Introduction
Chemotherapy has contributed to improvements in the
outcome of colorectal cancer (1,2), yet
the control of metastatic lesions is crucial for improving
outcomes. The metastatic mechanisms of colon cancer are still not
known in detail; however, previous studies by us and others have
identified the key molecules related to liver metastasis in colon
cancer (3–5), and pathological findings of budding
and venous invasion of tumors are known to be important in
predicting the outcome of colorectal cancer (6,7).
Clinically, 18F-fluorodeoxyglucose positron emission
tomography/computed tomography (18F-FDG-PET/CT) fusion
imaging is a useful tool for evaluating the stage, recurrence,
outcome, and effectiveness of treatment in human cancers (8–11).
Some studies have reported that PET/CT is useful in colon cancer
(12–14). However, PET/CT imaging has a
limitation in revealing hepatic metastasis due to normal uptake in
the liver (15).
Many studies have shown that in vivo studies
with subcutaneous xenografts in nude mice and severe combined
immunodeficient (SCID) mice are useful for analyzing human cancers
(16–19), whereas they are limited in
evaluating internal organ metastases associated with human cancers.
Immunodeficient mice commonly show poor metastatic lesions in
vivo. We reported that distant metastatic lesions were easily
reproduced by human cancer cell lines in newly developed
NOD/Shi-scid/IL-2Rγnull (NOG) mouse models
employing systemic injections (20–23).
It was confirmed that the experimental metastasis model of human
cancer using NOG mice was more sensitive and easier to achieve than
that using SCID mice due to their multiple immunological
dysfunctions not only in cytokine production capability, but also
in the functional competence of T, B and NK cells (24,25).
We previously developed a reliable new model for assaying hepatic
metastasis with superimmunodeficient NOG mice (26,27).
Some in vivo studies have evaluated
metastatic lesions of internal organs in mice with conventional
modalities such as bio-luminescence imaging (BLI), but difficulties
were found in revealing the mechanisms of metastasis (28). Therefore, a new in vivo
system to detect internal organ metastatic lesions is required to
simulate the clinical situation of metastatic lesions using
reliable and quantitative approaches.
In this study, we examined whether
18F-FDG-PET/CT scans could semi-quantitatively reveal
abnormal FDG uptakes in hepatic metastasis of human colon cancer
cell line HCT116 in NOG mice, thereby overcoming the above
described limitations encountered when evaluating metastatic
lesions in the liver. Moreover, liver metastasis in NOG mice was
studied by histopathological analysis. We showed here that the
evaluation of metastatic lesions in NOG mice by PET/CT may be an
extremely useful in vivo metastatic model.
Materials and methods
Cell culture
The HCT116 cell line was obtained from the American
Type Culture Collection (Manassas, VA, USA) and maintained in
McCoy’s 5A medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented
with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin
and 100 μg/ml streptomycin. Cells were incubated in a humidified
(37°C, 5% CO2) incubator and passaged on reaching 80%
confluence.
In vivo transplantation of human colon
cancer cell line, HCT116
NOG mice (9–12 weeks of age, male) were maintained
at the specific pathogen-free facilities of the Central Institute
for Experimental Animals (CIEA, Kawasaki, Japan). Experimental
liver metastases using NOG mice were generated by intrasplenic
injection of HCT116 cells (1.0×105 and
1.0×106/mouse, n=2) and splenectomy. All experiments
involving laboratory animals were performed in accordance with the
care and use guidelines of the CIEA, according to our previous
reports (26,27).
Whole-body imaging of NOG mice with
18F-FDG-PET and CT scans
Positron-emitting fluorine-18 (18F) was
produced by 18O(p, n) 18F nuclear reaction
using a cyclotron (HM-18; Sumitomo Heavy Industry, Osaka, Japan) at
Hamamatsu Photonics PET Center. 18F-FDG-PET and CT scans
were performed in NOG mice 14 days after transplantation. The
production of 18F-FDG was carried out according to a
method described elsewhere (29).
The kinetics and distribution patterns of the radiolabeled compound
were determined with a small animal PET scanner (ClairvivoPET;
Shimadzu Corp., Kyoto, Japan). This scanner consists of depth of
interaction (DOI) detector modules with an axial field of view
(FOV) of 151 mm, a transaxial FOV of 102 mm, and a transaxial
spatial resolution of 1.54 mm in the center (30). Anesthetized mice were placed in a
prone position on a fixation plate and then placed in the gantry
hole of the PET scanner. After transmission measurement with an
external 137Cs point source (22 MBq) for attenuation
correction, 18F-FDG at a dose of 6 MBq (0.2 ml) was
injected intravenously into each mouse via the tail vein. Data were
acquired in list-mode format for 60 min; full 3D sinograms with
corrected efficiency, scattering, attenuation, count losses and
decay were reconstructed using an iterative 3D dynamic raw-action
maximum likelihood algorithm (Drama). Summation images from 40 to
60 min after 18F-FDG injection were reconstructed, and
average and maximum values of the standardized uptake (SUVmean,
SUVmax) were semi-quantitatively calculated in various organs of
NOG mice. After PET scanning, a CT scan was performed with
ClairvivoCT (Shimadzu Corp.) in each NOG mouse.
Macroscopic and microscopic
examinations
Mice were autopsied after PET/CT scan examinations
to evaluate liver metastases. These metastatic lesions were also
histologically confirmed. Immunohistochemistry was carried out on 4
μm tissue sections using the Bond Polymer Refine Detection system
(Leica Microsystems, Tokyo, Japan) according to the manufacturer’s
instructions with minor modifications. In brief, 4 μm sections of
formalin-fixed, paraffin-embedded tissues were deparaffinized by
Bond Dewax Solution (Leica Microsystems) and an antigen retrieval
procedure was carried out using Bond ER solution (Leica
Microsystems) for 30 min at 100°C. Endogenous peroxidases were
quenched by incubation with hydrogen peroxide for 5 min. Sections
were incubated for 30 min at ambient temperate with primary
monoclonal antibodies for anti-HLA class 1-A, B, C (Hokudo,
Sapporo, Japan) using the biotin-free polymeric horseradish
peroxidase (HRP)-linker antibody conjugate system in a Bond-Max
automatic slide stainer (Leica Microsystems). Nuclei were
counterstained with hematoxylin.
Statistical analysis
Statistical comparisons of data sets were analyzed
by a one-way factorial ANOVA. Data are shown as means ± standard
error of mean (SEM). These analyses were performed using JMP
version 8 software (SAS Institute, Inc., Cary, NC, USA). P-values
<0.05 were considered to indicate statistically significant
differences.
Results
18F-FDG-PET findings in
control NOG mice
18F-FDG- PET/CT showed intense FDG
uptakes in the brain (SUVmean 1.519±0.083, SUVmax 2.140±0.127),
heart (SUVmean 1.343±0.035, SUVmax 1.868±0.016), kidney (SUVmean
2.163±0.125, SUVmax 3.148±0.150) and bladder (SUVmean 32.986±3.590,
SUVmax 49.692±8.152) of control NOG mice (Table I, Fig.
1).
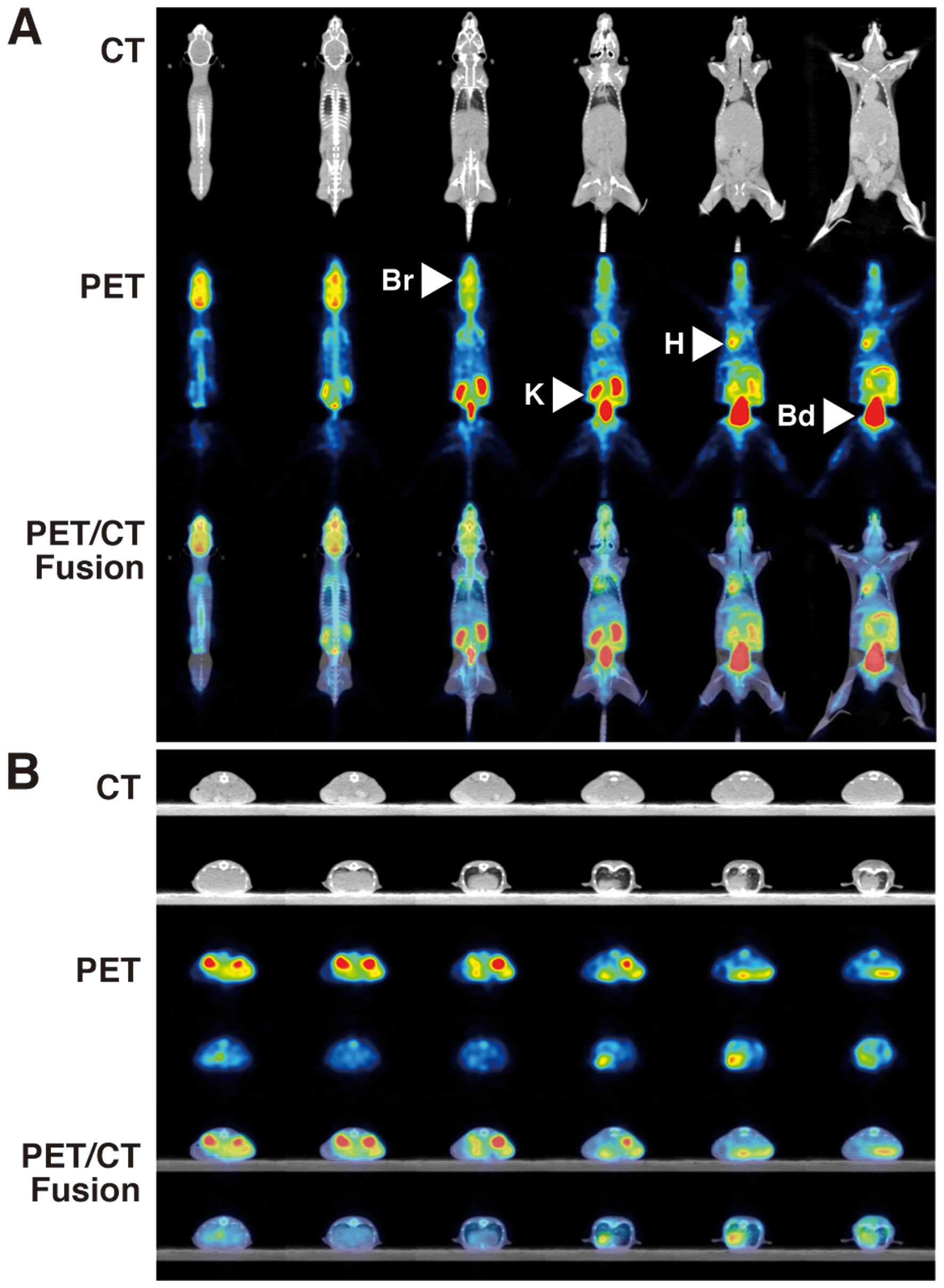 | Figure 1PET and CT findings in control NOG
mice. FDG-PET/CT shows intense FDG uptakes in the brain (Br, SUVmax
2.140±0.127), heart (H, SUVmax 1.868±0.016), kidney (K, SUVmax
3.148±0.150) and bladder (Bd, SUVmax 49.692±8.152) of control NOG
mice. (A) Coronal view, (B) axial view. Upper panels, CT; middle
panels, PET; lower panels, PET/CT fusion. |
 | Table ISUV values in the various organs of
control NOG mice by 18F-FDG-PET/CT. |
Table I
SUV values in the various organs of
control NOG mice by 18F-FDG-PET/CT.
| SUVmean | SUVmax |
|---|
| Brain | 1.519±0.083 | 2.140±0.127 |
| Heart | 1.343±0.035 | 1.868±0.016 |
| Kidney | 2.163±0.125 | 3.148±0.150 |
| Bladder | 32.986±3.590 | 49.692±8.152 |
| Lung | 0.577±0.064 | 1.384±0.104 |
| Liver | 0.450±0.033 | 0.635±0.017 |
| Muscle | 0.151±0.011 | 0.327±0.040 |
| Bone | 0.214±0.013 | 0.476±0.034 |
| Intestine | 0.824±0.033 | 2.061±0.111 |
| Testis | 0.510±0.022 | 1.212±0.335 |
18F-FDG-PET and CT findings of
tumor metastasis in NOG mice
18F-FDG-PET/CT showed higher multiple FDG
uptakes (SUVmean 0.853±0.087, SUVmax 1.254±0.237) in the livers of
NOG mice transplanted with 1.0×105 cells of the HCT116
cell line (Table II, Fig. 2) than those of control NOG mice
(SUVmean 0.450±0.033, SUVmax 0.635±0.017). CT scans also showed
marked liver swelling in NOG mice transplanted with
1.0×106 cells of HCT116 (Fig. 3). PET/CT showed diffuse higher FDG
uptakes (SUVmean 1.211±0.108, SUVmax 1.701±0.158) in the livers of
NOG mice with injection of 1.0×106 HCT116 cells than FDG
uptakes with 1.0×105 cells. There were significant
differences in FDG uptakes between the three groups (ANOVA, P=0.017
in SUVmean, P=0.044 in SUVmax). No other organs with abnormal FDG
uptake were noted besides that of the livers of NOG mice
transplanted with 1.0×105 and 1.0×106 cells
of HCT116.
 | Table IISUV values in the livers of NOG mice
by 18F-FDG- PET/CT. |
Table II
SUV values in the livers of NOG mice
by 18F-FDG- PET/CT.
| SUVmean | SUVmax |
|---|
| Control |
0.450±0.033a |
0.635±0.017b |
| 1.0×105
cells of HCT116 |
0.853±0.087a |
1.254±0.237b |
| 1.0×106
cells of HCT116 |
1.211±0.108a |
1.701±0.158b |
In vivo liver metastasis
Experimental liver metastases, which consisted of
solid white masses, were detected in all livers of NOG mice
transplanted with 1.0×105 and 1.0×106 cells
of the HCT116 cell line. Metastatic hepatomegaly transplanted with
1.0×106 cells was more severe than that with
1.0×105 cells (Fig. 4,
H&E). It was also immunohistochemically confirmed that
there were obviously more metastatic foci in the livers of NOG mice
transplanted with 1.0×106 than in mice transplanted with
1.0×105 HTC116 cells (Fig.
4, HLA).
Discussion
In this study, we clearly and quantitatively
demonstrated increased multiple 18F-FDG uptakes in
hepatic metastases of human colonic cancer cell line HCT116 in NOG
mouse models using a small animal PET/CT system. We previously
reported that 18F-FDG-PET/CT clinically is a very useful
imaging modality to evaluate human cancers, including rare
carcinoma cases (31–34). Many reports have shown that animal
PET imaging is useful for evaluating human cancer xenografts in
vivo. It is usually difficult to reveal metastases as
conventional in vivo models are limited in their evaluation
of metastases associated with human cancers (28,35).
It is easy to produce experimental liver metastases in
immunocompromised NOG mice because of their multiple immunological
dysfunctions in cytokine production capability as well as
functional competence of T, B and NK cells (24–27).
However, quantitative evaluation of experimental liver metastases
is still being developed. We previously reported the use of liver
weight as an index of metastatic hepatomegaly or microvessel counts
to reflect tumor cell activity (23,36).
We present herein that the model system with NOG mice and a small
animal PET/CT system has the feasibility of a quantitative
metastasis model to simulate clinical situations. Several studies
have reported the development of new anticancer therapies and novel
drugs for human cancers using animal PET (37,38).
This model system presented here with NOG mice and a small animal
PET/CT system may be useful for developing new anticancer therapies
and novel drugs for hepatic metastases of human cancers.
Although PET imaging with 18F-FDG is
routinely used for tumor detection and therapy monitoring, several
limitations have been reported with 18F-FDG, such as
high uptake in normal tissues of the brain and inflammatory tissues
(15,39). In contrast, positron-labeled amino
acids and their analogs are considered to be useful because of the
low rate of amino acid use in normal control tissues. Natural and
unnatural artificial labeled amino acids have been reported
(40,41). This hepatic metastasis model, using
intrasplenic injection of small numbers of cancer cells into NOG
mice, is reliable and quantitative, and more closely mimics in
vivo conditions (26). New
compounds, which are more sensitive and specific for the detection
of human cancers, are expected to replace 18F-FDG
(42,43). The hepatic metastasis model system
in NOG mice combined with small animal PET/CT analysis will be
useful in developing new labeling compounds for PET.
In conclusion, the hepatic metastasis model system
in NOG mice combined with PET/CT has the feasibility to simulate
clinical situations, and this model system is useful for analyzing
mechanisms of metastasis and developing new therapeutic approaches
for metastatic lesions of human cancers.
Acknowledgements
We are grateful to Hiroshi Iwata, Takeharu Kaiuchi,
Norihiro Harada and Dai Fukumoto for their technical assistance and
helpful discussions.
References
|
1
|
O’Connor ES, Greenblatt DY, LoConte NK, et
al: Adjuvant chemotherapy for stage II colon cancer with poor
prognostic features. J Clin Oncol. 29:3381–3388. 2011.PubMed/NCBI
|
|
2
|
Karoui M, Roudot-Thoraval F, Mesli F, et
al: Primary colectomy in patients with stage IV colon cancer and
unresectable distant metastases improves overall survival: results
of a multicentric study. Dis Colon Rectum. 54:930–938. 2011.
View Article : Google Scholar
|
|
3
|
Tokunaga T, Oshika Y, Abe Y, et al:
Vascular endothelial growth factor (VEGF) mRNA isoform expression
pattern is correlated with liver metastasis and poor prognosis in
colon cancer. Br J Cancer. 77:998–1002. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tokunaga T, Nakamura M, Oshika Y, et al:
Thrombospondin 2 expression is correlated with inhibition of
angiogenesis and metastasis of colon cancer. Br J Cancer.
79:354–359. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun L, Hu H, Peng L, et al: P-cadherin
promotes liver metastasis and is associated with poor prognosis in
colon cancer. Am J Pathol. 179:380–390. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanaka M, Hashiguchi Y, Ueno H, Hase K and
Mochizuki H: Tumor budding at the invasive margin can predict
patients at high risk of recurrence after curative surgery for
stage II, T3 colon cancer. Dis Colon Rectum. 46:1054–1059. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sato T, Ueno H, Mochizuki H, et al:
Objective criteria for the grading of venous invasion in colorectal
cancer. Am J Surg Pathol. 34:454–462. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cronin CG, Swords R, Truong MT, et al:
Clinical utility of PET/CT in lymphoma. Am J Roentgenol.
194:W91–W103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ueda S, Saeki T, Shigekawa T, et al:
18F-fluorodeoxyglucose positron emission tomography
optimizes neoadjuvant chemotherapy for primary breast cancer to
achieve pathological complete response. Int J Clin Oncol.
17:276–282. 2012. View Article : Google Scholar
|
|
10
|
Xie L, Saynak M, Veeramachaneni NK, et al:
Non-small cell lung cancer: prognostic importance of positive FDG
PET findings in the mediastinum for patients with N0–N1 disease at
pathologic analysis. Radiology. 261:226–234. 2011.PubMed/NCBI
|
|
11
|
Abe Y, Tamura K, Sakata I, et al: Clinical
implications of 18F-fluorodeoxyglucose positron emission
tomography/computed tomography at delayed phase for diagnosis and
prognosis of malignant pleural mesothelioma. Oncol Rep. 27:333–338.
2012.
|
|
12
|
Mori S and Oguchi K: Application of
(18)F-fluorodeoxyglucose positron emission tomography to detection
of proximal lesions of obstructive colorectal cancer. Jpn J Radiol.
28:584–590. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ducreux M and Dromain C: Non-invasive
imaging tools in colorectal cancer. Rev Prat. 60:1071–1073.
2010.(In French).
|
|
14
|
Treglia G, Calcagni ML, Rufini V, et al:
Clinical significance of incidental focal colorectal
(18)F-fluorodeoxyglucose uptake: our experience and a review of the
literature. Colorectal Dis. 14:174–180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kubota R, Kubota K, Yamada S, Tada M, Ido
T and Tamahashi N: Microautoradiographic study for the
differentiation of intratumoral macrophages, granulation tissues
and cancer cells by the dynamics of fluorine-18-fluorodeoxyglucose
uptake. J Nucl Med. 35:104–112. 1994.
|
|
16
|
Abe Y, Nakamura M, Ohnishi Y, Inaba M,
Ueyama Y and Tamaoki N: Multidrug resistance gene (MDR1) expression
in human tumor xenografts. Int J Oncol. 5:1285–1292.
1994.PubMed/NCBI
|
|
17
|
Abe Y, Ohnishi Y, Yoshimura M, et al:
P-glycoprotein-mediated acquired multidrug resistance of human lung
cancer cells in vivo. Br J Cancer. 74:1929–1934. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suto R, Abe Y, Nakamura M, et al:
P-glycoprotein-mediated acquired multidrug resistance of human
osteosarcoma xenografts in vivo. Int J Oncol. 12:287–291.
1998.
|
|
19
|
Fujimori S, Abe Y, Nishi M, et al: The
subunits of glutamate cysteine ligase enhance cisplatin resistance
in human non-small cell lung cancer xenografts in vivo. Int
J Oncol. 25:413–418. 2004.PubMed/NCBI
|
|
20
|
Miyakawa Y, Ohnishi Y, Tomisawa M, et al:
Establishment of a new model of human multiple myeloma using
NOD/SCID/gamma(c)(null) (NOG) mice. Biochem Biophys Res Commun.
313:258–262. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ikoma N, Yamazaki H, Abe Y, et al: S100A4
expression with reduced E-cadherin expression predicts distant
metastasis of human malignant melanoma cell lines in the
NOD/SCID/γCnull (NOG) mouse model. Oncol Rep.
14:633–637. 2005.PubMed/NCBI
|
|
22
|
Hamada K, Monnai M, Kawai K, et al: Liver
metastasis models of colon cancer for evaluation of drug efficacy
using NOD/Shi-scid IL2Rγnull (NOG) mice. Int J Oncol.
32:153–159. 2008.PubMed/NCBI
|
|
23
|
Chijiwa T, Abe Y, Ikoma N, et al:
Thrombospondin 2 inhibits metastasis of human malignant melanoma
through microenvironment-modification in
NOD/SCID/γCnull (NOG) mice. Int J Oncol.
34:5–13. 2009.PubMed/NCBI
|
|
24
|
Ito M, Hiramatsu H, Kobayashi K, et al:
NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model
for engraftment of human cells. Blood. 100:3175–3182. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hiramatsu H, Nishikomori R, Heike T, et
al: Complete reconstitution of human lymphocytes from cord blood
CD34+ cells using the NOD/SCID/gamma(c)(null) mice
model. Blood. 102:873–880. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suemizu H, Monnai M, Ohnishi Y, Ito M,
Tamaoki N and Nakamura M: Identification of a key molecular
regulator of liver metastasis in human pancreatic carcinoma using a
novel quantitative model of metastasis in
NOD/SCID/γcnull (NOG) mice. Int J Oncol.
31:741–751. 2007.PubMed/NCBI
|
|
27
|
Kubo A, Ohmura M, Wakui M, et al:
Semi-quantitative analyses of metabolic systems of human colon
cancer metastatic xenografts in livers of superimmunodeficient NOG
mice. Anal Bioanal Chem. 400:1895–1904. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Deroose CM, De A, Loening AM, et al:
Multimodality imaging of tumor xenografts and metastases in mice
with combined small-animal PET, small-animal CT, and
bioluminescence imaging. J Nucl Med. 48:295–303. 2007.PubMed/NCBI
|
|
29
|
Oberdorfer F, Hull WE, Traving BC and
Maier-Borst W: Synthesis and purification of
2-deoxy-2-[18F]fluoro-D-glucose and
2-deoxy-2-[18F]fluoro-D-mannose: characterization of products by
1H- and 19F-NMR spectroscopy. Int J Rad Appl Instrum A. 37:695–701.
1986.
|
|
30
|
Mizuta T, Kitamura K, Iwata H, et al:
Performance evaluation of a high-sensitivity large-aperture
small-animal PET scanner: ClairvivoPET. Ann Nucl Med. 22:447–455.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ueda S, Tsuda H, Asakawa H, et al:
Clinicopathological and prognostic relevance of uptake level using
18F-fluorodeoxyglucose positron emission tomography/computed
tomography fusion imaging (18F-FDG PET/CT) in primary breast
cancer. Jpn J Clin Oncol. 38:250–258. 2008. View Article : Google Scholar
|
|
32
|
Abe Y, Tamura K, Sakata I, et al:
Usefulness of 18F-FDG positron emission
tomography/computed tomography for the diagnosis of
pyothorax-associated lymphoma: a report of three cases. Oncol Lett.
1:833–836. 2010.
|
|
33
|
Abe Y, Tamura K, Sakata I, et al: Unique
intense uptake demonstrated by 18F-FDG positron emission
tomography/computed tomography in primary pancreatic lymphoma: a
case report. Oncol Lett. 1:605–607. 2010.PubMed/NCBI
|
|
34
|
Ozeki Y, Abe Y, Kita H, et al: A case of
primary lung cancer lesion demonstrated by F-18 FDG positron
emission tomography/computed tomography (PET/CT) one year after the
detection of metastatic brain tumor. Oncol Lett. 2:621–623.
2011.
|
|
35
|
Takamiya Y, Abe Y, Tanaka Y, et al: Murine
P-glycoprotein on stromal vessels mediates multidrug resistance in
intracerebral human glioma xenografts. Br J Cancer. 76:445–450.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hatanaka H, Oshika Y, Abe Y, et al:
Vascularization is decreased in pulmonary adenocarcinoma expressing
brain-specific angiogenesis inhibitor 1 (BAI1). Int J Mol Med.
5:181–183. 2000.PubMed/NCBI
|
|
37
|
Pantaleo MA, Nicoletti G, Nanni C, et al:
Preclinical evaluation of KIT/PDGFRA and mTOR inhibitors in
gastrointestinal stromal tumors using small animal FDG PET. J Exp
Clin Cancer Res. 29:1732010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Moroz MA, Kochetkov T, Cai S, et al:
Imaging colon cancer response following treatment with AZD1152: a
preclinical analysis of [18F]fluoro-2-deoxyglucose and
3′-deoxy-3′-[18F]fluorothymidine imaging. Clin Cancer Res.
17:1099–1110. 2011.PubMed/NCBI
|
|
39
|
Kubota K, Nakamoto Y, Tamaki N, et al:
FDG-PET for the diagnosis of fever of unknown origin: a Japanese
multi-center study. Ann Nucl Med. 25:355–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tsukada H, Sato K, Fukumoto D, Nishiyama
S, Harada N and Kakiuchi T: Evaluation of D-isomers of O-11C-methyl
tyrosine and O-18F-fluoromethyl tyrosine as tumor-imaging agents in
tumor-bearing mice: comparison with L- and D-11C-methionine. J Nucl
Med. 47:679–688. 2006.PubMed/NCBI
|
|
41
|
Tsukada H, Sato K, Fukumoto D and Kakiuchi
T: Evaluation of D-isomers of O-18F-fluoromethyl, O-18F-fluoroethyl
and O-18F-fluoropropyl tyrosine as tumour imaging agents in mice.
Eur J Nucl Med Mol Imaging. 33:1017–1024. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Perk LR, Stigter-van Walsum M, Visser GW,
et al: Quantitative PET imaging of Met-expressing human cancer
xenografts with 89Zr-labelled monoclonal antibody DN30. Eur J Nucl
Med Mol Imaging. 35:1857–1867. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang H, Liu B, Tian JH, et al: Monitoring
early responses to irradiation with dual-tracer micro-PET in
dual-tumor bearing mice. World J Gastroenterol. 16:5416–5423. 2010.
View Article : Google Scholar : PubMed/NCBI
|


















