone M,
Leopold L and Friedman JM: Positional cloning of the mouse obese
gene and its human homologue. Nature. 372:425–432. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sweeney G: Leptin signalling. Cell Signal.
14:655–663. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garofalo C and Surmacz E: Leptin and
cancer. J Cell Physiol. 207:12–22. 2006. View Article : Google Scholar
|
|
17
|
Riolfi M, Ferla R, Del Valle L, et al:
Leptin and its receptor are overexpressed in brain tumors and
correlate with the degree of malignancy. Brain Pathol. 20:481–489.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ishikawa M, Kitayama J and Nagawa H:
Enhanced expression of leptin and leptin receptor (OB-R) in human
breast cancer. Clin Cancer Res. 10:4325–4331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hoon Kim J, Lee SY, Myung SC, Kim YS, Kim
TH and Kim MK: Clinical significance of the leptin and leptin
receptor expressions in prostate tissues. Asian J Androl.
10:923–928. 2008.PubMed/NCBI
|
|
20
|
Uddin S, Bu R, Ahmed M, et al:
Overexpression of leptin receptor predicts an unfavorable outcome
in Middle Eastern ovarian cancer. Mol Cancer. 8:742009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koda M, Sulkowska M, Kanczuga-Koda L,
Surmacz E and Sulkowski S: Overexpression of the obesity hormone
leptin in human colorectal cancer. J Clin Pathol. 60:902–906. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu H, Wan D, Pan Z, et al: Expression and
biological significance of leptin, leptin receptor, VEGF, and CD34
in colorectal carcinoma. Cell Biochem Biophys. 60:241–244. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Uddin S, Bavi P, Siraj AK, et al: Leptin-R
and its association with PI3K/AKT signaling pathway in papillary
thyroid carcinoma. Endocr Relat Cancer. 17:191–202. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng SP, Chi CW, Tzen CY, et al:
Clinicopathologic significance of leptin and leptin receptor
expressions in papillary thyroid carcinoma. Surgery. 147:847–853.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koda M, Sulkowska M, Wincewicz A, et al:
Expression of leptin, leptin receptor, and hypoxia-inducible factor
1 alpha in human endometrial cancer. Ann NY Acad Sci. 1095:90–98.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koda M, Sulkowska M, Kanczuga-Koda L, et
al: Expression of the obesity hormone leptin and its receptor
correlates with hypoxia-inducible factor-1 alpha in human
colorectal cancer. Ann Oncol. 18(Suppl 6): vi116–119. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koda M, Kanczuga-Koda L, Sulkowska M,
Surmacz E and Sulkowski S: Relationships between hypoxia markers
and the leptin system, estrogen receptors in human primary and
meta-static breast cancer: effects of preoperative chemotherapy.
BMC Cancer. 10:3202010. View Article : Google Scholar
|
|
28
|
Garofalo C, Koda M, Cascio S, et al:
Increased expression of leptin and the leptin receptor as a marker
of breast cancer progression: possible role of obesity-related
stimuli. Clin Cancer Res. 12:1447–1453. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen C, Chang YC, Liu CL, Chang KJ and Guo
IC: Leptin-induced growth of human ZR-75-1 breast cancer cells is
associated with up-regulation of cyclin D1 and c-Myc and
down-regulation of tumor suppressor p53 and p21WAF1/CIP1. Breast
Cancer Res Treat. 98:121–132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheng SP, Liu CL, Hsu YC, Chang YC, Huang
SY and Lee JJ: Regulation of leptin receptor expression in human
papillary thyroid cancer cells. Biomed Pharmacother. 66:469–473.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng SP, Yin PH, Chang YC, Lee CH, Huang
SY and Chi CW: Differential roles of leptin in regulating cell
migration in thyroid cancer cells. Oncol Rep. 23:1721–1727.
2010.PubMed/NCBI
|
|
32
|
Cheng SP, Yin PH, Hsu YC, et al: Leptin
enhances migration of human papillary thyroid cancer cells through
the PI3K/AKT and MEK/ERK signaling pathways. Oncol Rep.
26:1265–1271. 2011.PubMed/NCBI
|