Introduction
The mammalian target of rapamycin (mTOR), a highly
conserved serine/threonine protein kinase, is critical for cell
growth, survival and angiogenesis (1). Members of the epidermal growth factor
receptor family, including human epidermal growth factor receptor 2
(HER2), use the phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR
pathway to promote cell growth and survival (2). mTOR is predominantly controlled by the
PI3K/Akt pathway and can be activated by Akt-mediated
phosphorylation (3,4). mTOR has two main downstream
messengers, the 40S ribosomal S6 kinase (S6K1) and the eukaryotic
translation initiation factor 4E-binding protein 1 (4E-BP1)
(5), both of which are activated
via phosphorylation by phosphorylated (p)-mTOR.
The eukaryotic initiation factor 4E (eIF4E) complex
is an initiation factor on the 5′ cap structure of mRNA that
recruits the small ribosomal subunit to mRNA. It contains three
initiation factors, eIF4E, eIF4G and eIF4A (2,6). To
assemble the eIF4E complex, eIF4E first binds to the 5′ cap to
recruit eIF4G and eIF4A. However, 4E-BP1 inhibits eIF4G binding to
eIF4E. The p-4E-BP1 loses its ability to bind to eIF4E and allows
the eIF4E complex to bind to the cap structure of mRNA (7–9),
subsequently initiating the protein translation. It has been shown
that eIF4E expression is associated with patient survival following
anthracycline chemotherapy treatment, and the influence of eIF4E on
cancer survival is modulated substantially by 4E-BP1 (10,11).
The PI3K/Akt/mTOR/4E-BP1 pathway has also been found to correlate
with HER2, and it may be used as a predictive marker for patient
prognosis (2). Another downstream
factor, S6K1, is associated with the translational machinery and
has been demonstrated to predict poor prognosis in hormonal
receptor-positive breast cancer patients (12,13).
Neoadjuvant chemotherapy (NAC), also known as
preoperative therapy, uses chemotherapy as the initial treatment of
malignant tumors, followed by surgery or other therapies. NAC is
now well established in the treatment of inoperable locally
advanced and inflammatory breast cancer. It is also used in
operable breast cancer treatment in order to obtain clinical and
pathological response to NAC, or to downstage tumors to allow
breast-conserving surgery (14,15). A
large number of studies have demonstrated the efficacy of NAC in
primary operable and locally advanced breast cancer patients, as
well as patients who have achieved pathological complete response
(pCR), which is regarded as a good surrogate predictor of
disease-free survival (DFS) and overall survival (OS) (16–19).
However, few molecular markers are available to predict the NAC
responses or survival gains. It has been demonstrated that
PI3K/Akt/mTOR is commonly deregulated in human cancers (20,21),
due to the mutation of PIK3CA, Akt and phosphatase and tensin
homolog (PTEN), or the loss of PTEN (22–25).
It has also been demonstrated that the combination of mTOR
inhibitors with cytotoxic agents can exert synergistic
antiproliferative activity in in vitro studies, irrespective
of HER2 status (26). A number of
non-randomized studies in HER2-positive trastuzumab-resistant
metastatic breast cancer have shown the antitumor activity of mTOR
inhibitors when used together with standard chemotherapy plus
trastuzumab (27,28). However, a study evaluating the
addition of mTOR inhibitors to paclitaxel treatment in
HER2-negative patients suggested that supplementing paclitaxel
treatment with everolimus did not significantly improve pCR rates
compared with those of paclitaxel alone (29). A number of other clinical trials
have been initiated to identify the most beneficial therapeutic
strategies to include mTOR inhibitors for different patient
subgroups (30).
Materials and methods
Patients and tissues
In total, 83 primary breast cancer patients treated
with NAC at the First Hospital of China Medical University
(Shenyang, China) between 2007 and 2010 were selected. Preoperative
chemotherapy was performed as follows: 37 patients were
administered docetaxel (75 mg/m2) with platinum (TP; 100
mg/m2) or cyclophosphamide (TC; 1.0 g) every three weeks
for three to five cycles, while the other 46 patients received
5-fluorouracil (1.0 g), epirubicin (80 mg/m2) and
cyclophosphamide (CEF; 1.0 g) every three weeks for three to four
cycles. Patients were followed-up for a median of 45 months after
their initial cancer surgery. Relevant clinical and pathological
parameters are described in Table
I. Archival formalin-fixed, paraffin-embedded breast tissues
were collected from core biopsies (pre-NAC) and matched resection
tissues (post-NAC). Six patients achieved pCR. All of the
carcinomas had been histologically confirmed as invasive breast
cancer according to the criteria of the World Health Organization
(31) and the molecular subtypes of
breast carcinoma were identified.
 | Table IClinical and pathological features of
the patients (n=83). |
Table I
Clinical and pathological features of
the patients (n=83).
| Characteristic | n (%) |
|---|
| Age, years |
| ≤45 | 49 (59.0) |
| >45 | 34 (41.0) |
| Pre-NAC stage (based
on ultrasound) |
| T2 | 41 (49.4) |
| T3 | 24 (28.9) |
| T4 | 18 (21.7) |
| Post-NAC stage (based
on resection pathology) |
| T0 | 6 (7.2) |
| T1 | 23 (27.7) |
| T2 | 33 (39.8) |
| T3 | 12 (14.5) |
| T4 | 9 (10.8) |
| Tumor size
changea |
| Increase | 8 (9.6) |
| Decrease | 69 (83.1) |
| pCR | 6 (7.2) |
| NAC regimen |
| TP | 32 (38.6) |
| TC | 15 (18.1) |
| CEF | 36 (43.4) |
| Positive axillary
metastasis | 61 (73.5) |
| Estrogen
receptor-positive | 37 (44.6) |
| Her2-positive | 51 (61.4) |
| Surgery |
| Breast
conserving | 2 (2.4) |
| Mastectomy | 81 (97.6) |
| Follow-up,
monthsb | 45 (32–78) |
| Recurrence | 28 (33.7) |
| Mortality | 23 (27.7) |
Immunohistochemical staining
Immunohistochemical examination was performed on
4-μm formalin-fixed, paraffin-embedded sections. Briefly, following
deparaffinization and rehydration, the endogenous peroxidase
activity was blocked using 3% H2O2 (reagent
A; UltraSensitive™ SP IHC kit; Maxim Biotech Inc., Fuzhou, China).
Next, antigen retrieval was performed and normal serum was applied
to the sections to block non-specific antibody binding (reagent B;
UltraSensitive™ SP IHC kit; Maxim Biotech Inc.). Sections were then
incubated overnight at 4°C with the primary antibodies. A goat
anti-rabbit polyclonal antibody against mTOR (phospho S2448) was
used for p-mTOR at a dilution of 1:500 (ab131538; Abcam, Cambridge,
UK), and a goat anti-rabbit polyclonal antibody against eIF4EBP1
(phospho Thr36) was used for p-4EBP1 at a dilution of 1:200
(ab47365; Abcam). Following overnight incubation at 4°C, sections
were incubated for 15 min with the secondary antibody solution
(reagent C; UltraSensitive™ SP IHC kit; Maxim Biotech Inc.). The
sections were then incubated with streptavidin-perosidase (reagent
D; UltraSensitive™ SP IHC kit; Maxim Biotech Inc.) for 15 min and
stained with 3,3-diaminobenzidine. Finally, sections were
counterstained with hematoxylin for 5 min and mounted. Negative
controls were processed with normal rabbit serum (Dako,
Carpinteria, CA, USA) in place of the primary antibody. Positive
controls were performed using breast cancer tissue sections that
had shown strong staining for the respective protein during
antibody optimization. This study was approved by the ethics
committee of the First Affiliated Hospital (Shenyang, China) and
written informed consemt was obtained from all patients.
Evaluation of immunohistochemistry
The immunohistochemical staining results were
evaluated and scored independently in a blinded manner by two
pathologists. Cases of disagreement were reviewed jointly to obtain
a consensus score. The score was the average of 10 distinct
high-power fields observed under the 40× objective. The staining
was considered positive when cytoplasmic and/or membranous staining
was observed in the malignant cells, and the staining was evaluated
using a semi-quantitative scoring system considering the extent and
intensity. The percentage of cells stained were scored as follows:
0, no cells stained; 1, 1–10% of cells stained; 2, 11–50% of cells
stained; 3, 51–80% of cells stained; and 4, >80% of cells
stained. Staining intensity was scored as follows: 0, negative; 1,
weak; 2, moderate; and 3, strong. The two parameters were
multiplied, resulting in an individual immunoreactivity score
ranging between 0 and 12 for every case. The six patients who
achieved pCR were regarded as negative post-NAC.
Statistical analysis
Statistical analyses were performed using SPSS
v.19.0 (SPSS, Inc., Chicago, IL, USA). Wilcoxon signed-rank test
was used to evaluate the independence between two groups of matched
samples, and Mann-Whitney U test was used to assess the
independence between two independent samples without any
distribution assumption. Spearman’s correlation coefficients were
used to reveal a correlation between two continuous variables.
Receiver Operating Characteristic (ROC) curve analyses were used to
select cut-off values (giving the highest combined sensitivity and
specificity) to dichotomize pre- and post-NAC expression scores for
the endpoint of DFS. DFS was recorded from the date of surgery to
the date of relapse or last follow-up, and estimated using the
Kaplan-Meier analyses. The statistical significance of the
differential survival was assessed using the log-rank (score) test.
Additionally, multivariate Cox regression analysis was performed
taking into account the pre-NAC expression of p-mTOR and p-4E-BP1,
and post-NAC expression of p-mTOR, as well as other
clinicopathological features, including tumor grade, receptor
status, pre-NAC tumor stage and axillary metastasis. All P-values
presented are two-sided, and P≤0.05 was considered to indicate a
statistically significant difference.
Results
p-mTOR and p-4E-BP1 are downregulated
following NAC and correlate with each other in pre-NAC samples
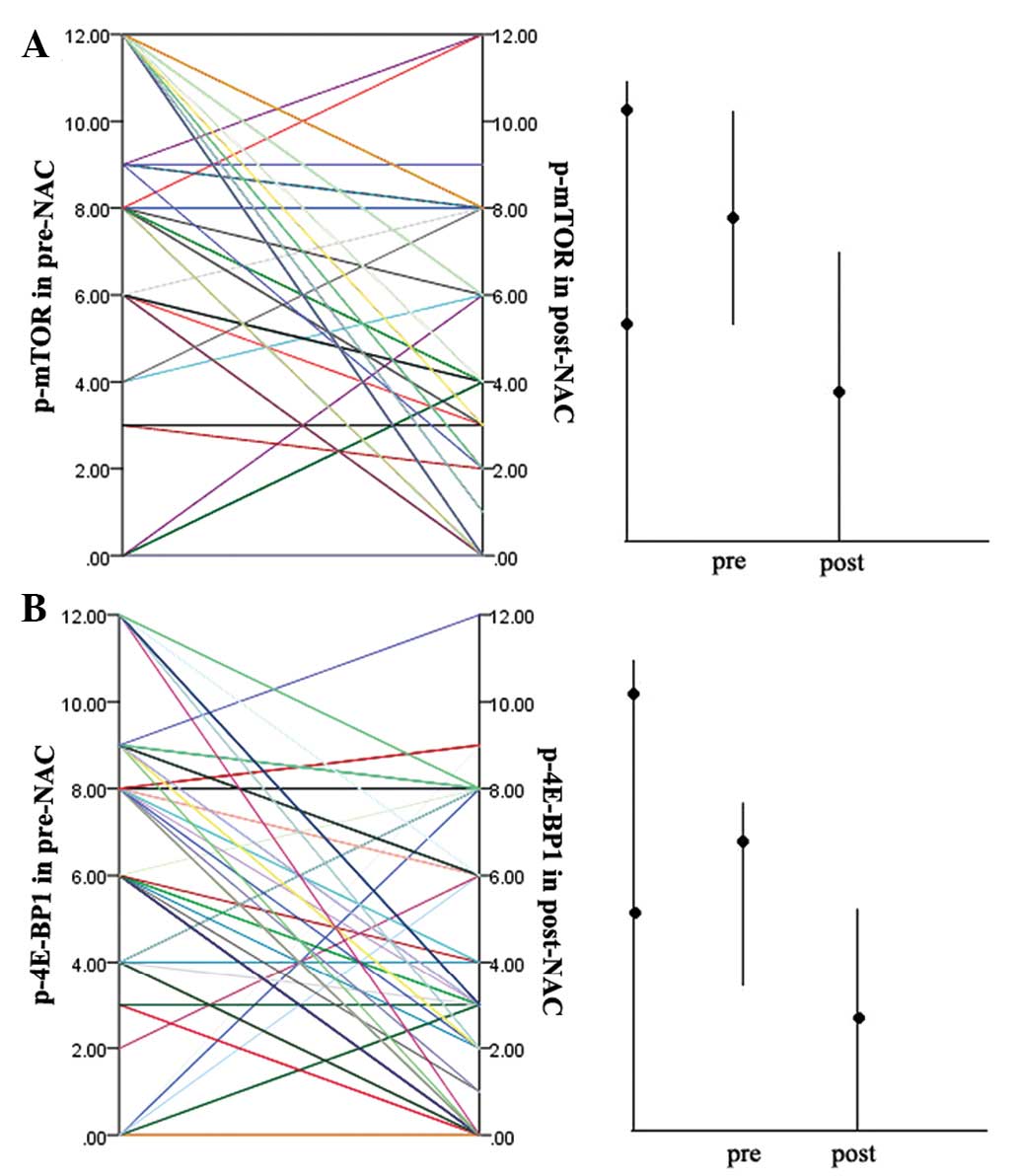
The expression of p-mTOR and p-4E-BP1 were examined
in the tumors of 83 cases of breast cancer patients. The patients
who had achieved pCR were not examined post-NAC. Tumors were
obtained from diagnostic biopsies pre-NAC and surgical resections
post-NAC. Table I shows the
clinical and pathological features of the patient cohort. The
staining of p-mTOR was predominantly cytoplasmic, and present in
71/83 cases (85.5%) and 58/83 cases (69.9%) pre-NAC (Fig. 1A) and post-NAC (Fig. 1B), respectively. The p-4E-BP1 was
detected in the nucleus and cytoplasm, with positive rates of
p-4E-BP1 in 69/83 cases (83.1%) and 58/83 cases (69.9%) pre-NAC
(Fig. 1C) and post-NAC (Fig. 1D), respectively. The scores and
their distributions are shown in Fig.
2. Scores were lower for p-mTOR (51/83 cases; 61.4%) and
p-4E-BP1 (56/83 cases; 67.5%) in post-NAC samples than in the
matched pre-NAC samples, indicating a decrease in their expression
in response to NAC. Wilcoxon signed-rank test showed significant
differences between pre- and post-NAC scores (P<0.001 for p-mTOR
and p-4E-BP1). It was also examined whether a correlation exists
between the expression of p-mTOR and p-4E-BP1 pre- and post-NAC, as
well as for the expression change following treatment (pre-NAC
minus post-NAC level). The expression of p-mTOR and p-4E-BP1 were
found to significantly correlate with each other in pre-NAC samples
(Spearman’s ρ analyses, P=0.004), which is consistent with previous
studies (2,32,33).
However, the two factors were not found to correlate with each
other in post-NAC samples, indicating that chemotherapy may have
changed the expression of the two factors to some degree. No
significant correlations were found between the changes of p-mTOR
and p-4E-BP1 following NAC. As would be expected, the pre- and
post-NAC levels were significantly associated with the expression
change of the respective factor.
Decrease of p-mTOR expression following
NAC positively correlates with HER2 expression and diminishing
tumor size
Pre-NAC expression, post-NAC expression and the
expression change following NAC of p-mTOR and p-4E-BP1 was
evaluated to identify correlations with patient or tumor
characteristics, including patient age at diagnosis, tumor grade
and stage, estrogen receptor status, and HER2 status of tumors from
core biopsy at diagnosis, as well as the tumor stage and presence
of axillary metastases from resection pathology. Spearman’s ρ
analyses were performed, and not only did the expression of p-mTOR
and p-4E-BP1 in pre-NAC samples correlate with HER2 [ρ coefficient,
0.181 (P=0.05) for p-mTOR; ρ coefficient, 0.193 (P=0.04) for
p-4E-BP1], which is consistent with the previous study, but the
change of mTOR expression was also found to significantly correlate
with HER2 (ρ coefficient, 0.275; P=0.006). Next, the changes of
p-mTOR in HER2-positive and -negative groups were compared, and the
expression of mTOR was found to decrease significantly following
treatment with NAC in the HER2-positive group. The changes of
p-mTOR expression had median values of 4 in the HER2-positive group
and 1 in HER2-negative group. The Mann-Whitney U test was used to
assess the differences between the two groups, and a P-value of
0.041 was obtained, suggesting some degree of cross-talk between
the PI3K/Akt/mTOR-related pathways and HER2. The expression of
p-mTOR and p-4E-BP1 pre- and post-NAC was also evaluated, as well
as the expression change of the two factors to identify
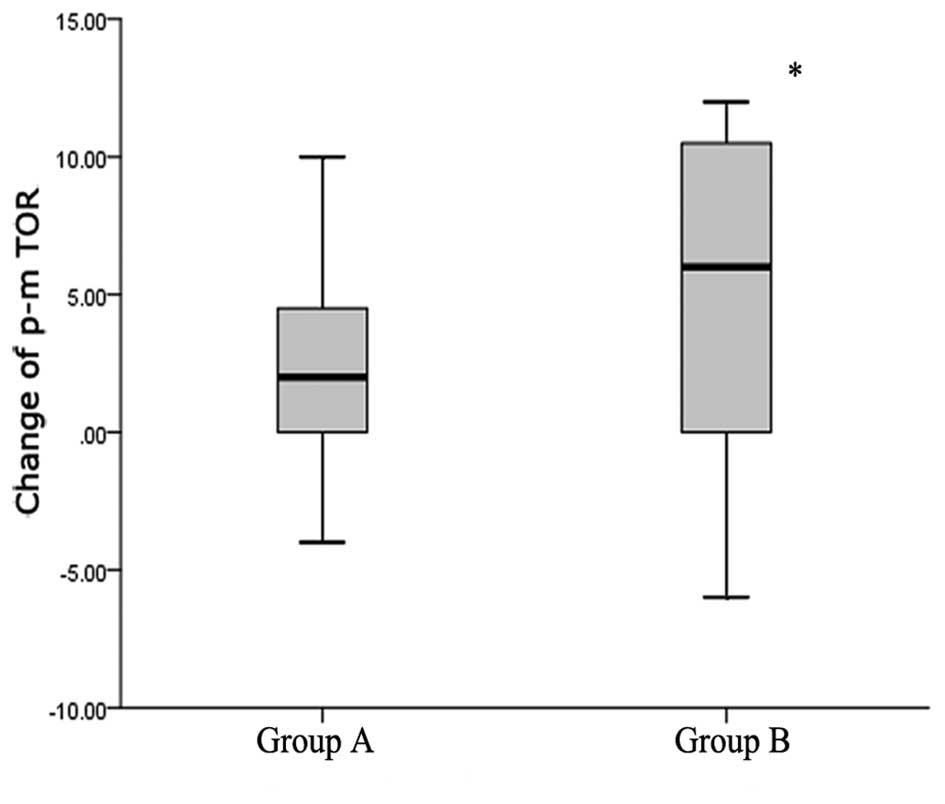
correlations with tumor size. Therefore, patients were classified
into different groups according to tumor size; patients with tumors
that had diminished by <1 cm, showed no change, or had increased
were defined as group A, while patients whose tumor sizes had
diminished by >1 cm, or patients who had achieved PCR were
defined as group B. The only significant correlation was found
between the expression change of mTOR and diminishing tumor size.
The median values of expression change of mTOR were 2 in group A
and 6 in group B (Fig. 3). The
Mann-Whitney U test showed a significant difference between groups
A and B (P=0.033). No significant correlations were found between
the two factors and other clinicopathological features.
High levels of p-mTOR and p-4E-BP1
pre-NAC correlate with poor DFS, and the high expression of p-mTOR
post-NAC has a significant association with poor DFS
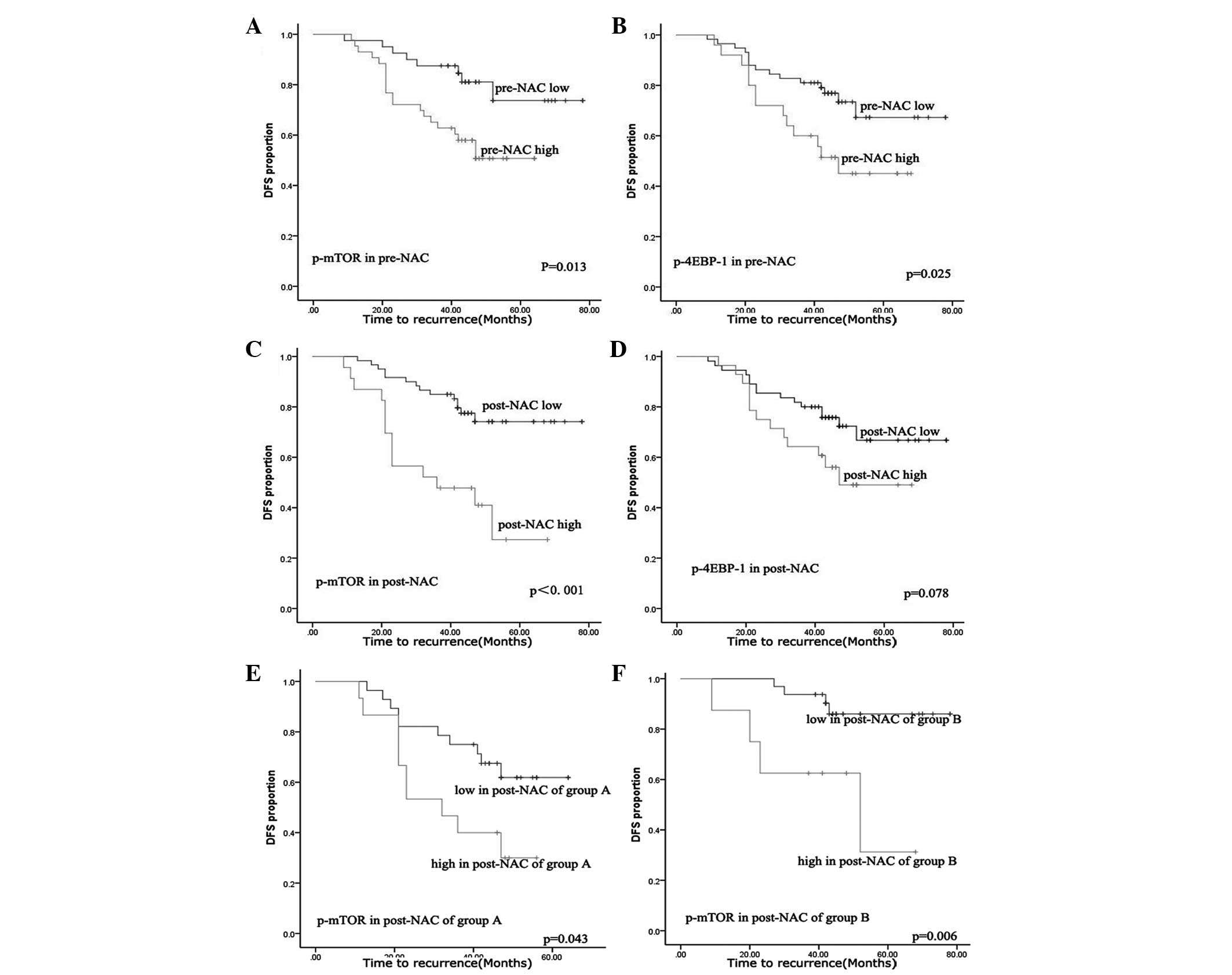
In order to assess the differential survival with
respect to pre- and post-NAC expression, as well as the expression
change following NAC for the two markers, Kaplan-Meier survival
analyses were performed. ROC curve analyses were used to
dichotomize the expression scores into high and low expression
groups. The cut-off values were obtained from the highest combined
sensitivity and specificity at the endpoint of DFS, and were as
follows: p-mTOR, 8 and p-4E-BP1, 9 for pre-NAC; and p-mTOR, 7 and
p-4E-BP1, 5 for post-NAC. A high expression of p-mTOR and p-4E-BP1
was found to significantly correlate with poor DFS pre-NAC
(Fig. 4A and B, log-rank, P=0.013
for p-mTOR and P=0.025 for p-4E-BP1). The expression of p-4E-BP1
post-NAC was not significantly correlated with DFS (Fig. 4D). By contrast, the high expression
of p-mTOR in post-NAC samples had a significant association with
poor DFS compared with the pre-NAC samples (Fig. 4C, log-rank, P<0.001). It was also
examined whether the expression changes of the factors correlate
with DFS. Changes in expression of the two factors were
dichotomized as up- or downregulated, but no significant
correlation was found. In order to identify the predictive value of
mTOR post-NAC, patients with high p-mTOR expression pre-NAC were
defined as group A, while those with low p-mTOR expression pre-NAC
were defined as group B. Next, the p-mTOR expression was compared
between groups A and B post-NAC. High expression of p-mTOR post-NAC
was found to correlate with poor DFS, regardless of whether the
patients were in group A or B (P=0.043 for group A and P=0.006 for
group B). Finally, multivariate Cox regression analysis was
performed taking into account p-mTOR and p-4E-BP1 expression
pre-NAC, p-mTOR expression post-NAC and other clinicopathological
features, including tumor grade, receptor status, tumor stage
pre-NAC and axillary metastasis. Post-NAC expression of p-mTOR was
identified as the only significant factor, with its high expression
associated with a hazard ratio of 3.073 (95% confidence interval,
1.4–6.8; P=0.006).
Discussion
Consistent with previous studies, the expression
levels of p-mTOR and p-4E-BP1 in pre-NAC samples were found to
significantly correlate with each other in this study. However,
following chemotherapy, the expression levels of the two factors
were found to decrease and no longer showed a correlation. This may
indicate that the PI3K/Akt/mTOR/4E-BP1 pathway can be suppressed by
chemotherapy in certain patients without treatment with mTOR
inhibitors. This result supports the previous finding that the
expression of eIF4E, as the downstream factor of p-4E-BP1, was
reduced following NAC (34). This
may also suggest that chemotherapy can suppress certain upstream
signals or regulators of mTOR, such as Akt, PTEN and TSC1/TSC2,
resulting in the inhibition of the mTOR/4E-BP1/eIF4E pathway.
HER2-mediated activation of the PI3K/Akt/mTOR pathway has been
implicated in the angiogenesis and metastasis of breast cancers
(35), and is predictive of tumor
progression (2). In an in
vitro study, HER2-overexpressing cells with an activated
Akt/mTOR/4E-BP1 pathway were more dependent on this pathway for
growth and, therefore, were more sensitive to mTOR inhibition
(2). A previous study has also
shown that the expression of p-mTOR and p-4E-BP1 correlate with
HER2 expression, which is consistent with the finding in this
study. The decrease of p-mTOR was also found to be significant in
HER2-positive patients compared with HER2-negative patients,
indicating that the PI3K/Akt/mTOR pathway may be suppressed more
effectively by chemotherapy in HER2-positive breast cancers. Based
on these results, we hypothesize that HER2-positive patients with
high p-mTOR expression following NAC may benefit more from the
addition of mTOR inhibitors to chemotherapy. However, further study
is required to investigate the specific association between the
PI3K/Akt/mTOR/4E-BP1 pathway and chemotherapy sensitivity.
It has been demonstrated that patients with
favorable responses to NAC exhibit improved DFS (36). Previous clinical trials have also
shown that patients who achieve pCR exhibit an improved DFS and OS
(16–19). Although a number of different
prognostic indicators are being developed, few molecular markers
are widely used to predict the NAC responses. In this study, the
change of p-mTOR expression was found to correlate with the change
of tumor size. Patients with lower levels of p-mTOR expression
following NAC are likely to have smaller tumor sizes. Although this
finding is not useful to predict the sensitivity of chemotherapy
prior to NAC, it can be used as a marker of the effect during the
course of NAC and as a reference to decide the chemotherapy regimen
following surgery. It was also noted that the PI3K/Akt/mTOR pathway
correlates with the resistance to chemotherapy based on the
analysis of the correlation between the change of p-mTOR expression
and the change of tumor size.
This study not only confirmed previous findings that
high levels of p-mTOR and p-4E-BP1 pre-NAC are significantly
associated with poor DFS, but also found that the high expression
of p-mTOR in post-NAC samples exhibits a significant association
with poor DFS. No significant correlation was found between the
changes of the two factors, or between the post-NAC expression of
4E-BP1 and DFS. In an effort to investigate the reason why the
change of p-mTOR was not found to correlate with DFS, it was
demonstrated that the p-mTOR expression significantly correlated
with DFS, regardless of whether its expression was high or low
pre-NAC. This result indicated that patients whose tumors contain
high levels of p-mTOR following NAC may be more resistant to
chemotherapy. These patients, particularly HER2-positive patients,
may be more appropriate candidates for adding mTOR inhibitors to
the chemotherapy. As a downstream factor of p-4E-BP1, eIF4E has
been demonstrated to correlate with DFS, regardless of its
expression in neoadjuvant (34) or
adjuvant chemotherapy (10).
However, the p-4E-BP1 expression post-NAC or the change of p-4E-BP1
was not found to correlate with DFS. Although the exact mechanism
is not clear, this may be due to the different chemotherapy
regimens.
The expression levels of p-mTOR and p-4E-BP1 were
significantly decreased following treatment with NAC, particularly
in HER2-positive samples. However, little is known with regard to
the mechanisms that drive the expression changes of the two
factors. The p-mTOR expression post-NAC may be a more reliable
predictor to DFS in NAC patients, and may be used as a reference to
select patients that are suitable for adding mTOR inhibitors to the
chemotherapy. It is known that mutations of PIK3CA, Akt and PTEN,
or the loss of PTEN may influence the expression of p-mTOR
following NAC. Therefore, these mutations may cause different
sensitivities to chemotherapy. Further study is required to clarify
the exact mechanisms and to evaluate the predictive value of the
PI3K/Akt/mTOR/4E-BP1 pathway in NAC.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81172199 and 81272920)
and the Hi-Tech Research Development Program of China (863 Program;
no. 2006AA02Z493).
References
|
1
|
Ma XM and Blenis J: Molecular mechanisms
of mTOR-mediated translational control. Nature Rev Mol Cell Biol.
10:307–318. 2009.
|
|
2
|
Zhou X, Tan M, Stone Hawthorne V, et al:
Activation of the Akt/mammalian target of rapamycin/4E-BP1 pathway
by ErbB2 overexpression predicts tumor progression in breast
cancers. Clinical Cancer Res. 10:6779–6788. 2004.
|
|
3
|
Meric-Bernstam F and Gonzalez-Angulo AM:
Targeting the mTOR signaling network for cancer therapy. J Clin
Oncol. 27:2278–2287. 2009.
|
|
4
|
Faivre S, Kroemer G and Raymond E: Current
development of mTOR inhibitors as anticancer agents. Nat Rev Drug
Discov. 5:671–688. 2006.
|
|
5
|
Margariti N, Fox SB, Bottini A and
Generali D: ‘Overcoming breast cancer drug resistance with mTOR
inhibitors’. Could it be a myth or a real possibility in the
short-term future? Breast Cancer Res Treat. 128:599–606. 2011.
|
|
6
|
Gingras AC, Raught B and Sonenberg N: eIF4
initiation factors: effectors of mRNA recruitment to ribosomes and
regulators of translation. Annu Rev Biochem. 68:913–963. 1999.
|
|
7
|
Hara K, Yonezawa K, Kozlowski MT, et al:
Regulation of eIF-4EBP1 phosphorylation by mTOR. J Biol Chem.
272:26457–26463. 1997.
|
|
8
|
von Manteuffel SR, Dennis PB, Pullen N, et
al: The insulin-induced signalling pathway leading to S6 and
initiation factor 4E binding protein 1 phosphorylation bifurcates
at a rapamycin-sensitive point immediately upstream of p70s6k. Mol
Cell Biol. 17:5426–5436. 1997.
|
|
9
|
Brunn GJ, Hudson CC, Sekulić A, et al:
Phosphorylation of the translational repressor PHAS-I by the
mammalian target of rapamycin. Science. 277:99–101. 1997.
|
|
10
|
Heikkinen T, Korpela T, Fagerholm R, et
al: Eukaryotic translation initiation factor 4E (eIF4E) expression
is associated with breast cancer tumor phenotype and predicts
survival after anthracycline chemotherapy treatment. Breast Cancer
Ress Treat. 141:79–88. 2013.
|
|
11
|
Coleman LJ, Peter MB, Teall TJ, et al:
Combined analysis of eIF4E and 4E-binding protein expression
predicts breast cancer survival and estimates eIF4E activity. Br J
Cancer. 100:1393–1399. 2009.
|
|
12
|
Jefferies HB, Fumagalli S, Dennis PB, et
al: Rapamycin suppresses 5′TOP mRNA translation through inhibition
of p70s6k. EMBO J. 16:3693–3704. 1997.
|
|
13
|
Kim EK, Kim HA, Koh JS, et al:
Phosphorylated S6K1 is a possible marker for endocrine therapy
resistance in hormone receptor-positive breast cancer. Breast
Cancer Res Treat. 126:93–99. 2011.
|
|
14
|
Fuksa L, Micuda S, Grim J, Ryska A and
Hornychova H: Predictive biomarkers in breast cancer: their value
in neoadjuvant chemotherapy. Cancer Invest. 30:663–678. 2012.
|
|
15
|
Mieog JS, van der Hage JA and van de Velde
CJ: Neoadjuvant chemotherapy for operable breast cancer. Br J Surg.
94:1189–1200. 2007.
|
|
16
|
Kuerer HM, Newman LA, Smith TL, et al:
Clinical course of breast cancer patients with complete pathologic
primary tumor and axillary lymph node response to doxorubicin-based
neoadjuvant chemotherapy. J Clin Oncol. 17:460–469. 1999.
|
|
17
|
Ogston KN, Miller ID, Payne S, et al: A
new histological grading system to assess response of breast
cancers to primary chemotherapy: prognostic significance and
survival. Breast. 12:320–327. 2003.
|
|
18
|
Thomas E, Holmes FA, Smith TL, et al: The
use of alternate, non-cross-resistant adjuvant chemotherapy on the
basis of pathologic response to a neoadjuvant doxorubicin-based
regimen in women with operable breast cancer: long-term results
from a prospective randomized trial. J Clin Oncol. 22:2294–2302.
2004.
|
|
19
|
Heys SD, Hutcheon AW, Sarkar TK, et al:
Neoadjuvant docetaxel in breast cancer: 3-year survival results
from the Aberdeen trial. Clin Breast Cancer. 3(Suppl 2): S69–S74.
2002.
|
|
20
|
Guertin DA and Sabatini DM: Defining the
role of mTOR in cancer. Cancer Cell. 12:9–22. 2007.
|
|
21
|
Dillon RL, White DE and Muller WJ: The
phosphatidyl inositol 3-kinase signaling network: implications for
human breast cancer. Oncogene. 26:1338–1345. 2007.
|
|
22
|
Cancer Genome Atlas Network. Comprehensive
molecular portraits of human breast tumours. Nature. 490:61–70.
2012.
|
|
23
|
Banerji S, Cibulskis K, Rangel-Escareno C,
et al: Sequence analysis of mutations and translocations across
breast cancer subtypes. Nature. 486:405–409. 2012.
|
|
24
|
Saal LH, Holm K, Maurer M, et al: PIK3CA
mutations correlate with hormone receptors, node metastasis, and
ERBB2, and are mutually exclusive with PTEN loss in human breast
carcinoma. Cancer Res. 65:2554–2559. 2005.
|
|
25
|
Stemke-Hale K, Gonzalez-Angulo AM, Lluch
A, et al: An integrative genomic and proteomic analysis of PIK3CA,
PTEN, and AKT mutations in breast cancer. Cancer Res. 68:6084–6091.
2008.
|
|
26
|
Mondesire WH, Jian W, Zhang H, et al:
Targeting mammalian target of rapamycin synergistically enhances
chemotherapy-induced cytotoxicity in breast cancer cells. Clinical
Cancer Res. 10:7031–7042. 2004.
|
|
27
|
Jerusalem G, Fasolo A, Dieras V, et al:
Phase I trial of oral mTOR inhibitor everolimus in combination with
trastuzumab and vinorelbine in pre-treated patients with
HER2-overexpressing metastatic breast cancer. Breast Cancer Res
Treat. 125:447–455. 2011.
|
|
28
|
Andre F, Campone M, O’Regan R, et al:
Phase I study of everolimus plus weekly paclitaxel and trastuzumab
in patients with metastatic breast cancer pretreated with
trastuzumab. J Clin Oncol. 28:5110–5115. 2010.
|
|
29
|
Huober J, Hanusch C, Fasching PA, et al:
Neoadjuvant chemotherapy of paclitaxel with or without Rad001:
Results of the non-responder part of the GEPARUINTO study. In:
Presented at 33rd Annual San Antonio Breast Cancer Symposium; San
Antonio, TX. 2011
|
|
30
|
Yardley DA: Combining mTOR inhibitors with
chemotherapy and other targeted therapies in advanced breast
cancer: Rationale, clinical experience, and future directions.
Breast Cancer (Auckl). 7:7–22. 2013.
|
|
31
|
Yang F and Li J: WHO classification of
tumors of the breast. Zhonghua Wai Ke Za Zhi. 52:1–3. 2014.(In
Chinese).
|
|
32
|
Chen J and Fang Y: A novel pathway
regulating the mammalian target of rapamycin (mTOR) signaling.
Biochemical Pharmacol. 64:1071–1077. 2002.
|
|
33
|
Mita MM, Mita A and Rowinsky EK: The
molecular target of rapamycin (mTOR) as a therapeutic target
against cancer. Cancer Biol Ther. 2(4 Suppl 1): S169–S177.
2003.
|
|
34
|
Hiller DJ, Chu Q, Meschonat C, et al:
Predictive value of eIF4E reduction after neoadjuvant therapy in
breast cancer. J Surg Res. 156:265–269. 2009.
|
|
35
|
Klos KS, Wyszomierski SL, Sun M, et al:
ErbB2 increases vascular endothelial growth factor protein
synthesis via activation of mammalian target of rapamycin/p70S6K
leading to increased angiogenesis and spontaneous metastasis of
human breast cancer cells. Cancer Res. 66:2028–2037. 2006.
|
|
36
|
Hanrahan EO, Hennessy BT and Valero V:
Neoadjuvant systemic therapy for breast cancer: an overview and
review of recent clinical trials. Expert Opin Pharmacother.
6:1477–1491. 2005.
|