Introduction
Acute myeloid leukemia (AML) represents a group of
myeloid neoplasms characterized by the accumulation of myeloblasts
in the bone marrow and/or blood (1).
Central diabetes insipidus (DI) is a rare complication observed in
<0.6% of patients with AML (2,3), which
typically occurs in patients with abnormalities in chromosomes 3 or
7 (4–7). Central DI is a condition characterized
by extreme thirst and excessive urination, that is caused by a
deficiency of antidiuretic hormone (ADH) (8). A number of case studies have described
an association between DI and AML with monosomy 7 (4,6,7,9,10); however, DI has been rarely reported in
cases of ectopic virus integration site-1 (EVI1)-positive
AML with monosomy 7. The EVI1 gene is located on chromosome
3q26 and encodes a 1,051-amino acid DNA-binding phosphoprotein,
which functions as a transcription factor (11,12).
Groschel et al (13) reported
the that EVI1 overexpression is observed in 10.7% AML
patients. The present study reports the case of an 18-year-old
female suffering from AML with monosomy 7 and EVI1
overexpression (−7/EVI1+) without 3q aberration,
who presented with central DI as the initial symptom. In addition,
the present study investigated the possible mechanisms associated
with EVI1, monosomy 7 and DI.
Case report
In December 2012, an 18-year-old female presented at
West China Hospital (Chengdu, China) with complaints of fatigue,
ecchymosis, polyuria and polydipsia lasting for two months. A
physical examination performed upon admission revealed dry skin,
indicating dehydration. A complete blood count revealed a white
cell count of 151.93×109/l, with 84% peripheral blasts,
a hemoglobin level of 80 g/l and a platelet count of
139×109/l. In addition, electrolyte tests identified a
serum sodium level of 162.4 mmol/l (indicating the presence of
hypernatremia), serum osmolality level of 342 mOsm/kg and urine
osmolality level of 128 mOsm/kg. Magnetic resonance imaging of the
brain revealed mild thickening of the pituitary stalk (Fig. 1), while cerebrospinal analysis was
negative for leukemia. Central DI was suspected, and the patient
was administered desmopressin acetate tablets (2 mg; Minirin;
Ferring International Center SA, Saint-Prex, Switzerland) orally,
three times a day. The polyuria, polydipsia and dry skin resolved,
with normalization of serum sodium and serum osmolality levels.
Bone marrow smear tests indicated a morphological diagnosis of AML
with 83.5% blasts (Fig. 2).
Cytochemical staining was negative for peroxidase and positive for
periodic acid-Schiff (Baso Diagnostics Inc., Zhuhai, Taiwan). Flow
cytometric analysis revealed 88% blasts in the total nucleated cell
population expressing cluster of differentiation (CD) 34, human
leukocyte antigen (HLA)-DR, CD13, CD117 and CD123 (data not shown),
and a diagnosis of AML with minimal differentiation
[French-American-British classification, AML-M0 (14)] was determined. Trilineage dysplasia
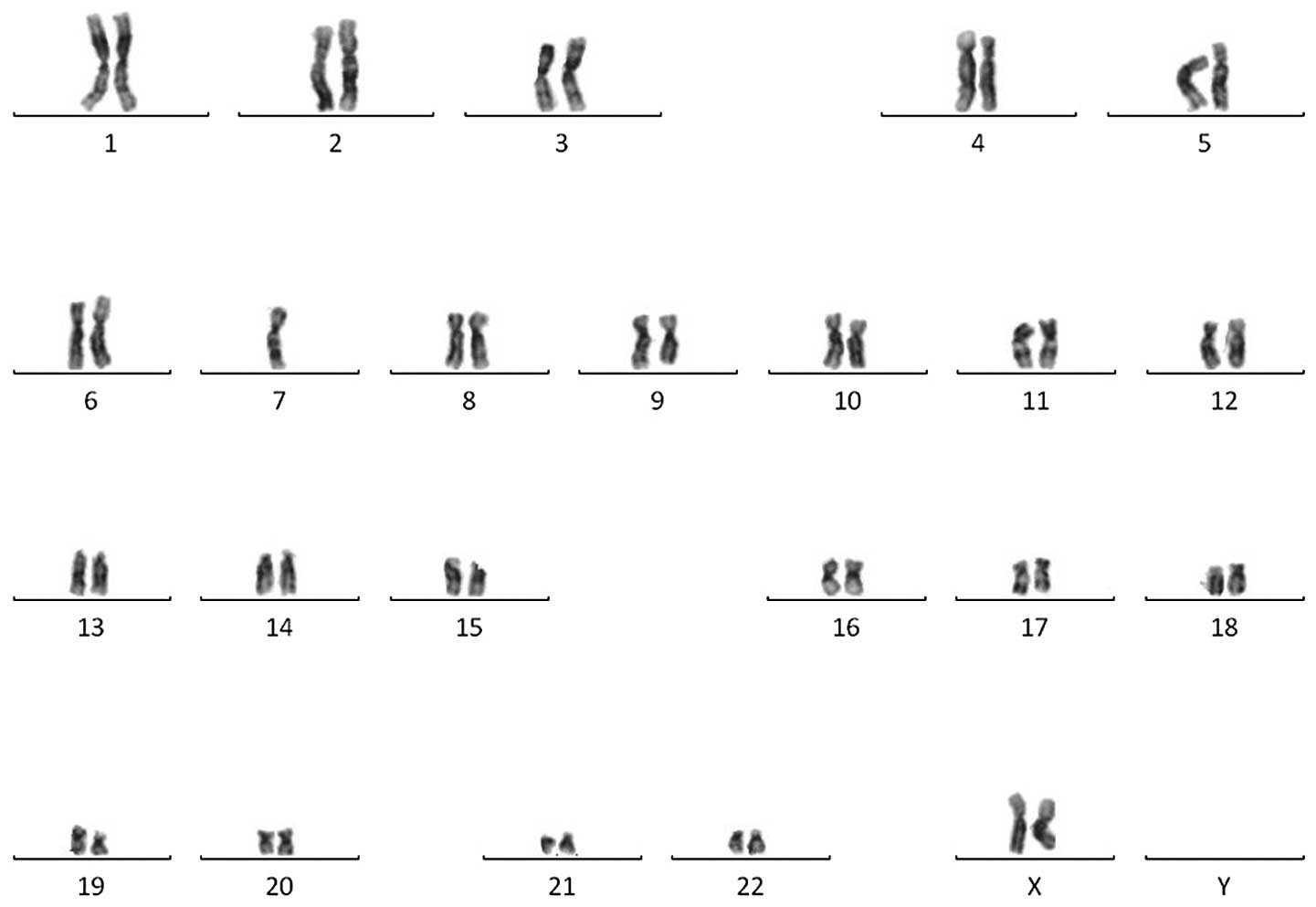
was unremarkable, while a cytogenetic analysis revealed a monosomy
karyotype, 45,XX,-7 (Fig. 3). The
patient was screened for fusion genes, revealing that EVI1
and T-cell leukemia homeobox 1 were positive, whereas
FLT3/ITD, C-KIT D816V, nucleophosmin and
CCAAT/enhancer binding protein α were negative. Furthermore, the
patient had a family history of acute lymphocytic leukemia (pre-B),
since the patient's father had been diagnosed with the disease at
West China Hospital one year before the present diagnosis.
Cytogenetic analysis and genetic screening of the bone marrow of
the patient's father was performed to identify whether a common
oncogene was present, however the results were unremarkable.
Subsequently, the patient was subjected to an
initial cycle of induction chemotherapy with daunorubicin (45
mg/m2, days 1–3) and cytarabine (200 mg/d, days 1–7);
however, remission was not achieved. A second cycle of induction
chemotherapy was administered using the FLAG regimen (50 mg
fludarabine, days 1–5; 2 g cytarabine, days 1–5; 300 µg granulocyte
colony-stimulating factor, days 0–15) with no response. Treatment
with arsenic trioxide (10 mg, days 1–21) and lenalidomide (10 mg,
days 1–21) also produced no response. Without improvement or
significant deterioration, the patient's platelet count remained
high (83×109/l–194×109/l) following
chemotherapy. Due to the refractoriness to chemotherapy and the
high risk associated with transplantation, the patient was
reluctant to undergo allogeneic stem-cell transplantation, despite
her sibling being an HLA match. Therefore, the patient continued to
take desmopressin acetate tablets, and palliative care with
low-dose cytarabine and supportive treatment were administered.
However, the patient succumbed to a severe pulmonary infection 8
months after diagnosis.
Discussion
AML associated with DI (DI-AML) rarely occurs. The
association between DI-AML and cytogenetic aberrations has been
reported in a number of studies (1–3). The most
common aberrations are monosomy 7 and 3q alterations. Montecucco
et al (15) and de la Chapelle
et al (9) reported that 77% of
DI-AML cases were associated with monosomy 7q and 44% of cases were
associated with 3q alterations. In addition, Piccin et al
(16) reviewed previously published
reports of DI-AML, revealing that all 76 cases had acquired
monosomy 7. The finding of the aforementioned studies indicate that
monosomy 7q alterations may be a common ‘chromosomal determinant’
for DI-AML onset. DI-AML patients with 3q aberrations have a number
of common characteristics, including age (29–52 years), normal or
high platelet count, hyperleukocytosis, trilineage myelodysplasia,
no central nervous system involvement, failure to respond to
first-line treatment or early relapse, and poor prognosis (17,18). These
features are termed 3q21q26 syndrome. The findings of the current
study are consistent with such features, with the exception of
trilineage myelodysplasia and 3q aberration. However, the patient
also demonstrated EVI1 overexpression and monosomy-7
(−7/EVI1+), consistent with one of the two cases
reported by Piccin (15). To the best
of our knowledge, this is the second case of DI-AML with
-7/EVI1+ without a 3q aberration.
The EVI1 gene is located on 3q26 and codes
for a 1,051-amino acid DNA-binding phosphoprotein, which functions
as a transcription factor (11,12).
Groschel et al (13)
demonstrated that EVI1+ is associated with
specific chromosome abnormalities, including inv(3)/t(3;3), monosomy 7 and 11q23
translocations. EVI1+ was detected in 21/23 AML
patients with inv(3)/t(3;3) and in
33/38 AML patients with monosomy 7. The role of EVI1 remains
unclear; however, inappropriate EVI1 activation, in
combination with other undefined genetic alterations, are
hypothesized to result in low levels of antidiuretic hormone (ADH)
(18). As ~90% of circulating ADH is
associated with platelets, it is postulated that platelet ADH
originates in the hypothalamus and that chromosome 3 abnormalities
are associated with dysthrombopoiesis, which may result in
alterations in ADH levels or function (7,19,20). However, this does not explain why
DI-AML patients without 3q alterations exhibit aberrations in ADH
levels or function.
In the present study, the patient exhibited no
cytogenetic evidence of chromosome 3 abnormalities. Curley et
al (21) reported a case of
DI-AML with t(3;3)(q21;q26) and monosomy 7 that presented
EVI1 overexpression at the onset; however, EVI1
overexpression was not detected upon AML relapse and DI did not
recur. This indicates that EVI1 overexpression may be
involved in the development of DI in AML patients. The prognosis of
DI-AML is poor and patient survival, regardless of karyotype, is
extremely low compared with a similar cohort of AML patients
without DI. Monosomy 7 separates the disease into two entities
(7). Patients with monosomy 7 tend to
have a poorer complete remission (CR) rate and outcome compared
with patients with other aberrations (7). Gröschel et al (13) reported that 31/33 patients with
-7/EVI1+ AML failed to achieve CR following the
first induction therapy, and 31 patients succumbed to the disease
after a median period of 8.6 months. All patients with
-7/EVI1+ AML had a poor survival rate [two-year
relapse-free survival (RFS), 0%; two-year overall survival, 0%].
Additionally, patients with EVI1+ AML who
received allogeneic stem cell transplantation during the first CR
had significantly improved five-year RFS (33±10% vs. 0%) (13). The response to treatment and survival
time in the present study are similar to the results of the study
by Groschel et al (13).
In the current study, the case of an AML patient
with monosomy 7 and EVI1 overexpression, who exhibited
central DI as the initial symptom, was presented. Treatment with
induction therapy was unsuccessful. This case suggests that the
presence of -7/EVI1+ may be involved in the
development of DI-AML, which is a rare syndrome, and indicates an
extremely poor prognosis. The association between EVI1
overexpression and poor prognosis of AML requires further
investigation and thus, the establishment of aggressive treatment
approaches, including stem cell transplantation, and novel clinical
trials are required.
Acknowledgements
The study was supported by funding provided by the
Office of Science and Technology in Sichuan Province (grant no.
2014SZ0006-6).
References
|
1
|
Hasserjian RP: Acute myeloid leukemia:
advances in diagnosis and classification. Int J Lab Hematol.
35:358–366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kimmel DW and O'Neill BP: Systemic cancer
presenting as diabetes insipidus. Clinical and radiographic
features of 11 patients with a review of metastatic-induced
diabetes insipidus. Cancer. 52:2355–2358. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maghnie M, Cosi G, Genovese E, et al:
Central diabetes insipidus in children and young adults. N Engl J
Med. 343:998–1007. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Castagnola C, Morra E, Bernasconi P,
Astori C, Santagostino A and Bernasconi C: Acute myeloid leukemia
and diabetes insipidus: results in 5 patients. Acta Haematol.
93:1–4. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nieboer P, Vellenga E, Adriaanse R and van
de Loosdrecht AA: Central diabetes insipidus preceding acute
myeloid leukemia with t(3;12)(q26;p12). Neth J Med. 56:45–47. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wössmann W, Borkhardt A, Gossen R, Göbel
FJ and Reiter A: Acute myeloid leukemia presenting with diabetes
insipidus. Eur J Pediatr. 161:161–162. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harb A, Tan W, Wilding GE, Battiwalla M,
Sait SN, Wang ES and Wetzler M: Acute myeloid leukemia and diabetes
insipidus with monosomy 7. Cancer Genet Cytogenet. 190:97–100.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saborio P, Tipton GA and Chan JC: Diabetes
insipidus. Pediatr Rev. 21:122–129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de la Chapelle A and Lahtinen R: Monosomy
7 predisposes to diabetes insipidus in leukaemia and
myelodysplastic syndrome. Eur J Haematol. 39:404–411. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Müller CI, Engelhardt M, Laubenberger J,
et al: Myelodysplastic syndrome in transformation to acute leukemia
presenting with diabetes insipidus: Due to pituitary infiltration
association with abnormalities of chromosome 3 and 7. Eur J
Haematol. 69:115–119. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martinelli G, Ottaviani E, Buonamici S, et
al: Association of 3q21q26 syndrome with different RPN1/EVI1 fusion
transcripts. Haematologica. 88:1221–1228. 2003.PubMed/NCBI
|
|
12
|
Jółkowska J and Witt M: The EVI-1 gene -
its role in pathogenesis of human leukemias. Leuk Res. 24:553–558.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gröschel S, Lugthart S, Schlenk RF, et al:
High EVI1 expression predicts outcome in younger adult patients
with acute myeloid leukemia and is associated with distinct
cytogenetic abnormalities. J Clin Oncol. 28:2101–2107. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Catovsky D, Matutes E, Buccheri V, Shetty
V, Hanslip J, Yoshida N and Morilla R: A classification of acute
leukaemia for the 1990s. Ann Hematol. 62:16–21. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Montecucco C, Cazzola M and Ascari E:
Diabetes insipidus in the preleukaemic phase of acute
non-lymphocytic leukaemia. A monosomy 7-associated condition? Scand
J Haematol. 33:326–327. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piccin A, Raimondi R, Laspina S, Marchi M,
Rodeghiero F and Rovigatti U: Erythroleukaemia, diabetes insipidus
and hypophyseal damage: Two case reports. Leuk Res. 31:1135–1139.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lavabre-Bertrand T, Bourquard P, Chiesa J,
Berthéas MF, Lefort G, Taïb J, Lavabre-Bertrand C, Navarro M and
Bureau JP: Diabetes insipidus revealing acute myelogenous leukaemia
with a high platelet count, monosomy 7 and abnormalities of
chromosome 3: a new entity? Eur J Haematol. 66:66–69. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Breccia M, Petti MC, Ottaviani E, Mancini
M, D'Elia GM, Mecarocci S and Alimena G: Diabetes insipidus as
first manifestation of acute myeloid leukaemia with EVI-1-positive,
3q21q26 syndrome and T cell-line antigen expression: what is the
EVI-1 gene role? Br J Haematol. 118:438–441. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nussey SS, Ang VT, Bevan DH and Jenkins
JS: Human platelet arginine vasopressin. Clin Endocrinol (Oxf).
24:427–433. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dilek I, Uysal A, Demirer T, Koç H, Ozcan
M, Keleş H, Numaoğlu N, Ustün C and Ciftçi E: Acute myeloblastic
leukemia associated with hyperleukocytosis and diabetes insipidus.
Leuk Lymphoma. 30:657–660. 1998.PubMed/NCBI
|
|
21
|
Curley C, Kennedy G, Haughton A, Love A,
McCarthy C and Boyd A: Acute myeloid leukemia, the 3q21q26 syndrome
and diabetes insipidus: a case presentation. Asia Pac J Clin Oncol.
6:77–79. 2010. View Article : Google Scholar : PubMed/NCBI
|