Introduction
Osteosarcoma is the most prevalent primary malignant
bone tumor in children and young adults, and is characterized by
aggressive invasion, early metastasis and resistance to existing
chemotherapeutics (1). In recent
years, the survival rate of osteosarcoma patients has improved due
to advances in aggressive systemic chemotherapy. However, the
survival rate of osteosarcoma patients is still low for those with
primary metastases and relapse compared to patients with localized
disease (2,3). Moreover, multidrug combination
chemotherapy for osteosarcoma leads to ototoxicity, cardiac
toxicity and secondary malignancies (2). Thus, it is vital to identify novel
approaches for both diagnosis and treatment that are more efficient
than the currently available methods to resolve these problems and
improve the prognosis of osteosarcoma (4). An understanding of the molecular
events that drive the progression and metastasis of osteosarcoma
would facilitate better diagnosis and treatment strategies.
Recently, NADP+-dependent isocitrate
dehydrogenases (IDHs), including IDH1 and IDH2, were found to be
down-regulated in glioma, melanoma and bladder cancer (5–8). By
providing NADPH, IDHs play an important role in controlling the
mitochondrial redox balance and mitigating cellular oxidative
damage (9,10). In our previous study, IDH1 was shown
to be a tumor-suppressor gene in osteosarcoma and inhibited the
malignant progression of osteosarcoma (11,12).
However, the role of IDH2 in osteosarcoma remains unknown.
IDH2, a mitochondrial NADP-dependent enzyme,
catalyzes oxidative decarboxylation and produces CO2,
NADPH and α-ketoglutarate from isocitrate in the mitochondria
(13). NADPH is a vital cofactor
for many enzymatic reactions, including fat and cholesterol
biosynthesis and glutathione metabolism (14). It was demonstrated that
mitochondrial NADP+-dependent isocitrate dehydrogenase
plays an important role in cellular defense against oxidative
damage by providing NADPH, which is needed to regenerate the
glutathione levels in mitochondria (15–17).
Reactive oxygen species (ROS), which are produced in mitochondria
as a natural by-product of normal energy metabolism, are involved
in over 150 human disorders (18).
Increased ROS levels promote cellular oxidative stress that
contributes to various processes in malignant tumors, including
carcinogenesis, aberrant growth, angiogenesis and metastasis
(19,20).
Tumor cell growth and invasion are important in
malignant progression and are regulated by many biological
regulators. One such regulator is NF-κB that regulates the
expression of various genes involved in immunity, stress responses,
inflammation and inhibition of apoptosis, thus providing
appropriate conditions for tumor cell progression (21,22).
NF-κB is constitutively activated in tumor cells, including
osteosarcoma cells, and contributes to maintain the highly
proliferative malignant phenotype as well as cellular invasion
(23,24). NF-κB regulates several
metastasis-related matrix metalloproteinases (MMPs), such as MMP1,
MMP3 and MMP-9 (25–29). MMP-9 is recognized as a classic
invasion- or metastasis-related NF-κB target gene and is described
as an important biomarker that is directly associated with the
metastatic processes in osteosarcoma (11,30,31).
In the present study, we first investigated the
expression and significance of IDH2 in osteosarcoma biopsies in
vivo. Next, we studied the role of IDH2 downregulation in
vitro in the Saos-2 and MG-63 human osteosarcoma cell lines.
Furthermore, we studied the related biological mechanisms that were
induced by IDH2 downregulation in osteosarcoma cells.
Materials and methods
Tissue specimens and clinical data
Fifty-one formalin-fixed, paraffin-embedded
osteosarcoma specimens (before the administration of neo-adjuvant
chemotherapy) were collected according to the Chinese National
Ethical Guidelines ('Code for Proper Secondary Use of Human
Tissue', Chinese Federation of Medical Scientific Societies). Due
to the limited available tumor material and follow-up information,
only 44 of these osteosarcoma tumor samples were eligible for the
present study. The mean age of the patients (mean ± SD) was
25.25±13.61 years (range 9–61; male/female ratio 32:12). Of the 44
eligible patients, 23 had non-metastatic tumors (53.3%).
Osteoblastic osteosarcoma was the most common histopathological
subtype and occurred in 29 patients (65.9%). The lower end of the
femur was the most common site and was observed in 13 patients
(29.5%). The patient distribution according to Rosen's histological
grade (19,20) included stage I in 5 (11.3%), II in
16 (36.4%), III in 16 (36.4%) and stage IV in 7 patients (15.9%).
Stages I and II were defined as low Rosen grade osteosarcoma
specimens and stages III and IV were defined as high grade
specimens. The patients were followed-up until death from the
disease, or until their most recent clinical therapy at the end of
the present study. The mean follow-up time (mean ± SD) was
4.26±1.99 years (range 0.5–9.0). All patients were diagnosed
according to the osteosarcoma criteria defined by the World Health
Organization. Written informed consent was obtained from each
patient before he/she entered into the present study, and all study
protocols were approved by the Ethics Committee for Clinical
Research of Wuhan University, China.
Immunohistochemistry and specimen
evaluation
The sections were cut from the formalin-fixed,
paraffin-embedded osteosarcoma tissue and hydrated through graded
alcohol solutions. For antigen unmasking, the sections were treated
in a trypsin solution at 37°C for 10 min. The sections were then
washed with deionized water and incubated with 3%
H2O2 for 5 min. They were then incubated with
the anti-IDH2 mAb at room temperature for 1 h, followed by a
secondary antibody and the peroxidase-conjugated
streptavidin-biotin complex (both from Santa Cruz Biotechnology,
Santa Cruz, CA, USA) at 37°C for 30 min. The immunoreactivity was
visualized with 3,3′-diaminobenzidine (DAB) (Zymed, South San
Francisco, CA, USA). The negative controls were obtained by
omitting the primary antibody.
IDH2 staining was detected in the mitochondria and
was scored by adding the number of cells displaying clear tumor
cell labeling; the intensity of staining was scored between 0 and 6
(32,33). The proportion score was as follows:
0 indicates negative staining; +1 indicates ≤25% positive labeling
in tumor cells; +2 indicates 25–50% positive tumor cells; and +3
indicates >50% positive tumor cells. The intensity score was as
follows: 0 indicates no staining; +1 indicates weak staining; +2
indicates intermediate staining; and +3 indicates strong staining
(32,33). For statistical analysis, the
osteosarcoma patients were grouped as either the low expression
group (scored 1–4) or the high expression group (scored 5–6). At
least 5 separated neoplastic infiltration foci were analyzed in
each specimen, followed by the evaluation of 10 slides/patient and
6 sections/slide. The immunostaining was assessed by three
independent observers. The slides were scanned using a microscope
(Carl Zeiss AG, Germany) by reviewing the entire spot and the
images were recorded using a digital camera (DC500; Leica) and
Leica FW4000 software. The images were processed using Adobe
Photoshop.
Cell lines, culture and lentiviral
infection
The Saos-2 and MG63 tumor cells (ATCC, LGC
Promochem, Germany) were grown in Roswell Park Memorial Institute
(RPMI)-1640 medium (Sigma-Aldrich, USA) with 10% fetal bovine serum
(FBS) (Amresco, USA) and 0.1% penicillin/streptomycin and
maintained in an atmosphere with 5% CO2 at 37°C.
To downregulate IDH2, small interfering RNA (siRNA)
sequences: LV-KD, 5′-GTGGACATCCAGCTAAAGTAT-3′, were inserted into
the pLL3.7 shRNA lentiviral vector (Genesil, Wuhan, China). The
pLL3.7 lentiviral vector LV-mIκB (Genesil), which was aimed at
overexpressing the mutant IκB (mIκB), was constructed as previously
described to suppress NF-κB activity (13). siRNA sequences for MMP-9
downregulation were: 5′-ACCACAACAUCACCUAUUGTT-3′ (34). The empty lentiviral vector
(Genesil), LV-EV was used as a control. In some experiments,
non-treated cells, named the NT cells, were used as another
control. The lentiviral stocks were added to the Saos-2 and MG63
osteosarcoma cell lines.
The cells were infected with the lentivirus and
selected with an 800 μg/ml G418 solution for the Saos-2
cells and a 500 μg/ml solution for the MG63 cells. The
efficiency of the highest infection, as determined by G418
selection, was obtained at a multiplicity of infection (MOI) of 10
for the Saos-2 cells and 50 for the MG63 cells. The cells
transfected with LV-KD or LV-EV were named the KD or EV cells,
respectively. After selection, the efficiency of infection was
verified by western blotting. Polyclonal populations and clones
displaying higher levels of IDH2 downregulation were chosen for the
subsequent studies. After IDH2 downregulation, LV-mIκB or
LV-siMMP-9 was transfected into the cells for the NF-κB and MMP-9
assays, respectively.
Protein isolation and western blot
analysis
The cell lysates were prepared using lysis buffer
from the Dual-Luciferase assay kit (Promega, Madison, WI, USA)
according to the manufacturer's instructions. The lysates were
separated on 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) gels and transferred onto polyvinylidene
difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The
membranes were then blocked with 5% skim milk in Tris-buffered
saline with 0.05% Tween (TBST) and washed 6 times with TBST. The
IDH2 and MMP-9 proteins were detected using rabbit polyclonal
primary antibodies (Protein Technology Group, USA). NF-κB, Bcl-2,
JNK, p-JNK, ERK, p-ERK, IκBα and p-IκBα were detected by mouse
monoclonal primary antibodies (Santa Cruz Biotechnology). The
β-actin proteins were recognized by a monoclonal β-actin-specific
mouse IgG (Santa Cruz Biotechnology) and used as the internal
loading control. The antibodies were diluted according to the
manufacturer's instructions and incubated overnight at 4°C,
followed by incubations with the peroxidase-conjugated goat
anti-rabbit or anti-mouse immunoglobulins (1:2,000; Santa Cruz
Biotechnology) in TBST for 1 h. The signals were developed using an
enhanced chemiluminescent reagent (Pierce Biotechnology, Rockford,
IL, USA).
MTT assay
A total of 3.5×103 cells were seeded in
each test well in a 96-well plate to detect cell growth. After 1–6
days of culture, the cells were washed with phosphate-buffered
saline (PBS). MTT (5 mg/ml) was then added to each well (including
the control) and the mixture was incubated at 37°C for 4 h. The
culture medium was then replaced with an equal volume of
dimethylsulfoxide (DMSO). After shaking the plate at room
temperature for 10 min, the absorbance of each well was determined
at 570 nm using a VersaMax microplate reader (Molecular Devices,
Sunnyvale, CA, USA).
Cell cycle analysis with flow
cytometry
The cells were harvested by trypsinization, fixed
with 70% pre-chilled alcohol and stored at 4°C overnight. The
alcohol was then removed by centrifugation at 1,000 rpm for 5 min,
and the cells were treated with 0.1% Triton-X and DNase-free RNase
(10 mg/ml) for 30 min (35). The
cell DNA was stained with 1 mg/ml propidium iodide (PI) for 15 min
in the dark and analyzed using a flow cytometer (FACScan;
Becton-Dickinson, New York, NY, USA). The relative proportions of
cells in the G0–G1, S and G2-M phases of the cell cycle were
determined from the flow cytometry data.
Cell invasion assay
Cell invasion was determined using a two-chamber
Transwell (Corning, New York, NY, USA). The upper surface of a
polycarbonate membrane with 8-μm pores was coated with 1
mg/ml Matrigel (36). The cells
(~10×105) were suspended in RPMI-1640 serum-free medium
(Gibco, USA) and placed in the upper chamber; RPMI-1640 medium
containing 10% FBS was placed in the lower chamber. The cells were
incubated at 37°C for 48 h with 5% CO2 in an incubator.
At the end of the incubation, the cells on the upper surface of the
membrane were completely removed by wiping with a cotton swab.
Then, the membrane was fixed with methanol and stained with 0.1%
crystal violet. The cells that invaded the Matrigel and reached the
lower surface of the membrane were counted and photographed under a
microscope.
NF-κB activity assay
An NF-κB reporter plasmid, NF-κB-Luc, was
constructed by cloning five repeats of the NF-κB regulatory
elements into the pLuc plasmid (Stratagene, La Jolla, CA, USA) to
drive luciferase expression. The empty plasmid, Luc, containing a
minimal TATA box, was used as a negative control. Approximately
2.0×106 cells were transiently transfected with 10
μg of the NF-κB reporter plasmid by electroporation
(37). Then, the cells were seeded
and incubated in 24-well plates at 37°C. After 24 h, the luciferase
activity was analyzed using the Luciferase assay system (Promega)
according to the manufacturer's instructions.
Intracellular ROS assay
To obtain dissociated Saos2 and MG63 cells for the
ROS assay, the culture medium was first removed and the cells were
washed two times with RPMI-1640 serum-free medium (Sigma-Aldrich).
DCFH-DA, diluted to a final concentration of 10 μM with
RPMI-1640 medium, was added to the medium and incubated for 20 min
at 37°C. The fluorescence was read at 488 nm for excitation and 530
nm for emission by flow cytometry (FACScan). The increase in the
value compared to the control was viewed as the increase in
intracellular ROS levels.
MMP-9 activity assay
The IDH2 and/or MMP-9 down-regulated osteosarcoma
cells were seeded in 6-well plates and incubated at 37°C. After 24
h, the medium was removed. Then, the cells were washed and
incubated in serum-free medium for 48 h (37). The MMP-9 activity in the medium and
cell lysate was detected using the Fluorokine E Human MMP-9
Activity assay kit (R&D Systems) according to the
manufacturer's protocol.
Statistical analysis
The statistical analyses were performed using the
SPSS 13.0 software package for Windows (SPSS, Inc., Chicago, IL,
USA). Comparisons between groups were analyzed by the t-test or
Mann-Whitney U test. Associations were assessed by Pearson's or
Spearman's correlation coefficients. Event-free survival was
calculated from the start of treatment to relapse or metastasis.
Overall survival was calculated from the beginning of treatment to
the last follow-up or death of the patient. The Kaplan-Meier method
was used for the survival analysis. P<0.05 was considered to
indicate a statistically significant result. P<0.01 was
considered to indicate a highly statistically significant
result.
Results
In vivo tissues
IDH2 correlates with Rosen's
histological grade and metastasis in osteosarcoma
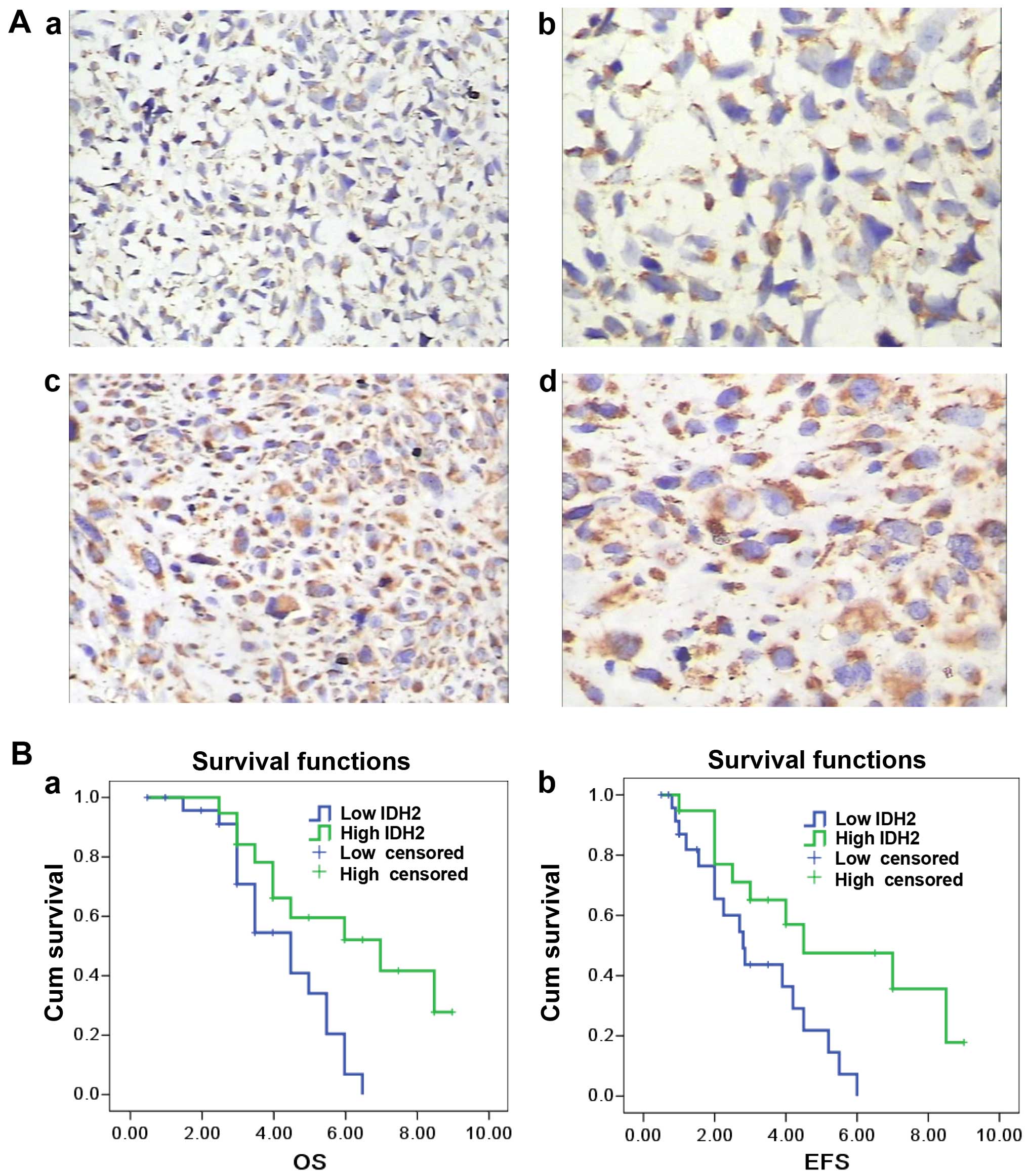
IDH2 was mainly found in the mitochondria (Fig. 1A). Of the 44 osteosarcoma specimens,
41 cases were IDH2-positive using immunohistochemistry (93.2%) and
19 exhibited high levels of staining (43.1%). The average IDH2
immunostaining score was 3.15 (SD, 1.46; range from 0 to 6). Lower
IDH2 expression was observed in high Rosen's histological grade
(38,39) osteosarcoma compared to the low grade
specimens (P=0.005; r=−0.505) (Fig.
1A). IDH2 expression was inversely correlated with metastasis
(P=0.026; r=−0.334). There was no significant correlation between
IDH2 expression and overall survival (P=0.063) or event-free
(relapse and metastasis included) survival (P=0.074) (Fig. 1B). IDH2 expression did not correlate
with other clinical features, such as age, location of the primary
tumor and histological type (P>0.05).
In vitro cell lines
IDH2 downregulation promotes cell
proliferation
IDH2 downregulation was verified by western
blotting. The IDH2 protein was significantly decreased in the
Saos-2 KD cells compared to the EV or NT cells (P<0.01)
(Fig. 2A), respectively; similar
results were obtained in the MG63 cells (Fig. 2A). There was no difference in IDH2
expression between the NT and EV cells, either in the Saos-2 or
MG63 cells (P>0.05) (Fig.
2A).
IDH2 downregulation increased the cell growth rate
in the Saos-2 KD cells by 1.7-fold and in the MG63 KD cells by
1.5-fold on day 6 compared to the Saos-2 EV or MG-63 EV cells
(P<0.01) (Fig. 2B). IDH2
downregulation promoted the growth of osteosarcoma cells.
IDH2 downregulation decreases the
distribution of cells in the S phase and increases the distribution
in the G2/M phase
The DNA of the cell populations was analyzed after
stable transfection of IDH2 siRNAs into the Saos-2 and MG63 cells.
IDH2 downregulation induced an increase in the G2/M population in
the Saos-2 and MG-63 cell lines by 180.4±3.5 and 60.3±2.2%
(P<0.05), respectively, whereas the S phase population was
reduced by 53.2±5.8 and 69.6±2.7% (P<0.05), respectively,
compared to the empty vector control (Fig. 2C and D). The population in the G0/G1
phase was not significantly changed in the present study
(P>0.05) (Fig. 2C and D). The EV
cells did not show significant changes in the cell cycle
distribution in the Saos-2 and MG63 cells compared to the NT cells
(P>0.05) (Fig. 2C and D). IDH2
downregulation in the osteosarcoma cells induced cell cycle
progression from the S to the G2/M phase.
IDH2 downregulation exacerbates cell
invasion
Next, the effect of IDH2 on cell invasion was
investigated. As shown in Fig. 3A and
B, IDH2 downregulation promoted the cell invasive activity of
the Saos-2 KD cells by 2.8-fold and the MG63 KD cells by 2.2-fold
compared to the Saos-2 EV or MG-63 EV cells (P<0.01). The EV
cells did not show significant changes in invasion activity in the
Saos-2 and MG63 cells compared to the NT cells (P>0.05)
(Fig. 3A and B). IDH2
downregulation promoted the invasion of osteosarcoma cells.
IDH2 downregulation does not change
the intracellular ROS levels
The intracellular ROS levels in the Saos-2 and MG63
cell lines were also investigated. IDH2 downregulation did not
induce a significant difference in the ROS levels in the Saos-2 KD
or MG63 KD cells (P>0.05), respectively, compared to the EV and
NT cells (Fig. 3C and D).
IDH2 downregulation increases NF-κB
activation and IkBα phosphorylation
In both the Saos-2 and MG63 cells, the degradation
of inactivated NF-κB was increased by IDH2 downregulation compared
to the EV cells (Fig. 4A). However,
there was no significant difference in the protein expression of
other regulators, such as Bcl-2, JNK and ERK (Fig. 4A). NF-κB transcriptional activity
was analyzed by comparing the luciferase activity of the pNF-κB-Luc
transfected EV cells to the basal activity. We investigated the
overexpression of mIκB (a constitutive NF-κB inhibitor) on the
cells with IDH2 downregulation. NF-κB activity was promoted in the
Saos-2 and MG63 KD cells with stable IDH2 downregulation compared
to the respective EV and/or NT cells (P<0.01). The increased
NF-κB activity induced by IDH2 downregulation was inhibited by mIκB
overexpression (P<0.01) (Fig.
4B). In addition, Fig. 4C shows
that IDH2 downregulation increased the expression of p-IκBα
(P<0.01). The significant p-IκBα upregulation induced by IDH2
downregulation was inhibited by mIκB overexpression (Fig. 4C).
IDH2 downregulation elevates MMP-9
activation
Western blot analysis and an MMP-9 activity assay
were conducted. We found that the MMP-9 protein levels were
markedly increased in the IDH2 downregulated Saos-2 or MG63 cells
(Fig. 5A and B). In addition, there
was a 3.5- and 2.7-fold increase in the MMP-9 activity (Fig. 5A and B) in these cells,
respectively. Furthermore, the increased protein expression and
activity of MMP-9 induced by IDH2 downregulation were significantly
inhibited by MMP-9 downregulation (Fig.
5A and B).
Discussion
To date, the alteration of IDH2 expression levels
has been identified in several types of carcinoma (5,41–44).
In melanoma, IDH2 was found to be frequently downregulated, and an
increase in tumor-free survival resulted from the overexpression of
IDH2 (9). IDH2 was also reported to
be downregulated in early phase colon carcinoma compared to
peritumoral tissues (45). However,
the expression and significance of IDH2 in osteosarcoma remain
unknown. In our study, it was observed that IDH2 was expressed at a
lower level in high-grade osteosarcoma, and 3 of the 44
osteosarcoma patients did not express IDH2. There was a similar
trend toward increased metastasis in patients with low IDH2
expression, although no significant correlation was observed
between IDH2 expression and survival. Our previous study indicated
that patients with low IDH1 expression tended to have higher
pathological grade tumors with increased metastatic potential
(12). A higher 5-year survival
rate was also found in the IDH1 high expression group vs. the IDH1
low expression group, although there was no significant correlation
between IDH1 expression and overall survival (12). The significant similarity of IDH1
and IDH2 in osteosarcoma suggests that IDH1 and IDH2 could both be
potential biomarkers for assessing malignant progression and
predicting the risk of metastasis in osteosarcoma.
Next, cell proliferation and metastasis studies were
performed to explore the biological significant of IDH2
downregulation in osteosarcoma. In our study, IDH2 downregulation
increased cell growth in osteosarcoma. Furthermore, it decreased
the proportion of cells in the S phase and increased the proportion
in the G2/M phase, suggesting a pro-progression effect. We also
found that IDH2 downregulation exacerbated cell invasion in the
Saos-2 and MG63 osteosarcoma cell lines. In colon cancer, IDH2
downregulation has been reported to inhibit the growth of colon
carcinoma cells (45). Kim et
al demonstrated that the reduction in IDH2 levels in malignant
melanoma cells has antitumorigenic effects (13). However, our previous study showed
that IDH1 upregulation inhibited cell proliferation in the 143B and
MG63 osteosarcoma cell lines, whereas IDH1 downregulation
exacerbated cell proliferation (12). In the present study, we found a
similar biological significance of IDH1 and IDH2 downregulation in
osteosarcoma cells. Moreover, this suggested that, similar to IDH1,
IDH2 downregulation exacerbated cell proliferation and metastasis
in osteosarcoma cells.
The levels of reactive oxygen species (ROS) were
analyzed in the osteosarcoma cell lines to explore the mechanism of
IDH2 downregulation in cell proliferation and metastasis.
Mitochondrial NADP+-dependent isocitrate dehydrogenase
was reported to have an important role in cellular defense against
oxidative damage by supplying NADPH, which is needed to produce
glutathione (15–17). Cellular oxidative stress arising
from high levels of ROS contributes to the development and
progression of malignant tumors, including carcinogenesis, aberrant
growth, angiogenesis and metastasis (19). Thus, mitochondrial
NADP+-dependent isocitrate dehydrogenase is
fundamentally important for the defense against ROS, which was
detected in our study. The result showed that there was no
significant difference in the intracellular ROS levels between the
downregulated osteosarcoma cells and non-treated cells. ROS, which
were involved in the effects of IDH2 downregulation in melanoma
cells, were significantly elevated (19). In addition, this surprising
difference may suggest different mechanisms of IDH2 in different
tumor cells.
NF-κB is constitutively activated and is implicated
in cellular proliferation and invasion in osteosarcoma cells
(21,23). NF-κB activation occurs via
phosphorylation of IκBα and IκBβ and IκBα phosphorylation is
essential for the release of active NF-κB (46). Therefore, we examined whether and
how IDH2 affected NF-κB activity. Notably, IDH2 downregulation
increased NF-κB degradation in osteosarcoma cells, suggesting that
NF-κB may be involved in the IDH2 downregulation-induced
pro-proliferation effect. IDH2 downregulation increased NF-κB
activity as well as IκBα phosphorylation in the Saos-2 and MG63
cells compared to the control cells. This result is supported by
the finding that increased NF-κB activity and IκBα phosphorylation
can be inhibited by overexpression of mIκB (a constitutive NF-κB
inhibitor). IDH2 downregulation increased the activity of NF-κB,
which was likely due to IκBα phosphorylation, and, therefore,
contributed to the IDH2 downregulation-induced pro-growth function
in the osteosarcoma cell lines.
In our study, we also examined the effects of IDH2
downregulation on the invasion of osteosarcoma cells. We found that
IDH2 downregulation promoted the invasion of osteosarcoma cells
into Matrigel. MMPs are involved in the processes of tumor cell
invasion and metastasis (25) and
are directly associated with the metastatic processes in
osteosarcoma (30,31). We found, for the first time, that
IDH2 downregulation increased MMP-9 expression at the protein level
and also activated MMP-9. This is supported by the finding that
increased MMP-9 expression and activation can be inhibited by
siMMP-9. These findings suggest that the potential pro-metastatic
activities following IDH2 downregulation could be partially
interpreted by the elevated MMP-9 activity. In addition, NF-κB
activity was increased following IDH2 downregulation in our study.
Based on our results, IDH2 downregulation likely promoted cell
invasion, at least in part, through increased activation of NF-κB
and its target genes: MMPs.
The limitation of the present study is that it is a
retrospective study with limited samples. In addition, the lower
IDH2 expression in the higher grade osteosarcoma samples may not
indicate a mutation in this gene. Furthermore, IDH2 siRNA was used
only in a small number of cell lines. Further studies are needed to
confirm the precise molecular regulation of IDH2 and NF-κB and
their interaction to elucidate the role of IDH2 in cell growth and
invasion in animal models. However, it may still be valuable to
study the role of IDH2 in osteosarcoma. In addition, the
pro-proliferation and pro-invasion activities as well as the
potential effects of IDH2 on cell immortalization and the
inflammatory response in osteosarcoma remain to be elucidated in
relevant models.
In conclusion, IDH2 downregulation may indicate high
pathological grade and metastasis. IDH2 downregulation induced
malignant progression via increased NF-κB and MMP-9 activity in
osteosarcoma in vitro. IDH2 may be an effective target by
which to develop new therapeutic strategies against
osteosarcoma.
Acknowledgments
We thank Guorong Yu, Shengxiang Tao, Zhenyu Pan and
Weidong Xiao for technical assistance. The present study was
supported by Hubei Province's Outstanding Medical Academic Leader
Program.
Abbreviations:
|
IDH2
|
isocitrate dehydrogenase 2
|
|
NF-κB
|
nuclear factor-κB
|
|
MMPs
|
matrix metalloproteinases
|
|
MMP-9
|
matrix metalloproteinase-9
|
|
JNK
|
Jun N-terminal kinase
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
MAPKs
|
mitogen-activated protein kinases
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Raymond AK and Jaffe N: Osteosarcoma
multidisciplinary approach to the management from the pathologist's
perspective. Cancer Treat Res. 152:63–84. 2009. View Article : Google Scholar
|
|
2
|
Gorlick R, Anderson P, Andrulis I, Arndt
C, Beardsley GP, Bernstein M, Bridge J, Cheung NK, Dome JS, Ebb D,
et al: Biology of childhood osteogenic sarcoma and potential
targets for therapeutic development: Meeting summary. Clin Cancer
Res. 9:5442–5453. 2003.PubMed/NCBI
|
|
3
|
Kager L, Zoubek A, Pötschger U, Kastner U,
Flege S, Kempf-Bielack B, Branscheid D, Kotz R, Salzer-Kuntschik M,
Winkelmann W, et al: Primary metastatic osteosarcoma: Presentation
and outcome of patients treated on neoadjuvant Cooperative
Osteosarcoma Study Group protocols. J Clin Oncol. 21:2011–2018.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bacci G, Longhi A, Versari M, Mercuri M,
Briccoli A and Picci P: Prognostic factors for osteosarcoma of the
extremity treated with neoadjuvant chemotherapy: 15-Year experience
in 789 patients treated at a single institution. Cancer.
106:1154–1161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Memon AA, Chang JW, Oh BR and Yoo YJ:
Identification of differentially expressed proteins during human
urinary bladder cancer progression. Cancer Detect Prev. 29:249–255.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cancer Genome Atlas Research Network; Brat
DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA, Rheinbay
E, Miller CR, Vitucci M, Morozova O, et al: Comprehensive,
integrative genomic analysis of diffuse lower-grade gliomas. N Engl
J Med. 372:2481–2498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ikota H, Nobusawa S, Arai H, Kato Y,
Ishizawa K, Hirose T and Yokoo H: Evaluation of IDH1 status in
diffusely infiltrating gliomas by immunohistochemistry using
anti-mutant and wild type IDH1 antibodies. Brain Tumor Pathol.
32:237–244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lian CG, Xu Y, Ceol C, Wu F, Larson A,
Dresser K, Xu W, Tan L, Hu Y, Zhan Q, et al: Loss of
5-hydroxymethylcytosine is an epigenetic hallmark of melanoma.
Cell. 150:1135–1146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park SY, Lee SM, Shin SW and Park JW:
Inactivation of mitochondrial NADP+-dependent isocitrate
dehydrogenase by hypochlorous acid. Free Radic Res. 42:467–473.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jo SH, Son MK, Koh HJ, Lee SM, Song IH,
Kim YO, Lee YS, Jeong KS, Kim WB, Park JW, et al: Control of
mitochondrial redox balance and cellular defense against oxidative
damage by mitochondrial NADP+-dependent isocitrate
dehydrogenase. J Biol Chem. 276:16168–16176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu X, Liu Y, Qin C, Pan Z, Luo J, Yu A and
Cheng Z: Up-regulated isocitrate dehydrogenase 1 suppresses
proliferation, migration and invasion in osteosarcoma: In vitro and
in vivo. Cancer Lett. 346:114–121. 2014. View Article : Google Scholar
|
|
12
|
Hu X, Yu AX, Qi BW, Fu T, Wu G, Zhou M,
Luo J and Xu JH: The expression and significance of IDH1 and p53 in
osteosarcoma. J Exp Clin Cancer Res. 29:432010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim SH, Yoo YH, Lee JH and Park JW:
Mitochondrial NADP+-dependent isocitrate dehydrogenase
knockdown inhibits tumorigenicity of melanoma cells. Biochem
Biophys Res Commun. 451:246–251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koh HJ, Lee SM, Son BG, Lee SH, Ryoo ZY,
Chang KT, Park JW, Park DC, Song BJ, Veech RL, et al: Cytosolic
NADP+-dependent isocitrate dehydrogenase plays a key
role in lipid metabolism. J Biol Chem. 279:39968–39974. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakamura H: Thioredoxin and its related
molecules: Update 2005. Antioxid Redox Signal. 7:823–828. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kirsch M and De Groot H: NAD(P)H, a
directly operating antioxidant? FASEB J. 15:1569–1574. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Avery AM, Willetts SA and Avery SV:
Genetic dissection of the phospholipid hydroperoxidase activity of
yeast gpx3 reveals its functional importance. J Biol Chem.
279:46652–46658. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nishikawa M: Reactive oxygen species in
tumor metastasis. Cancer Lett. 266:53–59. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim S, Kim SY, Ku HJ, Jeon YH, Lee HW, Lee
J, Kwon TK, Park KM and Park JW: Suppression of tumorigenesis in
mitochondrial NADP+-dependent isocitrate dehydrogenase
knock-out mice. Biochim Biophys Acta. 1842:135–143. 2014.
View Article : Google Scholar
|
|
20
|
Kim ES and Moon A: Ursolic acid inhibits
the invasive phenotype of SNU-484 human gastric cancer cells. Oncol
Lett. 9:897–902. 2015.PubMed/NCBI
|
|
21
|
Inoue R, Matsuki NA, Jing G, Kanematsu T,
Abe K and Hirata M: The inhibitory effect of alendronate, a
nitrogen-containing bisphosphonate on the PI3K-Akt-NFkappaB pathway
in osteosarcoma cells. Br J Pharmacol. 146:633–641. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ghosh S and Karin M: Missing pieces in the
NF-kappaB puzzle. Cell. 109(Suppl 1): S81–S96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eliseev RA, Schwarz EM, Zuscik MJ, O'Keefe
RJ, Drissi H and Rosier RN: Smad7 mediates inhibition of Saos2
osteosarcoma cell differentiation by NFkappaB. Exp Cell Res.
312:40–50. 2006. View Article : Google Scholar
|
|
24
|
Andela VB, Sheu TJ, Puzas EJ, Schwarz EM,
O'Keefe RJ and Rosier RN: Malignant reversion of a human
osteosarcoma cell line, Saos-2, by inhibition of NFkappaB. Biochem
Biophys Res Commun. 297:237–241. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han YP, Tuan TL, Wu H, Hughes M and Garner
WL: TNF-alpha stimulates activation of pro-MMP2 in human skin
through NF-(kappa)B mediated induction of MT1-MMP. J Cell Sci.
114:131–139. 2001.
|
|
26
|
Sun P, Mu Y and Zhang S: A novel
NF-κB/MMP-3 signal pathway involves in the aggressivity of glioma
promoted by Bmi-1. Tumour Biol. 35:12721–12727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park SL, Kim WJ and Moon SK: p21WAF1
mediates the IL-15-induced migration and invasion of human bladder
cancer 5637 cells via the ERK1/2/NF-κB/MMP-9 pathway. Int
Immunopharmacol. 22:59–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Z, Guo Y, Jiang H, Zhang T, Jin C,
Young CY and Yuan H: Differential regulation of MMPs by E2F1, Sp1
and NF-kappa B controls the small cell lung cancer invasive
phenotype. BMC Cancer. 14:2762014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Poudel B and Kim DK, Ki HH, Kwon YB, Lee
YM and Kim DK: Downregulation of ERK signaling impairs U2OS
osteosarcoma cell migration in collagen matrix by suppressing MMP9
production. Oncol Lett. 7:215–218. 2014.
|
|
30
|
Wang IC, Chen YJ, Hughes DE, Ackerson T,
Major ML, Kalinichenko VV, Costa RH, Raychaudhuri P, Tyner AL and
Lau LF: FoxM1 regulates transcription of JNK1 to promote the
G1/S transition and tumor cell invasiveness. J Biol
Chem. 283:20770–20778. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu J, Wu S and Shi X: Expression of matrix
metalloproteinase regulator, RECK, and its clinical significance in
osteosarcoma. J Orthop Res. 28:1621–1625. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hirotsu M, Setoguchi T, Sasaki H,
Matsunoshita Y, Gao H, Nagao H, Kunigou O and Komiya S: Smoothened
as a new therapeutic target for human osteosarcoma. Mol Cancer.
9:52010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luo Y, Liang F and Zhang ZY: PRL1 promotes
cell migration and invasion by increasing MMP2 and MMP9 expression
through Src and ERK1/2 pathways. Biochemistry. 48:1838–1846. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang R, Dong K, Lin F, Wang X, Gao P, Wei
SH, Cheng SY and Zhang HZ: Inhibiting proliferation and enhancing
chemosensitivity to taxanes in osteosarcoma cells by RNA
interference-mediated downregulation of stathmin expression. Mol
Med. 13:567–575. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Koppikar P, Lui VW, Man D, Xi S, Chai RL,
Nelson E, Tobey AB and Grandis JR: Constitutive activation of
signal transducer and activator of transcription 5 contributes to
tumor growth, epithelial-mesenchymal transition, and resistance to
epidermal growth factor receptor targeting. Clin Cancer Res.
14:7682–7690. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Boye K, Grotterød I, Aasheim HC, Hovig E
and Maelandsmo GM: Activation of NF-kappaB by extracellular S100A4:
Analysis of signal transduction mechanisms and identification of
target genes. Int J Cancer. 123:1301–1310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huvos AG, Rosen G and Marcove RC: Primary
osteogenic sarcoma: Pathologic aspects in 20 patients after
treatment with chemotherapy en bloc resection, and prosthetic bone
replacement. Arch Pathol Lab Med. 101:14–18. 1977.PubMed/NCBI
|
|
39
|
Rosen G, Marcove RC, Caparros B, Nirenberg
A, Kosloff C and Huvos AG: Primary osteogenic sarcoma: The
rationale for preoperative chemotherapy and delayed surgery.
Cancer. 43:2163–2177. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pessôa IA, Sagica FE, Anselmo NP, Brito JR
and de Oliveira EH: IDH1 and IDH2 mutations in different histologic
subtypes and WHO grading gliomas in a sample from Northern Brazil.
Genet Mol Res. 14:6533–6542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Waitkus MS, Diplas BH and Yan H:
Isocitrate dehydrogenase mutations in gliomas. Neuro Oncol. Jul
16–2015.(Epub ahead of print). pii: nov136. PubMed/NCBI
|
|
42
|
Chan SM, Thomas D, Corces-Zimmerman MR,
Xavy S, Rastogi S, Hong WJ, Zhao F, Medeiros BC, Tyvoll DA and
Majeti R: Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2
dependence in acute myeloid leukemia. Nat Med. 21:178–184. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Aref S, Kamel Areida el S, Abdel Aaal MF,
Adam OM, El-Ghonemy MS, El-Baiomy MA and Zeid TA: Prevalence and
clinical effect of IDH1 and IDH2 mutations among cytogenetically
normal acute myeloid leukemia patients. Clin Lymphoma Myeloma Leuk.
15:550–555. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu WR, Tian MX, Jin L, Yang LX, Ding ZB,
Shen YH, Peng YF, Zhou J, Qiu SJ, Dai Z, et al: High expression of
5-hydroxymethylcytosine and isocitrate dehydrogenase 2 is
associated with favorable prognosis after curative resection of
hepatocellular carcinoma. J Exp Clin Cancer Res. 33:322014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lv Q, Xing S, Li Z, Li J, Gong P, Xu X,
Chang L, Jin X, Gao F, Li W, et al: Altered expression levels of
IDH2 are involved in the development of colon cancer. Exp Ther Med.
4:801–806. 2012.PubMed/NCBI
|
|
46
|
Simeonidis S, Stauber D, Chen G,
Hendrickson WA and Thanos D: Mechanisms by which IkappaB proteins
control NF-kappaB activity. Proc Natl Acad Sci USA. 96:49–54. 1999.
View Article : Google Scholar : PubMed/NCBI
|