Introduction
Intraductal papillary mucinous neoplasm (IPMN) of
the pancreas is a relatively new entity that is being diagnosed
with increasing frequency (1). IPMN
is characterized by intraductal proliferation of neoplastic
mucinous cells, which show varying degrees of atypia and usually
form papilla that lead to cystic dilatation of pancreatic ducts and
subsequently to clinically detectable masses (2). IPMN has been established as a
precursor of pancreatic adenocarcinoma via the hyperplasia,
dysplasia and invasive carcinoma sequence. However, the incidence
of this full progression varies greatly with the site of origin
(main duct or branch duct), and IPMN grade may be difficult to
distinguish clinically, particularly in the absence of surgery
(3). Therefore, all IPMN cases
without exception should be considered potentially malignant
(4,5).
In 2004, an international conference defined the
International Consensus Guidelines (ICG) for selecting patients for
immediate surgery or a surveillance strategy (6). Main pancreatic duct-type IPMN cases
are frequently malignant and the optimal management of such tumors
is now widely acknowledged to be resection of the lesion, provided
that the patient is fit enough for this intervention (7–10).
However, it is has been suggested that branch duct-type IPMN
lesions >3 cm in size and with suspicious radiological changes,
including the presence of mural nodules inside the cystic lesion, a
dilated main pancreatic duct or positive cytological findings,
should be recommended for surgical resection due to their higher
risk of malignant potential. However, this is a rapidly evolving
field and there are a significant number of areas where there
remains no consensus (11).
Early studies of IPMN suggested that patients
resected for branch duct-type IPMN had a more benign neoplasm
compared with those with main duct-type IPMN (12-14).
Short- and mid-term follow-up (median, <5 years) studies led to
the conclusion that morphological changes are rare events with
branch duct-type IPMN; however, the long-term evolution and/or
biological behavior of this tumor subgroup remain unknown (15,16).
Establishing such data on branch duct-type IPMN is thus of great
importance for the patients and clinical management teams. SMAD4 is
a tumor suppressor gene on chromosome 18q21.1 that is inactivated
in >50% of pancreatic malignancies, and SMAD4 protein
overexpression suppresses cell proliferation in malignant
pancreatic neoplasms (17,18). However, it remains unclear how SMAD4
is retained in intraductal lesions, while its loss is frequently
observed in invasive IPMNs.
The purpose of this study was to characterize the
clinicopathological features of patients with branch duct-type IPMN
resected at our institution with detailed examination of
histopathological and molecular investigations, and to compare the
outcomes of these patients with those with main duct-type IPMN.
Furthermore, we discuss the malignant potential of invasive
intraductal papillary mucinous carcinoma (IPMC) derived from branch
duct-type IPMN based on our analysis of the TGF-β/SMAD4
pathway.
Patients and methods
Patients, surgery and pathological
classification
This is a study of prospectively collected,
retrospectively analyzed data. The diagnosis of IPMN was suspected
following imaging and endoscopic analysis and was confirmed by
pathological analysis. We retrospectively reviewed the surgical
pathology database of Kochi Medical School to identify patients who
underwent resection for IPMN. Case selection was restricted to
patients who underwent resection in or after 2000, as since then
all clinical diagnoses of IPMN of the pancreas were evaluated using
standardized diagnostic modalities, including computed tomography
(CT), magnetic resonance imaging (MRI), endoscopic retrograde
cholangiopancreatography (ERCP) and in particular endoscopic
ultrasonography (EUS). All IPMN patients who had undergone a
pancreatic resection in the Department of Surgery at Kochi Medical
School between January 2000 and December 2011 were included in this
study.
The indication for resection or surveillance was
verified a posteriori for all patients in accordance with
the ICG (6). All patients with main
duct-type IPMN, symptomatic branch duct-type IPMN or asymptomatic
branch duct-type IPMN >30 mm in size and/or with mural nodules
and/or a dilated main pancreatic duct were referred for immediate
surgical resection. Patients with asymptomatic branch duct-type
IPMN <30 mm in size without mural nodules or dilated main
pancreatic duct were placed under careful monitoring and
surveillance. Patients placed under surveillance underwent clinical
examination, laboratory tests, including for carcinoembryonic
antigen (CEA) expression and carbohydrate antigen (CA) 19-9 serum
levels, as well as CT, MRI and EUS every 6 months for 2 years, and
yearly thereafter. Surgery was also performed when cysts showed
significant growth or when suspicion of malignancy was increased,
even if the original size of the cystic pancreatic lesion was
<30 mm (19).
The diagnosis was validated on the basis of the
histological findings in a surgical specimen, or the outcome of
surveillance. The lesions were classified into three categories
according to the World Health Organization classification: slight
dysplasia and intraductal papillary mucinous adenoma, moderate
dysplasia or borderline malignancy (borderline IPMN), severe
dysplasia or IMPC in situ (non-invasive IPMC) and invasive
carcinoma (invasive IPMC). When more than one pathological type was
present, the tumor was classified according to the worst lesion
present.
The study was approved by the ethics committee of
Kochi Medical School. Written informed consent was obtained from
the patients.
Clinical pre- and post-operative
evaluation in patients with IPMN of the pancreas
Medical records were reviewed retrospectively for
the following information: patient characteristics, clinical
history, physical examination, laboratory investigations, surgical
management, pathology examinations and post-operative course. Any
history of a previous extra-pancreatic neoplasm or ordinary
pancreatic carcinoma was investigated thoroughly. Body mass index
(BMI) was calculated as weight (kg) divided by height squared
(m2). A self-administered questionnaire was used to
determine the smoking and drinking habits of all IPMN patients.
Data of patient outcomes were obtained through retrospective review
of a prospectively maintained pancreatic resection database,
electronic hospital charts and medical records.
RNA isolation and real-time reverse
transcription polymerase chain reaction (RT-PCR) for TGF-β
Representative formalin-fixed and paraffin-embedded
(FFPE) sections of all IPMN tumors were collected from the surgical
pathology archives. In the present study, we extracted RNA from the
samples of both main and branch duct-type IPMN of the pancreas
using the RNeasy FFPE Kit (Qiagen, Hilden, Germany) (20). Total RNA yield and purity was
estimated by UV spectroscopy (Nanodrop ND-1000 Spectrophotometer;
Nanodrop Technologies, Wilmington, DE, USA) and RNA quality was
assessed on an Agilent 2100 Bioanalyzer (Agilent Technologies,
Santa Clara, CA, USA). First-strand cDNA synthesis was then
performed with 2.5 μg total RNA using the superscript
first-strand synthesis system for RT-PCR (Invitrogen, Carlsbad, CA,
USA) according to the manufacturer’s instructions. We measured the
expression of TGF-β with normalization as previously described
(21). Real-time RT-PCR was carried
out using the Power SYBR-Green PCR Master mix (Applied Biosystems,
Warrington, UK) as described previously (21). Primers used for PCR were as follows:
TGF-β-forward: 5′-gcagcacgtggagctgta-3′; TGF-β-reverse:
5′-cagccggttgctgaggta-3′. PCR conditions for all genes were as
follows: 95°C initial activation for 10 min followed by 40 cycles
of 95°C for 15 sec and 60°C for 60 sec, and fluorescence
determination at the melting temperature of the product for 20 sec
on an ABI PRISM 7000 (Applied Biosystems).
Immunohistochemistry for SMAD4
protein
The streptavidin-biotin-peroxidase method with the
Dako kit (Carpinteria, CA, USA) was used to detect SMAD4 protein
expression on three serially cut representative sections. Following
inactivation of endogenous peroxidase and blocking of nonspecific
antibody binding, the specimens were treated with biotinylated
antibodies specific for SMAD4 (1:100, Q13485, Epitomics, Abcam,
Cambridge, MA, USA) at 4°C overnight. Subsequently, sections were
incubated with the streptavidin-biotin-peroxidase complex reagent
for 30 min at room temperature. Diaminobenzidine tetrahydrochloride
was used as the chromogen and hematoxylin was used for
counterstaining.
Statistical analysis
Continuous variables are presented as mean ± SD.
Dichotomous variables are presented as both number and percentage
values. Data were analyzed using Student’s t-test (two-tailed),
with dichotomous variables analyzed by the χ2 test
(two-tailed) or Fisher’s exact test (two-tailed) by a biostatistics
specialist, as appropriate. Survival probabilities were determined
using the Kaplan-Meier method and compared using the log-rank test.
Survival analysis excluded patients who died in the 30-day
postoperative period. Cause of mortality was not available for all
patients, so only overall survival was calculated. P<0.05 was
considered to indicate a statistically significant result. All
analyses were performed using SPSS® (SPSS, Inc.,
Chicago, IL, USA).
Results
Patient characteristics
Of the 100 patients enrolled in the present study,
33 (33.0%) were found to have main duct-type IPMN (69.7% male) and
67 (67.0%) had branch duct-type IPMN (68.7% male; Table I). Mortality 30 days after
pancreatic resection was 1.0%; the one patient who died had
borderline IPMN of the head of the pancreas and underwent
pancreaticoduodenectomy. However, septic shock developed as a
consequence of bacterial endocarditis and the patient died on the
14th post-operative day. There were no significant differences in
age, gender or BMI between patients with main duct-type IPMN or
with branch duct-type IPMN. As shown in Table I, patients with main duct-type IPMN
had a significantly higher incidence of abdominal pain (51.5 vs.
20.9%; P=0.002), while those with branch duct-type IPMN had a
significantly higher incidence of enlarged tumor growth (37.3 vs.
12.1%; P=0.017). There were no significant differences in past
medical history, including diabetes mellitus and hypertension
incidence, alcohol consumption and cigarette smoking, between the
groups. Among the 100 patients with IPMN, 12 patients (12.0%) had
an ordinary pancreatic carcinoma; 2 cases (6.1%) in patients with
main duct-type IPMN and 10 cases (14.9%) in patients with branch
duct-type IPMN. Notably, patients suffering from IPMN had the
highest incidence of malignancy compared with their family history
in the two groups; 57.6% in patients with main duct-type IPMN and
52.2% in patients with branch duct-type IPMN (Table I). Indeed, 29 patients with IPMN
(29.0%) had a past medical history of other neoplasms (Table I).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | Main duct-type IPMN
(n=33) | Branch duct-type IPMN
(n=67) | P-value |
|---|
| Patient details | | | |
| Age (years), mean ±
SD | 68.7±6.8 | 66.9±10.8 | 0.381 |
| Male (%) | 69.7 | 68.7 | 0.916 |
| Body mass index,
mean ± SD | 22.8±3.8 | 21.9±3.0 | 0.187 |
| Presenting
sign/symptoms (%) | | | |
| Abdominal pain | 51.5 | 20.9 | 0.002 |
| Tumor enlarged | 12.1 | 37.3 | 0.017 |
| Group
examination | 24.2 | 22.4 | 0.964 |
| Past medical history
(%) | | | |
| Diabetes
mellitus | 48.5 | 40.3 | 0.437 |
| Hypertension | 50.0 | 38.8 | 0.594 |
| Cigarette
smoking | 63.6 | 67.2 | 0.726 |
| Alcohol
consumption | 66.7 | 56.7 | 0.340 |
| Other
neoplasms | 36.4 | 25.4 | 0.255 |
| Suffering from
pancreatic cancer (%) | 6.1 | 14.9 | 0.157 |
| Family history of
malignancy (%) | 57.6 | 52.2 | 0.615 |
Comparison of clinicopathological
findings
Table II
demonstrates the clinicopathological variables and tumor and
treatment characteristics of the 100 patients who underwent
surgical resection of IPMN. There were no significant differences
in expression of the pre-operative tumor markers CEA and CA 19-9
between main and branch duct-type IPMN cases. Immediate surgery was
performed in 81.8% of patients with main duct-type IPMN and in
53.7% of patients with branch duct-type IPMN (P=0.027), although
postoperative follow-up periods were significantly longer in the
latter group of patients (median, 1.6 years; range, 1–2 years) than
in the former (median, 3.6 years; range, 1–10 years; P=0.021).
There was no significant difference in the surgical procedures
between the groups. In total, pancreaticoduodenectomy was performed
in 48 patients, distal pancreatectomy with splenectomy in 34
patients and 6 patients underwent a total pancreatectomy. Notably,
minimally invasive pancreatic surgery was performed in only 10
patients with branch duct-type IPMN, comprising duodenum-preserving
pancreatic head resection in 5 patients, central pancreatectomy in
3 patients, inferior head resection in 1 patient and
spleen-preserving distal pancreatectomy in 1 patient. The incidence
of malignant change in patients with main duct-type IPMN (69.7%)
was significantly higher than that in patients with branch
duct-type IPMN (17.9%), as expected. Of the 33 main duct-type IPMN
cases, 10 (30.3%) were diagnosed as borderline IPMN, 14 (42.4%)
were non-invasive IPMC and 9 (27.3%) were invasive IPMC. Of the 67
branch duct-type IPMN cases, 55 (82.1%) were borderline IPMN, 3
(4.5%) were non-invasive IPMC and 9 (13.4%) were invasive IPMC,
with 10 of these 12 malignant IPMC patients exhibiting mural
nodules in cystic lesions of the pancreas that were found to be
malignant on pathological findings obtained from surgical
resection. However, the remaining 2 patients also had a malignant
neoplasm derived from branch duct-type IPMN, although the cystic
lesions of the pancreas had no mural nodules.
 | Table IIComparison of clinicopathological
findings between patients with main duct-type IPMN and branch
duct-type IPMN. |
Table II
Comparison of clinicopathological
findings between patients with main duct-type IPMN and branch
duct-type IPMN.
| Characteristic | Main duct-type IPMN
(n=33) | Branch duct-type
IPMN (n=67) | P-value |
|---|
| Tumor marker, blood
chemistry | | | |
| Carcinoembryonic
antigen (ng/ml) | 3.0±3.0 | 2.7±2.1 | 0.566 |
| Carbohydrate
antigen 19–9 (U/ml) | 55.5±88.0 | 48.1±182.7 | 0.850 |
| Surgical
period | | | |
| Immediately
(%) | 81.8 | 53.7 | 0.027 |
| Follow-up
(%) | 18.2 | 46.3 | |
| Follow-up, median
years (range) | 1.6 (1–2) | 3.6 (1–10) | 0.021 |
| Surgical procedure
(n) | | | |
| Total
pancreatectomy | 4 | 2 | 0.143 |
|
Pancreaticoduodenectomy | 17 | 31 | |
| Distal
pancreatectomy | 10 | 24 | |
| Minimal invasive
surgery | 0 | 10 | |
| Pathology | | | |
| Adenoma | 10 | 55 | |
| Non-invasive
carcinoma | 14 | 3 | 0.001 |
| Invasive
carcinoma | 9 | 9 | |
Survival
Overall survival following resection was analyzed in
patients with IPMN (n=87) after excluding patients who died in the
30-day post-operative period (1/100, 1.0%) and those suffering from
ordinary pancreatic carcinoma (12/100, 12.0%). Patient follow-up as
of December 2011 ranged from 4 to 196 months, with a median of 54
months (mean, 63.1 months). In general, patients with an invasive
IPMC had a significantly worse outcome compared with those with
borderline or non-invasive IPMC (Fig.
1A). The cumulative 5-year survival rate following curative
resection of invasive adenocarcinoma derived from IPMN was 44.4%
(median survival, 37.0 months), whereas borderline IPMN and
non-invasive IPMC behaved more favorably. The cumulative survival
following curative resection in patients with adenocarcinoma
derived from IPMN was then sub-analyzed. Notably, patients with an
invasive adenocarcinoma derived from main duct-type IPMN had a
significantly better outcome (66.7% surviving at 5 years; median
survival, 78.0 months) than those with invasive adenocarcinoma
derived from branch duct-type IPMN (0.0% surviving at 5 years;
median survival, 15.0 months; Fig.
1B).
Evaluation of TGF-β/SMAD4 signaling in
patients with IPMN
The overall survival in patients with an invasive
adenocarcinoma derived from branch duct-type IPMN was significantly
worse than in those patients with invasive adenocarcinoma derived
from main duct-type IPMN. We therefore examined TGF-β/SMAD4
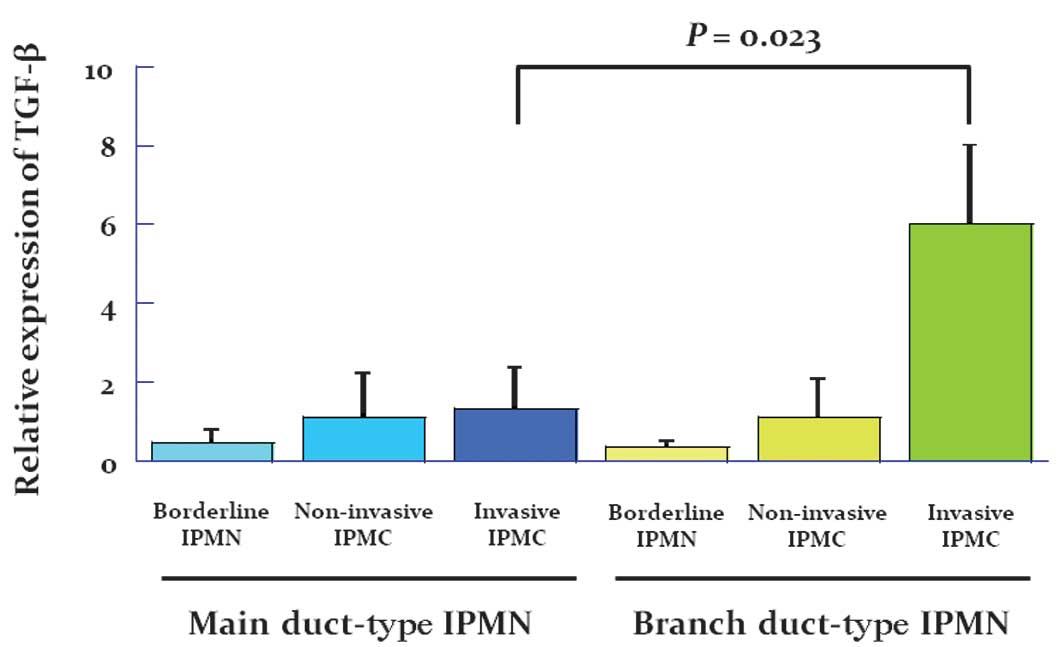
signaling in all patients. Fig. 2
shows the expression in arbitrary units as a ratio of the target
gene transcripts to TGF-β transcripts by real time RT-PCR. Notably,
the mRNA expression of TGF-β was significantly increased in
patients with adenocarcinoma derived from branch duct-type IPMN
compared with patients with borderline IPMN and especially with
those with adenocarcinoma derived from main duct-type IPMN.
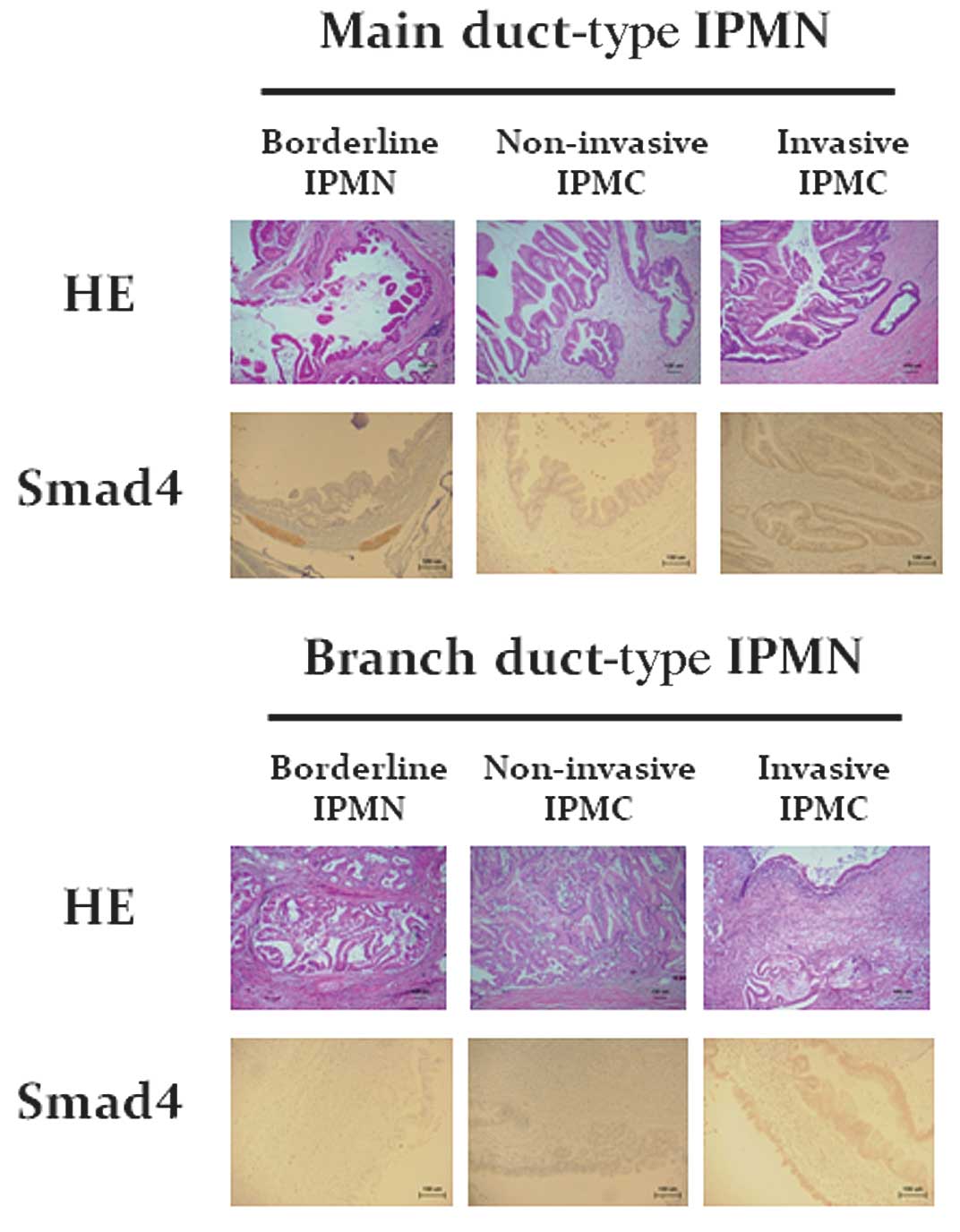
Immunohistochemical staining for SMAD4 protein in tissue sections
of the pancreas obtained from patients with IPMN showed that the
number of SMAD4-positive cells was increased in patients with
adenocarcinoma derived from branch duct-type IPMN (Fig. 3).
Discussion
In this retrospective study, we found that invasive
carcinoma derived from branch duct-type IPMN was more aggressive
than that derived from main duct-type IPMN, once invasive
morphological change was apparent. This study also clarified the
progression pattern of TGF-β/SMAD4 signaling in IPMNs.
As observed in previous studies, 55 (82.1%) of the
67 patients in this study with branch duct-type IPMN had a benign
neoplasm at the time of initial pre-operative surgical indication
(12–14). The most noteworthy finding in the
present study is, therefore, that patients with invasive carcinoma
derived from branch duct-type IPMNs, excluding patients with
ordinary pancreatic adenocarcinoma, had an extremely poor
prognosis, whereas patients with malignant IPMNs derived from main
duct-type IPMN had a relatively better prognosis following surgical
treatment. In recent years, an increasing number of studies
concerning follow-up clinical and imaging data for branch duct-type
IPMN have indicated that few such patients develop malignancy
(16,22,23). A
previous study reported that deletion of DPC4 (a tumor-suppressor
gene) increased aggressive cancer and decreased survivability
(24). However, our results with
regard to the increased invasive nature of branch duct-type IPMN
are in apparent contradiction with those of the previous study.
Furthermore, our results also indicate that ICG was an
unsatisfactory method to select patients with malignant IPMN,
prompting us to challenge the molecular analysis of TGF-β/SMAD4
signaling in IPMN (25).
TGF-β is a potent inhibitor of epithelial cell
growth and survival through modulating the expression of cell cycle
regulators and activating apoptosis, although these effects are
highly dependent on cellular context (26). However, TGF-β enhances the malignant
growth of certain established epithelial tumors, promoting tumor
cell proliferation, migration and the epithelial-to-mesenchymal
transition, which is a process by which advanced carcinomas acquire
a highly invasive, undifferentiated and metastatic phenotype
(27). Therefore, TGF-β signaling
may have biphasic stage-specific effects: inhibiting carcinoma
initiation while promoting the high-grade advancement and
dissemination of established tumors (28). In the present study, real-time
RT-PCR revealed significantly increased mRNA expression of TGF-β in
patients with carcinoma derived from branch duct-type IPMN, and
patients expressing SMAD4 had significantly worse outcomes.
TGF-β/SMAD4 signaling may therefore have pleiotropic and
context-dependent roles in IPMN and the present study suggested
that determining the TGF-β and/or SMAD4 status of a tumor at
initial diagnosis may be of value for stratifying patients into
treatment regimens (surgical management vs. conservative
follow-up).
Pancreatic surgery is burdened by significant
morbidity and mortality, even at specialized centers (29). Resecting premalignant or potentially
premalignant lesions affords an unprecedented opportunity to
perform a greater number of minimally invasive pancreatic
surgeries. However, the indication of such surgery for pancreatic
neoplasms remains controversial and is not described in the ICG. In
the present study, minimally invasive pancreatic surgery was
performed only in those patients with borderline IPMN, and these
patients had no recurrence. The most important consideration is not
allowing patients with borderline or non-invasive IPMN to succumb
to recurrent disease following curative surgery, even if patients
with invasive carcinoma arising in the setting of an IPMN appear to
have a more favorable outcome than patients with resectable
ordinary pancreatic carcinoma (8,30).
Therefore, surgeons should select IPMN patients for minimally
invasive pancreatic surgery based on an array of histological
features and a spectrum of biological behaviors, as optimal
diagnosis and risk stratification are often challenging.
The prognosis and recurrence rate of IPMN depend
mainly on tumor invasiveness and the type of duct involved. The
recurrence rate is higher for invasive lesions of the branch duct,
and such lesions must therefore be treated surgically as soon as
feasibly possible, similar to classic pancreatic adenocarcinoma.
Advances in imaging technologies have increased the number of
diagnoses of asymptomatic lesions, thus more stringent and careful
criteria should be included in the ICG to increase their
specificity and define malignancy risk pre-operatively. As it
stands, the ICG definition of differential clinical diagnosis for
IPMN of the pancreas is unsatisfactory with regard to malignant
status. Indeed, patients with IPMN, invasive or not, should be
submitted for lifetime follow-up checking for recurrence in the
remnant pancreas and for associated cancers.
Acknowledgements
This work was supported by the Kochi
Organization for Medical Reformation and Renewal Fund, and the
Center for Innovative and Translational Medicine, Regenerative
Medicine Group.
References
|
1
|
Tanaka M: Controversies in the management
of pancreatic IPMN. Nat Rev Gastroenterol Hepatol. 8:56–60. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mino-Kenudson M, Fernández-del Castillo C,
Baba Y, et al: Prognosis of invasive intraductal papillary mucinous
neoplasm depends on histological and precursor epithelial subtypes.
Gut. 60:1712–1720. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fernández-del Castillo C and Adsay VN:
Intraductal papillary mucinous neoplasms of the pancreas.
Gastroenterology. 139:708–713. 2010.
|
|
4
|
Loftus EV Jr, Olivares-Pakzad BA, Batts
KP, et al: Intraductal papillary-mucinous tumors of the pancreas:
clinicopathologic features, outcome, and nomenclature. Members of
the Pancreas Clinic, and Pancreatic Surgeons of Mayo Clinic.
Gastroenterology. 110:1909–1918. 1996. View Article : Google Scholar
|
|
5
|
Azar C, Van de Stadt J, Rickaert F, et al:
Intraductal papillary and mucinous tumour of the pancreas. Clinical
and therapeutic issues in 32 patients. Gut. 39:457–464. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanaka M, Chari S, Adsay V, et al
International Association of Pancreatology: International Consensus
Guidelines for management of intraductal papillary mucinous
neoplasms andmucinous cystic neoplasms of the pancreas.
Pancreatology. 6:17–32. 2006. View Article : Google Scholar
|
|
7
|
Salvia R, Fernández-del Castillo C, Bassi
C, et al: Main-duct intraductal papillary mucinous neoplasms of the
pancreas: clinical predictors of malignancy and long-term survival
following resection. Ann Surg. 239:678–685. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okabayashi T, Kobayashi M, Nishimori I, et
al: Clinicopathological features and medical management of
intraductal papillary mucinous neoplasms. J Gastroenterol Hepatol.
21:462–467. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Uehara H, Ishikawa O, Ikezawa K, et al: A
natural course of main duct intraductal papillary mucinous neoplasm
of the pancreas with lower likelihood of malignancy. Pancreas.
39:653–657. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arlix A, Bournet B, Otal P, et al:
Long-term clinical and imaging follow-up of nonoperated branch duct
form of intraductal papillary mucinous neoplasms of the pancreas.
Pancreas. 41:295–301. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garcea G and Dennison AR: Branch-type
intraductal papillary mucinous neoplasms: an update. Pancreatology.
11:336–342. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okabayashi T, Nishimori I, Maeda H and
Hanazaki K: Incidence of and predictive risk factors for
intraductal papillary mucinous neoplasm of the pancreas with
ordinary pancreatic cancer. J Clin Gastroenterol. 44:75–76. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matthaei H, Norris AL, Tsiatis AC, et al:
Clinicopathological characteristics and molecular analyses of
multifocal intraductal papillary mucinous neoplasms of the
pancreas. Ann Surg. 255:326–333. 2012. View Article : Google Scholar
|
|
14
|
Maguchi H, Tanno S, Mizuno N, et al:
Natural history of branch duct intraductal papillary mucinous
neoplasms of the pancreas: a multicenter study in Japan. Pancreas.
40:364–370. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tanno S, Nakano Y, Nishikawa T, et al:
Natural history of branch duct intraductal papillary-mucinous
neoplasms of the pancreas without mural nodules: long-term
follow-up results. Gut. 57:339–343. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rautou PE, Lévy P, Vullierme MP, et al:
Morphologic changes in branch duct intraductal papillary mucinous
neoplasms of the pancreas: a midterm follow-up study. Clin
Gastroenterol Hepatol. 6:807–814. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hahn SA, Schutte M, Hoque AT, et al: DPC4,
a candidate tumor suppressor gene at human chromosome 18q21.1.
Science. 271:350–353. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yachida S, Jones S, Bozic I, et al:
Distant metastasis occurs late during the genetic evolution of
pancreatic cancer. Nature. 467:1114–1117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang MJ, Jang JY, Kim SJ, et al: Cyst
growth rate predicts malignancy in patients with branch duct
intraductal papillary mucinous neoplasms. Clin Gastroenterol
Hepatol. 9:87–93. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Linton K, Hey Y, Dibben S, et al: Methods
comparison for high-resolution transcriptional analysis of archival
material on Affymetrix Plus 2.0 and Exon 1.0 microarrays.
Biotechniques. 47:587–596. 2009. View Article : Google Scholar
|
|
21
|
Yang J, Ikezoe T, Nishioka C, et al:
Long-term exposure of gastrointestinal stromal tumor cells to
sunitinib induces epigenetic silencing of the PTEN gene. Int J
Cancer. 130:959–966. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rodriguez JR, Salvia R, Crippa S, et al:
Branch-duct intraductal papillary mucinous neoplasms: observations
in 145 patients who underwent resection. Gastroenterology.
133:72–79. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Salvia R, Crippa S, Falconi M, et al:
Branch-duct intraductal papillary mucinous neoplasms of the
pancreas: to operate or not to operate? Gut. 56:1086–1090. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iacobuzio-Donahue CA, Klimstra DS, Adsay
NV, et al: Dpc-4 protein is expressed in virtually all human
intraductal papillary mucinous neoplasms of the pancreas:
comparison with conventional ductal adenocarcinomas. Am J Pathol.
157:755–761. 2000. View Article : Google Scholar
|
|
25
|
Pedrazzoli S, Sperti C, Pasquali C,
Bissoli S and Chierichetti F: Comparison of International Consensus
Guidelines versus 18-FDG PET in detecting malignancy of intraductal
papillary mucinous neoplasms of the pancreas. Ann Surg.
254:971–976. 2011. View Article : Google Scholar
|
|
26
|
Bierie B and Moses HL: Tumour
microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer.
Nat Rev Cancer. 6:506–520. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zavadil J and Böttinger EP: TGFbeta and
epithelial-to-mesenchymal transitions. Oncogene. 24:5764–5774.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bardeesy N, Cheng KH, Berger JH, et al:
Smad4 is dispensable for normal pancreas development yet critical
in progression and tumor biology of pancreas cancer. Genes Dev.
20:3130–3146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Braga M, Capretti G, Pecorelli N, et al: A
prognostic score to predict major complications after
pancreaticoduodenectomy. Ann Surg. 254:702–707. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Poultsides GA, Reddy S, Cameron JL, et al:
Histopathologic basis for the favorable survival after resection of
intraductal papillary mucinous neoplasm-associated invasive
adenocarcinoma of the pancreas. Ann Surg. 251:470–476. 2010.
View Article : Google Scholar
|