Introduction
Euphorbia hylonoma Hand.-Mazz.
(Euphorbiaceae), commonly known as Jiu Niuzao, has long been used
as a folk medicine in China. It grows wildly in the Northwestern
region of China and is used in antineoplastic intervention, as well
as in the treatment of hepatocirrhosis, edema and incontinence
(1). The bioactive and chemical
constituents from the Euphorbia genus have been widely
studied (2–7). It has been previously reported that
several Euphorbia species are used in the treatment of skin
diseases, gonorrhea, migraines (8)
and cancerous conditions (9–11).
Previous studies have reported the isolation of the chemical
constituents from this plant, including tannins, ferulic acid
esters and sesquiterpenoids (12,13).
There have also been studies on the pharmacognosy of this plant
(14,15).
However, studies concerning the antitumor components
from this plant and their mechanisms of action are rare. In the
current study, 3,3′-di-O-methyl ellagic
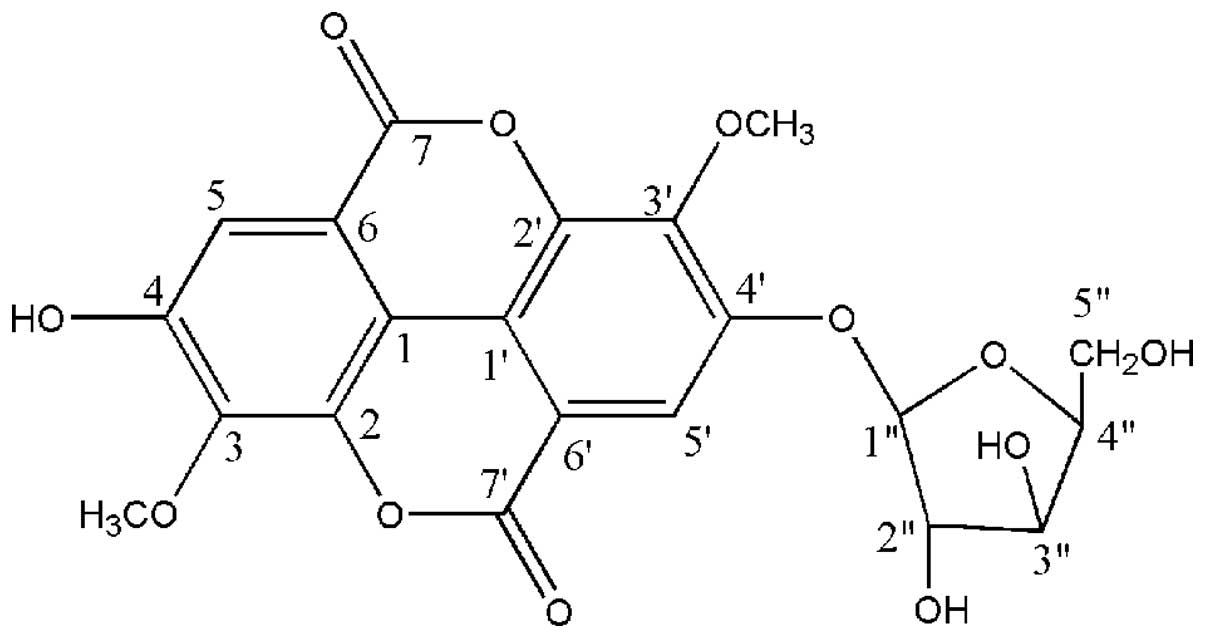
acid-4′-O-β-d-xylopyranoside(JNE2), an ellagic acid derivative, was
isolated from Euphorbiaceae during the anticancer screening study
of traditional Chinese medicine (TCM). After analyzing its chemical
features and comparing the spectral data with those of previous
studies (16,17), this compound was identified as JNE2.
The present study investigated the cytotoxic activity and the
antitumor mechanisms of JNE2. Cell cycle, apoptosis and western
blot analyses and cell invasion assay were employed, and the HepG2
human hepatoma cell line was adopted as the cell model.
Materials and methods
General materials
Nuclear magnetic resonance (NMR) spectra were
recorded on a Bruker Avance III 500 NMR spectrometer (Bruker
Corporation, Billerica, MA, USA) with tetramethylsilane as an
internal standard. High-resolution electrospray ionization mass
spectrometry was conducted using a Micromass Autospec-Ultima TOF
mass spectrophotometer (Micromass UK Ltd., Altrincham, UK). The
melting point was acquired using micro melting point apparatus
(Beijing Tech Instrument Co., Ltd., Beijing, China). The materials
used for column chromatography (CC) were silica gel
(SiO2; 200–300 mesh; Qingdao Marine Chemical Factory,
Qingdao, China) and Sephadex LH-20 (18–111 μm; GE Healthcare,
Amersham, UK). Thin-layer chromatography (TLC) was conducted using
glass precoated with silica gel (GF254; 10–40 mm; Qingdao Marine
Chemical Factory).
Plant material
The roots of Euphorbia hylonoma were
collected from Qinling Mountain, Shaanxi, China, in September 2010
and were identified by Professor Juxian Lu, Faculty of Pharmacy,
Medical College of Xi’an Jiaotong University (Xi’an, China). The
voucher specimen was retained at the Department of Pharmacy,
Medical School of Xi’an Jiaotong University for future
reference.
Cell culture
The HepG2 human hepatoma cell line was obtained from
the Shanghai Institute of Biochemistry and Cell Biology, Chinese
Academy of Sciences (Shanghai, China). HepG2 cells
(5.0×104 cells/ml) were cultured in RPMI-1640
supplemented with 10% fetal bovine serum (FBS), containing 2.0
mmol/l glutamine and 1% penicillin-streptomycin in 5%
CO2 at 37°C, and were allowed to adhere for 24 h. The
experiments were divided into the following five groups in the cell
proliferation assay (MTT assay): Negative control (dimethyl
sulfoxide; DMSO); positive control (15 μmol/l oxaliplatin);
low-dosage (22.5 μmol/l JNE2); middle-dosage (45 μmol/l JNE2); and
high-dosage (90 μmol/l JNE2). However, the low-dosage group was
cut-off in other assays due to its low efficiency in the MTT
assay.
Extraction and isolation
The dried and powdered roots (1 kg) of Euphorbia
hylonoma were extracted with acetone three times (24 h per
extraction) at room temperature to obtain 212-g extracts. A portion
of the acetone extracts (20 g) was chromatographed on a silica gel
(500-g) column. The column was eluted with a gradient of petroleum
ether-ethyl acetate (100:1 to 1:100) and methanol. The volume of
each elution was 250 ml and underwent TLC inspection. The fractions
with the same TLC spectrum behavior were combined to obtain seven
fractions, A–G. Subsequently, fraction D (4.3 g) was further
isolated on a silica gel column and eluted with petroleum-ether
acetate (7:3). Further CC of subfraction B (1.2 g) from fraction D
was performed on a Sephadex LH-20 column that was eluted with
methanol. Compound JNE2 (0.6 g) was obtained from subfraction B-6.
The identification of this compound was performed through the
analysis of the spectroscopic features and comparing the spectral
data with those of previous studies (16,17).
This compound was identified as JNE2 (Fig. 1) as follows as follows: White
powder, m.p. 241–243°C; 1H NMR (500 MHz,
DMSO-d6) δ ppm: 7.48 (1H, d, J=5.3, H-5), 7.73
(1H, d, J=3.2, H-5), 4.05 (1H, s, 3-OCH3), 4.08
(1H, s, 3′-OCH3), 10.8 (1H, s, 4-OH); 13C NMR
(125 MHz, DMSO-d6) δ ppm: 111.07 (C-1), 140.94(C-2),
140.19(C-3), 151.26 (C-4), 111.65 (C-5), 111.82 (C-6), 158.37
(C-7), 114.19 (C-1′), 141.58 (C-2′), 141.98 (C-3′), 152.84 (C-4′),
112.02(C-5′), 112.72(C-6′), 152.40 (C-7′), 101.94(C-1″),
73.09(C-2″), 76.17(C-3″), 69.31(C-4″), 65.84 (C-5″),
61.48(3-OCH3), 61.02 (3′-OCH3).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bro mide (MTT)
assay
HepG2 cells (2×104 cells/well) were
seeded in 96-well plates. Following overnight incubation, test
substances were added and the incubation was continued at 37°C in
an atmosphere containing 5% CO2 for 3 days.
Subsequently, 20 μl MTT (Sigma-Aldrich, St. Louis, MO, USA)
solution (5 g/l) was added into each well and incubated for an
additional 4 h. Supernatants were removed and formazan crystals
were dissolved in 200 μl DMSO. The optical density was measured at
490 nm using a POLARstar Optima (BMG Labtech GmbH, Ortenberg,
Germany).
Cell cycle and apoptosis analysis
Cell seeding and treatment were the same as MTT
assay. After three days of treatment, cells were harvested by
trypsinization and 1×106 cells were counted and used for
the analysis. Cells were fixed in ice-cold ethanol overnight at 4°C
following washing with PBS. The cells were then washed in PBS again
and incubated in 1 ml staining solution (20 μg/ml propidium iodide
and 10 U/ml RNase A) for 30 min at room temperature. The cells were
examined by fluorescence-activated cell sorting (FACS) using a flow
cytometer (FACSort; Becton-Dickinson, Franklin Lakes, NJ, USA), and
the cell cycle populations were determined using ModFit software
(Verity Software House, Turramurra, Australia).
For the analysis of apoptotic cell populations,
cells were trypsinized and washed in PBS. Staining with Alexa-Fluor
647 Annexin V (Invitrogen Life Technologies, Carlsbad, CA, USA) and
propidium iodide was performed in 20 mmol/l HEPES buffer (pH 7.4),
containing 150 mM NaCl and 2.5 mmol/l CaCl2, for 15 min
at room temperature. The cells were examined by FACS using a flow
cytometer (FACSort; Becton-Dickinson), and the apoptotic
populations were determined using ModFit software (Verity Software
House).
Cell invasion assay
Cell invasion was evaluated using the Chemicon QCM™
24-well collagen-based cell invasion assay (Millipore, Billerica,
MA, USA) according to the manufacturer’s instructions. In brief,
0.3 ml serum-free medium was added to the interior of each insert
to rehydrate the collagen layer for 30 min at room temperature. The
medium was then replaced with 0.3 ml prepared serum-free cell
suspension containing 3.0×105 cells and the
corresponding test substances. Medium (500 μl) containing 10% FBS
was added to the lower chamber and the cells were incubated for 24
h at 37°C. Following this, all non-invaded cells were removed from
the interior of the insert and the invaded cells were stained with
crystal violet. The stained cells were analyzed on an Olympus
fluorescence microscope (BX43; Olympus Corporation, Tokyo,
Japan).
Western blot analysis
Cell seeding and treatment were the same as MTT
assay. After three days of treatment, cells were harvested and
washed in PBS. Cell protein lysates were separated in 10%
SDS-polyacrylamide gels and electrophoretically transferred to
polyvinylidene difluoride membranes (Roche Diagnostics, Mannheim,
Germany). The lysates were then detected using rabbit polyclonal
antibodies that were specific for Bcl-2, BAX, caspase-3 and CCND1
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and a
commercial ECL kit (Pierce Biotechnology, Inc., Rockford, IL, USA).
Protein loading was estimated by human anti-β-actin monoclonal
antibody (Santa Cruz Biotechnology, Inc.).
Statistical analysis
Statistical analysis was performed by one-way ANOVA
test followed by Fisher’s protected least significant difference
post hoc test for multiple comparisons using the StatView program
(Abacus Concepts, Berkeley, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Cell growth inhibition
To evaluate the antitumor role of JNE2 on human
hepatoma cells, MTT and colony formation assays were employed to
detect the growth of HepG2 cells at various time points following
treatment with JNE2 at various concentrations. The results showed
that JNE2 exhibited a growth inhibitory effect on HepG2 cells in a
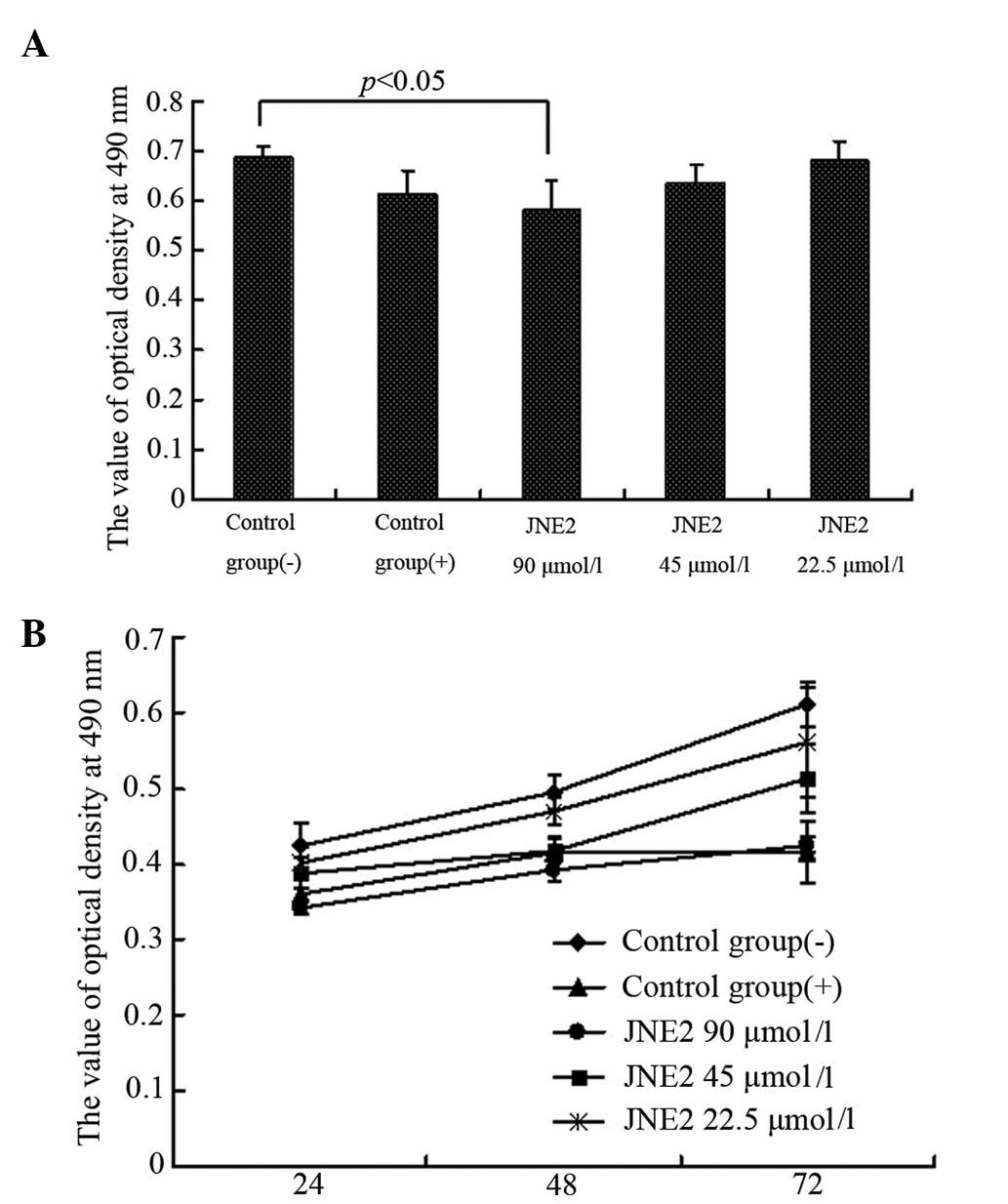
dose- and time-dependent manner. Following 24 h of treatment, the
OD value of the high-dosage group (JNE2, 90 μmol/l) was
significantly lower than that of the negative control group
(P<0.05) and also lower than that of the positive control group,
but without significant difference (Fig. 2A). The time-effect curve also
demonstrated the antiproliferation ability of JNE2 (Fig. 2B). Furthermore, the colony formation
results shown in Fig. 3 confirmed
that a high dosage of JNE2 may inhibit the growth of HepG2 cells.
These results suggested that JNE2 exhibits an inhibitory effect on
the proliferation of hepatoma cells.
JNE2-induces G0/G1 cell cycle arrest
To explore the mechanism underlying JNE2-suppressed
cell proliferation, the impact of JNE2 on cell cycle progression
was investigated by FACS and the results are presented in Fig. 4A. Following treatment with high and
middle dosages of JNE2, the cell cycle shifted from a high S phase
population to a high G1 phase population, together with an
accumulation of a G2/M phase population. Whereas, little effect on
the cell cycle was observed in the control groups. These results
indicated that JNE2 blocks the G1/S transition.
JNE2-induces HepG2 cell apoptosis
To examine the effect of JNE2 on apoptosis, hepatoma
cells were treated with various concentrations of JNE2. Compared
with the cells that were treated with the negative control, the
cells that were treated with a high dosage of JNE2 exhibited a
similar apoptotic rate to those treated with oxaliplatin, whereas
the cells that were treated with 45 μmol/l JNE2 exhibited slightly
lower apoptotic rates. In the positive and JNE2-treated groups, the
number of cells at the early and late stages of apoptosis
marginally increased. However, no significant differences were
identified (Fig. 4B) between the
groups. These results showed that JNE2 induces apoptosis in human
hepatoma cells in a dose-dependent manner.
JNE2 inhibits cancer invasion in
vitro
Cancer invasion is the process by which cells break
away from the primary tumor and migrate through the surrounding
tissue. This enables the cancer cells to move into blood vessels
and travel through the body, possibly establishing a secondary
tumor at an additional site (18).
To determine whether JNE2 inhibits invasion, HepG2 cells were
treated with various concentrations of JNE2 and the control groups
were treated separately. Evidently, the invasion was inhibited by a
high concentration of JNE2, whereas, migration was not altered in
the cells that were treated with the negative control (Fig. 5). These results markedly suggest
that JNE2 inhibits the invasion of HepG2 cells.
Western blot analysis
To clarify the apoptotic mechanism of JNE2 on HepG2
cells, western blot analysis was performed to examine the protein
expression levels of Bcl-2, Bax, caspase-3 and CCND1 (cyclin D1).
The cells that were treated with JNE2 exhibited upregulated levels
of Bax and caspase-3 expression when compared with the negative
control cells. This result may explain why JNE2 inhibited the
growth of HepG2 cells, and this function may be associated with
mitochondrial pathway-induced apoptosis. Conversely, the protein
levels of Bcl-2 and CCND1 were decreased with JNE2 treatment
(Fig. 6). The apoptotic effect of
JNE2 was confirmed by the upregulation of Bax and downregulation of
Bcl-2. Based on the G0/G1 arresting capability, the downregulation
of CCND1 may provide an explanation. These results suggested that
JNE2 may exhibit its apoptotic effect through the upregulation of
Bax and caspase-3, and the downregulation of Bcl-2 and CCND1.
Discussion
Ellagic acid, a type of polyphenol compound that
widely exists in herbs and numerous types of fruits and nuts, has
recently gained increasing attention, although it was once
considered useless in TCM. It has been well established that
ellagic acid exhibits anticancer (19), antimutagen (20–22)
and antimicrobial functions (23),
as well as others. Numerous studies concerning the antimutagen and
anticancer effects of ellagic acid were performed in the 1970s
(24–27). In previous in vitro and in
vivo experiments, ellagic acid has shown significant abilities
in inhibiting the growth of a number of types of tumor, such as
skin, esophagus and lung, as well as other tumors that are caused
by carcinogens (28–32). Its anticancer activities are
partially based on the quenching of reactive oxygen species,
thereby protecting critical cellular targets (i.e. DNA, proteins
and lipids) from oxidative insult (33–35).
Ellagic acid may also interfere with intracellular signaling
pathways, such as those that regulate proliferation, induce
apoptosis and respond to oxidative stress (36–39).
In the present study, JNE2, an ellagic acid derivative that was
isolated from a traditional Chinese herb, Euphorbia hylonoma
Hand.-Mazz. (Euphorbiaceae), showed significant antitumor effects
on the HepG2 human hepatocellular carcinoma cell line. The
antitumor mechanisms of JNE2 were also investigated.
The results of the current study showed that JNE2
may inhibit the proliferation of HepG2 cells. Specifically, JNE2
affected the cell cycle by arresting the cells in the G0/G1 phase,
thus, inducing apoptosis. Cancer cell invasion was also inhibited
by JNE2, particularly at a high concentration (90 μmol/l). Overall,
these results demonstrated the outstanding antitumor ability of
JNE2. The regulatory effects of JNE2 upon apoptosis-related protein
targets, including Bcl-2, Bax, caspase-3 and CCND1, were measured
to determine its antitumor mechanism at the molecular level.
Bcl-2 family proteins that comprise proapoptotic
proteins (such as Bax, Bad and Bid) and antiapoptotic proteins
(such as Bcl-2 and Bcl-xL) tightly regulate the mitochondrial
apoptosis pathway. A number of anticancer drugs trigger
mitochondria-mediated apoptosis in cancer cells through the
downregulation of Bcl-2/Bcl-xL or the upregulation of Bax/Bad/Bid.
Bcl-2 is an antiapoptosis gene (40–44)
and is closely associated with cellular apoptosis (45,46),
as well as with the mitochondrion (47,48).
Bax induces cellular apoptosis and the ratio of Bcl-2/Bax is the
determining factor of antiapoptosis for cells (49). The results of the present study
showed that JNE2 treatment significantly upregulated the expression
of Bax protein and downregulated that of Bcl-2. This suggested that
JNE2 acts on the Bcl-2/Bax genes to exert its apoptotic effect.
Caspases, a family of cysteine acid proteases, act
as important mediators of apoptosis. They are induced by various
stimuli and contribute to the overall apoptotic phenotype by
cleaving various cellular substrates (50–52).
Caspase-3, a key regulatory protease upon which a number of
signaling pathways merge for the execution of apoptosis, is
involved in apoptosis induced by Bcl-2/Bax, p38 and JAK-STAT
(53,54). In the current study, the protein
expression of caspase-3 was detected in HepG2 cells following
treatment with JNE2 and the upregulated effect was identified.
These results suggested that JNE2 induced the apoptosis of human
hepatoma HepG2 cells via the mitochondrial pathway.
CCND1 is a cell cycle control protein that mainly
affects G1 progression and G1/S transition. It forms complexes with
CDK4 and CDK6, and additionally with RB1. The phosphorylation of
RB1 by CCND1/CDK4 prevents cell cycle arrest at the G1/S start
point. The overexpression of CCND1 causes oncogenesis due to its
stimulation of the expression of Bcl-1, which accelerates cell
transition through the G1 phase. Therefore, JNE2 shows the ability
to block the G1/S phase transition by downregulating the expression
of CCND1.
However, among the downregulated and upregulated
expression ratios of Bcl-2/Bax, caspase-3 and CCND1, the
predominant mechanism by which JNE2 induces mitochondria-mediated
apoptosis in HepG2 cells is uncertain and other regulatory
mechanisms, including at the receptor level, require further
investigation.
Acknowledgements
The present study was supported by grants from the
National 863 Plan (no. 2012AA02A400) and the National Natural
Science Foundation of China (no. 81172957).
References
|
1
|
Jiangsu New Medical College. Dictionary
Traditional Drugs. Shanghai Scientific and Technical Publishers;
Shanghai: pp. 411993
|
|
2
|
Whelan LC and Ryan MF: Ethanolic extracts
of Euphorbia and other ethnobotanical species as inhibitors
of human tumour cell growth. Phytomedicine. 10:53–58. 2003.
|
|
3
|
Bruni R, Muzzoli M, Ballero M, Loi MC,
Fantin G, Poli F and Sacchetti G: Tocopherols, fatty acids and
sterols in seeds of four Sardinian wild Euphorbia species.
Fitoterapia. 75:50–61. 2004. View Article : Google Scholar
|
|
4
|
Hore SK, Ahuja V, Mehta G, Kumar P, Pandey
SK and Ahmad AH: Effect of aqueous Euphorbia hirta leaf
extract on gastrointestinal motility. Fitoterapia. 77:35–38.
2006.
|
|
5
|
Shi HM, Williams ID, Sung HH, Zhu HX, Ip
NY and Min ZD: Cytotoxic diterpenoids from the roots of
Euphorbia ebracteolata. Planta Med. 71:349–354. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruan HL, Zhang Y and Zhang YH: Studies on
constituents from roots of Euphorbia hylonoma. Zhongguo
Zhong Yao Za Zhi. 31:742–744. 2006.(In Chinese).
|
|
7
|
Cao D, SU YL and Yang JS: Triterpene
constituents from Euphorbia nemtocypha Hand-Mazz. Yao Xue
Xue Bao. 27:445–451. 1996.(In Chinese).
|
|
8
|
Chaabi M, Michel VF, Frossard N,
Randriantsoac A, Andriantsitohainad R and Lobstein A:
Anti-proliferative effect of Euphorbia stenoclada in human
airway smooth muscle cells in culture. J Ethnopharmacol.
109:134–139. 2007.
|
|
9
|
Valente C, Pedro M, Duarte A, Nascimento
MS, Abreu PM and Ferreira MJ: Bioactive diterpenoids, a new
jatrophane and two ent-Abietanes, and other constituents from
Euphorbia pubescens. J Nat Prod. 67:902–904. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang WF, Cui Z and Zhu Q: Cytotoxicity
and antiviral activity of the compounds from Euphorbia
kansui. Planta Med. 64:754–756. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yasukawa K, Akihisa T, Yoshida ZY and
Takido M: Inhibitory effect of euphol, a triterpene alcohol from
the roots of Euphorbia kansui, on tumor promotion by
12-O-Tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in
mouse skin. J Pharm Pharmacol. 52:119–124. 2000.PubMed/NCBI
|
|
12
|
Guo ZJ, Zhu R, Lu JX and Li YL: Chemical
constituents of Euphorbia hylonoma Hand. -Mazz. Zhongguo
Zhongyao Zazhi. 20:744–745. 1995.(In Chinese).
|
|
13
|
Ruan HL, Zhou XF, Zhang YH, Pi HF, Wu JZ
and Sun HD: Ferulic acid esters from Euphorbia hylonoma.
Fitoterapia. 78:72–73. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo ZJ, Lu Juxian, Li YL, et al: Studies
on pharmacognosy of Euphorbia hylonoma Hand-Nazz. J Xi’an
Med Univ. 3:313–315. 3181996.(In Chinese).
|
|
15
|
Guo ZJ, Lu X, Li YL and Zhu R: The studies
on resource of Euphorbia genus in Shaanxi province.
Northwest Pharm J. 11(Suppl 1): 6–7. 1996.
|
|
16
|
Liu RH, Chen LL and Kong LY: Ellagic Acid
Derivatives from the Stem Bark of Sapius sebiferum. Zhong
Guo Yao Ke Da Xue Xue Bao Bian Ji Bu. 5:370–373. 2002.(In
Chinese).
|
|
17
|
Hong YX and Wei GY: Studies on the
chemical constituents from leaves of Diplopanax stachyathus.
Zhong Cao Yao Bian Ji Bu. 2:125–127. 2004.(In chinese).
|
|
18
|
Duffy MJ, McGowan PM and Gallagher WM:
Cancer invasion and metastasis: changing views. J Pathol.
214:283–293. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maas JL and Galletta GJ: Ellagic acid, an
anticarcinogen in fruits, especially in strawberries: a review.
Hortscience. 26:10–14. 1991.
|
|
20
|
Thresiamma KC, George J and Kuttan R:
Protective effect of curcumin, ellagic acid and bixin on radiation
induced genotoxicity. J Exp Clin Cancer Res. 17:431–434.
1998.PubMed/NCBI
|
|
21
|
Mandal S and Stoner GD: Inhibition of
N-nitrosobenzylmethylamine-induced esophageal tumorigenesis in rats
by ellagic acid. Carcinogenesis. 11:55–61. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sohn EH, Koo HJ, Hang DT, et al:
Protective effects of ellagic acid on ethanol-induced toxicity in
hepatic HepG2 cells. Mol Cell Toxicol. 9:249–256. 2013. View Article : Google Scholar
|
|
23
|
Lu HF, Tsou MF, Lin JG, Ho CC, Liu JY,
Chuang JY and Chung JG: Ellagic acid inhibits growth and arylamine
N-acetyltransferase activity and gene expression in
Staphylococcus aureus. In Vivo. 19:195–199. 2005.PubMed/NCBI
|
|
24
|
Sayer JM, Yaji H, Wood AW, et al:
Extremely facile reaction between the ultimate carcinogen
benzo[a]pyrene-7,8-diol 9,10-epoxide and ellagic acid. J Am Chem
Soc. 104:5562–5564. 1982.
|
|
25
|
Take Y, Imouye Y, Nakamura S, et al:
Comparative studies of the inhibitory properties of antibiotics on
human immunodeficiency virus and avian myeloblastosis virus reverse
transcriptases and cellular DNA polymerases. J Antibiot (Tokyo).
42:107–115. 1989. View Article : Google Scholar
|
|
26
|
Stoner GD and Morse MA: Isothiocyanates
and plant polyphenols as inhibitors of lung and esophageal cancer.
Cancer Lett. 114:113–119. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Narayanan BA, Geoffroy O, Willingham MC,
Re GG and Nixon DW: P53/p21 (WAF1/CIP1) expression and its possible
role in G1 arrest and apoptosis in ellagic acid treated cancer
cells. Cancer Lett. 136:215–221. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ahn D, Putt D, Kresty L, Stoner GD, Fromm
D and Hollenberg PF: The effects of dietary ellagic acid on rat
hepatic and esophageal mucosal cytochromes P450 and phase II
enzymes. Carcinogenesis. 17:821–828. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Falsaperla M, Morgia G, Tartarone A,
Ardito R and Romano G: Support ellagic acid therapy in patients
with hormone refractory prostate cancer (HRPC) on standard
chemotherapy using vinorelbine and estramustine phosphate. Eur
Urol. 47:449–454. 2005. View Article : Google Scholar
|
|
30
|
Labrecque L, Lamy S, Chapus A, et al:
Combined inhibition of PDGF and VEGF receptors by ellagic acid, a
dietary-derived phenolic compound. Carcinogenesis. 26:821–826.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Arulmozhi V, Pandian K and Mirunalini S:
Ellagic acid encapsulated chitosan nanoparticles for drug delivery
system in human oral cancer cell line (KB). Colloids Surf B
Biointerfaces. 110:313–320. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rommel A and Wrolstad RE: Ellagic acid
content of red rasp2 berry juice as influenced by cultivar,
processing, and environ2 mental factors. J Agric Food Chem.
41:1951–1960. 1993. View Article : Google Scholar
|
|
33
|
Rafter JJ: Scientific basis of biomarkers
and benefits of functional foods for reduction of disease risk:
cancer. Br J Nutr. 88(Suppl 2): S219–S224. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Potter JD: Cancer prevention: epidemiology
and experiment. Cancer Lett. 114:7–9. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roy M, Chakrabarty S, Sinha D,
Bhattacharya RK and Siddiqi M: Anticlastogenic, antigenotoxic and
apoptotic activity of epigallocatechin gallate: a green tea
polyphenol. Mutat Res. 523–524:33–41. 2003.PubMed/NCBI
|
|
36
|
Kong AN, Owuor E, Yu R, Hebbar V, Chen C,
Hu R and Mandlekar S: Induction of xenobiotic enzymes by the MAP
kinase pathway and the antioxidant or electrophile response element
(ARE/EpRE). Drug Metab Rev. 33:255–271. 2001. View Article : Google Scholar
|
|
37
|
Park AM and Dong Z: Signal transduction
pathways: targets for green and black tea polyphenols. J Biochem
Mol Biol. 36:66–77. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Loo G: Redox-sensitive mechanisms of
phytochemical mediated inhibition of cancer cell proliferation. J
Nutr Biochem. 14:64–73. 2003.PubMed/NCBI
|
|
39
|
Heber D and Lu QY: Overview of mechanisms
of action of lycopene. Exp Biol Med (Maywood). 227:920–923.
2002.PubMed/NCBI
|
|
40
|
O’Neill JW and Hockenbery DM:
Bcl-2-related proteins as drug targets. Current Med Chem.
10:1553–1562. 2003.
|
|
41
|
Cory S and Adams JM: Killing cancer cells
by flipping the Bcl-2/Bax switch. Cancer Cell. 8:5–6. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fathi NA, Hussein MR, Hassan HI, Mosad E,
Galal H and Afifi NA: Glomerular expression and elevated serum
Bcl-2 and Fas proteins in lupus nephritis: preliminary findings.
Clin Exp Immunol. 146:339–343. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang R, Xue YY, Lu SD, Wang Y, Zhang LM,
Huang YL, Signore AP, Chen J and Sun FY: Bcl-2 enhances
neurogenesis and inhibits apoptosis of newborn neurons in adult rat
brain following a transient middle cerebral artery occlusion.
Neurobiol Dis. 24:345–356. 2006. View Article : Google Scholar
|
|
44
|
Karlnoski R, Wilcock D, Dickey C, Ronan V,
Gordon MN, Zhang W, Morgan D and Taglialatela G: Up-regulation of
Bcl-2 in APP transgenic mice is associated with neuroprotection.
Neurobiol Dis. 25:179–188. 2007. View Article : Google Scholar
|
|
45
|
Bernas T, Asem EK, Robinson JP, Cook PR
and Dobrucki JW: Confocal fluorescence imaging of photosensitized
DNA denaturation in cell nuclei. Photochem Photobiol. 81:960–969.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yoshida A, Takemura H, Inoue H, Miyashita
T and Ueda T: Inhibition of glutathione synthesis overcomes
Bcl-2-mediated topoisomerase inhibitor resistance and induces
nonapoptotic cell death via mitochondrial-independent pathway.
Cancer Res. 66:5772–5780. 2006. View Article : Google Scholar
|
|
47
|
Degli EM: Mitochondria in apoptosis: past,
present and future. Biochem Soc Trans. 32:493–495. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dias N and Bailly C: Drugs targeting
mitochondrial functions to control tumor cell growth. Biochem
Pharmacol. 70:1–12. 2005.PubMed/NCBI
|
|
49
|
Sedlak TW, Oltvai ZN, Yang E, Wang K,
Boise LH, Thompson CB and Korsmeyer SJ: Multiple Bcl-2 family
members demonstrate selective dimerizations with Bax. Proc Natl
Acad Sci USA. 92:3834–3838. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nunẽza G, Benedict MA, Hu Y and Inohara
N: Caspases: the proteases of the apoptotic pathway. Oncogene.
17:3237–3245. 1998.
|
|
52
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 37:719–727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dassé E, Bridoux L, Baranek T, Lambert E,
Salesse S, Sowa ML, Martiny L, Trentesaux C and Petitfrère E:
Tissue inhibitor of metalloproteinase-1 promotes hematopoietic
differentiation via caspase-3 upstream the MEKK1/MEK6/p38alpha
pathway. Leukemia. 21:595–603. 2007.PubMed/NCBI
|
|
54
|
Lanvin O, Gouilleux F, Mullié C, Mazière
C, Fuentes V, Bissac E, Dantin F, Mazière JC, Régnier A, Lassoued K
and Gouilleux-Gruart V: Interleukin-7 induces apoptosis of 697
pre-B cells expressing dominant-negative forms of STAT5: evidence
for caspase-dependent and -independent mechanisms. Oncogene.
23:3040–3047. 2004. View Article : Google Scholar
|