Introduction
Although numerous new anticancer strategies have
been developed during recent years, chemotherapy remains one of the
most important means for the treatment of malignant tumors.
Doxorubicin (Dox) is an anthracycline antibiotic isolated from
Streptomyces peucetius. Due to its broad antitumor spectrum
and effective antineoplastic activity, it has already been widely
used in various hematologic malignancies and solid tumors, such as
lymphoma (1), breast (2) and ovarian cancer (3), and soft tissue sarcomas (4). However, its clinical application is
seriously constrained by its instability, unselective distribution
and serious systemic toxicities, particularly dose-limiting
myocardial toxicity, which could result in cardiomyopathy and
congestive heart failure (5). These
limit the clinical application and cumulative lifetime total dose
of Dox (6). Hence, it is necessary
to develop new strategies to decrease the toxicity and improve the
therapeutic effect of Dox.
Among the various strategies to improve the
therapeutic index of chemotherapy drugs, a drug delivery system
based on biodegradable polymeric nanoparticles appears to be a
promising direction which has attracted increased attention due to
its superior cell uptake, passive targeting, improved anticancer
efficiency and biological safety of the cargo (7).
The monomethoxy poly(ethylene
glycol)-poly(ε-caprolactone) (MPEG-PCL) micelle is an amphiphilic,
biocompatible and biodegradable copolymer nanoparticle. These
features make MPEG-PCL micelles an ideal candidate for drug
delivery system development. Previous studies have preliminarily
showed the excellent drug loading capability of the MPEG-PCL
micelle and its superior pharmacokinetics and drug release
characteristics for drug encapsulation (8). These suggest that MPEG-PCL micelles
may serve as a desired drug carrier for Dox. However, related
research is rare. Only a few studies were limited to in
vitro testing or subcutaneous tumor models treated with
intratumoral injection. There are few systematic studies concerning
its potential use by intravenous injection in lung metastasis
models.
In the present study, we successfully prepared
Dox-loaded MPEG-PCL (Dox/MPEG-PCL) nanoparticles using a
self-assembly method. By encapsulating Dox in MPEG-PCL
nanoparticles, Dox obtained superior cell uptake, improved
anticancer effect and reduced toxicity to the myocardium compared
with its free state. These results suggest a promising application
of biodegradable MPEG-PCL nanoparticles to modify existing
traditional chemotherapy drugs to decrease their systemic
toxicities and improve their therapeutic index.
Materials and methods
Monomethoxy poly(ethylene glycol) (MPEG; Mn=2,000)
was purchased from Sigma-Aldrich (St. Louis, MO, USA).
ε-caprolactone (ε-CL) was purchased from Alfa Aesar, USA.
Dulbecco's modified Eagle's medium (DMEM) and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were both purchased from Sigma (St. Louis, MO, USA). Dox was
purchased from Hisun Pharmaceutical Co. (Zhejiang, China). The lung
carcinoma cell line A549 and the malignant melanoma cell line
B16-F10 were purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA). The cells were incubated in a 95%
air-humidified atmosphere containing 5% CO2 at 37°C. The
female C57BL/6 mice (6–8 weeks old) were purchased from the
Experimental Animal Center of Sichuan University (Chengdu, Sichuan,
China). The mice were keep at room temperature with a humidity of
50–60%. All animal care and experimental procedures in the present
study were conducted according to institutional animal care and use
guidelines.
Preparation of Dox-loaded MPEG-PCL
nanoparticles
Dox-loaded MPEG-PCL (Dox/MPEG-PCL) nanoparticles
were synthesized by a self-assembly process according to previous
studies (8,9). In brief, empty MPEG-PCL micelles
without encapsulated drug were initially prepared by a
self-assembly procedure triggered by heat rise. Firstly, MPEG-PCL
diblock copolymer was fully dissolved in cold water. Then, the
temperature of the MPEG-PCL solution was increased to 50°C. Due to
the amphiphilic property of its molecular structure, the MPEG-PCL
may automatically assemble into nano-sized micelles (with the PCL
segment formed the hydrophobic core of the micelle and PEG segment
became the hydrophilic shell of the micelle) triggered by a
temperature increase in the water solution under moderate stirring.
After the blank MPEG-PCL micelles were prepared, the Dox/MPEG-PCL
nanoparticles were prepared by a pH sensitive self-assembly
process. In short, Dox dissolved in water was slowly added into the
blank MPEG-PCL micelles in phosphate-buffered saline (PBS) (pH 7.4)
drop by drop. Due to the sudden change in the low solubility of Dox
at pH 7.4, Dox was encapsulated into the hydrophobic core of the
MPEG-PCL micelles. Finally, the Dox/MPEG-PCL nanoparticles were
purified and lyophilized for future use.
Physicochemical property and morphologic
characterization
Dox was successfully incorporated into the MPEG-PCL
nanoparticles. The basic characteristics of particle size and zeta
potential of the Dox/MPEG-PCL nanoparticles was determined by Nano
ZS90 (Malvern Instruments, UK) at room temperature. The ultra
microstructure of the Dox/MPEG-PCL nanoparticles was observed by a
transmission electron microscope (TEM) (H-6009IV; Hitachi, Tokyo,
Japan).
Cellular uptake in vitro and in vivo
As a model drug, one important feature of Dox which
makes it easily detectable is its fluorescence. Dox-derived
fluorescence can be excited at a 488-nm wavelength and detected at
a 564-nm wavelength (red fluorescence). In order to compare the
difference in cellular uptake between free Dox and Dox/MPEG-PCL
nanoparticles, fluorescence microscopy was adopted. Briefly, A549
cells were plated on a coverglass into 6-well plates at a density
of 1×105 and incubated overnight. Then, the cells were
treated with free Dox or Dox/MPEG-PCL nanoparticles, respectively.
After co-incubation for 6 h, the cells on the coverglass were taken
out for detection under a fluorescence microscope. Before
observation under a fluorescence microscope, cell nuclei were
pre-stained with DAPI. For in vivo cellular uptake,
2×104 B16-F10 cells were injected into a zebra fish 72 h
post fertilization (hpf), and 2 days later, Dox/MPEG-PCL
nanoparticles were administered by micro-injection. Twenty-four
hours later, the fish were observed under a fluorescence
microscope.
In vitro antitumor effect
The in vitro antitumor effect of the
Dox/MPEG-PCL nanoparticles was determined by MTT assay in B16-F10
cells. Briefly, the cells were plated in 96-well plates at a
density of 2×103 cells/well in 100 µl DMEM and
grown overnight. Then, 100 µl DMEM containing a series of
ascending concentrations of free Dox or Dox/MPEG-PCL nanoparticles
were added to each well, respectively. Each concentration was set
with 3 duplicate wells. Untreated cells were used as the control.
After incubation for 48 h, 20 µl of MTT solution (5 mg/ml)
was added to each well, and then incubated at 37°C for 4 h. Then,
MTT solution was removed and 150 µl DMSO was added to each
well. Absorbance of the formazan crystals was detected at 570 nm.
Cell viability was compared with the control cells and calculated
as a percentage.
In vivo antitumor effect
In order to verify the in vivo anticancer
effect and investigate the possible mechanism of Dox/MPEG-PCL
nanoparticles, we tested the Dox/MPEG-PCL nanoparticles on a highly
malignant multiple pulmonary metastasis model of melanoma. To
generate the multiple pulmonary metastatic model of melanoma,
1.5×105 B16-F10 cells fully resuspended in 0.2 ml of
normal saline (NS) were intravenously injected into the lateral
dorsal tail vein of C57BL/6 mice. Five days after inoculation, the
mice were randomly divided into 4 groups (6 mice/group, with an
average body weight of 20 g) and treated with an intravenous
injection of the following regimens, respectively; i) 100 µl
of PBS; ii) 2 mg of blank MPEG-PCL in 100 µl PBS; iii) 0.1
mg free Dox in 100 µl PBS (Dox dose, 5 mg/kg); and iv) 0.1
mg Dox encapsulated in 2 mg MPEG-PCL in 100 µl PBS (Dox
dose, 5 mg/kg). The administrations were repeated twice a week for
a total number of 6 times. Three days after the last time of
treatment, the mice were sacrificed, and both lungs of every mouse
were dissected for further evaluation. Since metastatic lesions of
B16-F10 melanoma are black or brown they are readily visually
detected. The numbers of surface metastatic lesions of each mice
were counted under a stereoscopic microscope (magnification, ×10)
by two independent investigators who were blinded to the group
assignment. The metastasis index of each mice was defined as the
sum of scores of all visible metastatic lesions in both lungs as
previously detailed: metastatic lesions ≤1 mm were scored as 1,
metastatic lesions >1 mm but ≤2 mm were scored as 3, and lesions
>2 mm were scored as 10 (10).
In the survival analysis, the experiment was repeated, and the
observation period was extended to as long as 50 days after
inoculation.
Systemic toxicity evaluation
To detect the possible toxicities of the
Dox/MPEG-PCL nanoparticles, at the end of the in vivo
experiment, major organs (heart, liver, spleen, lung and kidney) of
each group were also harvested at the same time. Paraffin-embedded
sections of the above organs were stained with hematoxylin and
eosin (H&E) method. The toxicities that induced pathological
changes to the major organs were observed under a microscope by two
experienced pathologists in a blinded manner. Since the myocardial
toxicity of Dox is a dose accumulated toxicity and usually occur at
a period of time after treatment, it is usually difficult for the
mice to survive over 30 days once inoculated with B16-F10 cells.
Thus, in order to reveal the potential myocardial protection of the
Dox/MPEG-PCL nanoparticles, we repeated the treatment regimens on
two groups of mice without tumors and fed them as long as 10 weeks
after the last intravenous administration.
Statistical analyses
All data are expressed as mean ± SD. Each
experimental group contained 6 mice. Comparison of the numbers of
metastatic lesions and the metastasis index between each group was
performed by analysis of variance (ANOVA). For survival analysis,
data were analyzed using the Kaplan-Meier method. Statistical
analysis was carried out using Prism GraphPad software and SPSS
statistical software (version 22.0; SPSS, Inc., IBM Co., Armonk,
NY, USA). P<0.05 was considered to indicate a statistically
significant result.
Results
Characterization of the Dox-loaded
MPEG-PCL nanoparticles
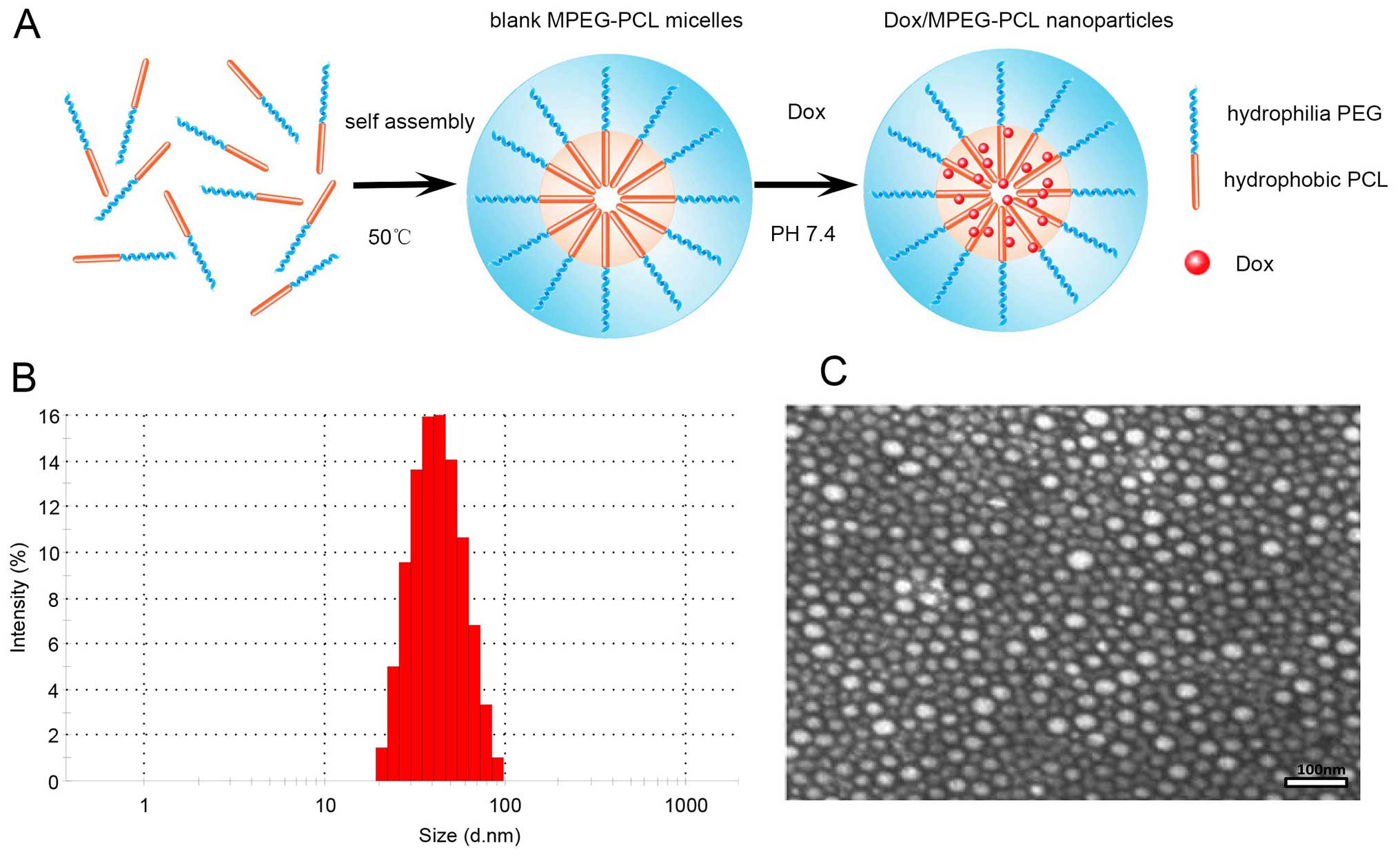
The Dox/MPEG-PCL nanoparticles were easily prepared
by simple heat triggering and then a pH-sensitive self-assembly
method as mentioned above. The process is illustrated as a sketch
map in Fig. 1A. Briefly, blank
MPEG-PCL micelles without Dox were first prepared ahead using a
heat-induced self-assembly process at 50°C, and then Dox was
encapsulated into blank MPEG-PCL micelles by a rapid pH-sensitive
automatically self-assembly process under moderate stirring. The
technological requirements for the whole process are simple, safe
and mild, without introducing any unwanted organic solvents and
surfactants. The whole procedure did not require any extreme
conditions or complicated physicochemical process. All the above
features enable the preparation of Dox/MPEG-PCL nanoparticles to be
easily scaled up for mass production.
For characterization of the Dox/MPEG-PCL
nanoparticles, we used Zetasizer Nano Z90 (Malvern Instruments) to
determine the particle size and zeta potential. As shown in
Fig. 1B, the Dox/MPEG-PCL
nanoparticles we prepared were relatively neat and uniform, with a
mean particle size of ~41.9 nm. Its zeta potential was −5.6 mV at
pH 7.0. Under transmission electron microscopy (Fig. 1C), Dox/MPEG-PCL nanoparticles showed
classical spherical shape (mean diameter ~35 nm) and at a
mono-dispersed state did not form severe aggregation. Severe
aggregation results in pulmonary embolism and is dangerous for
intravenous administration. By repeated examinations, the MPEG-PCL
had an average drug-loading content [the ratio of incorporated drug
to polymer (w/w)] of 5.33±0.54 and an entrapment efficiency as high
as 94% for Dox at room temperature.
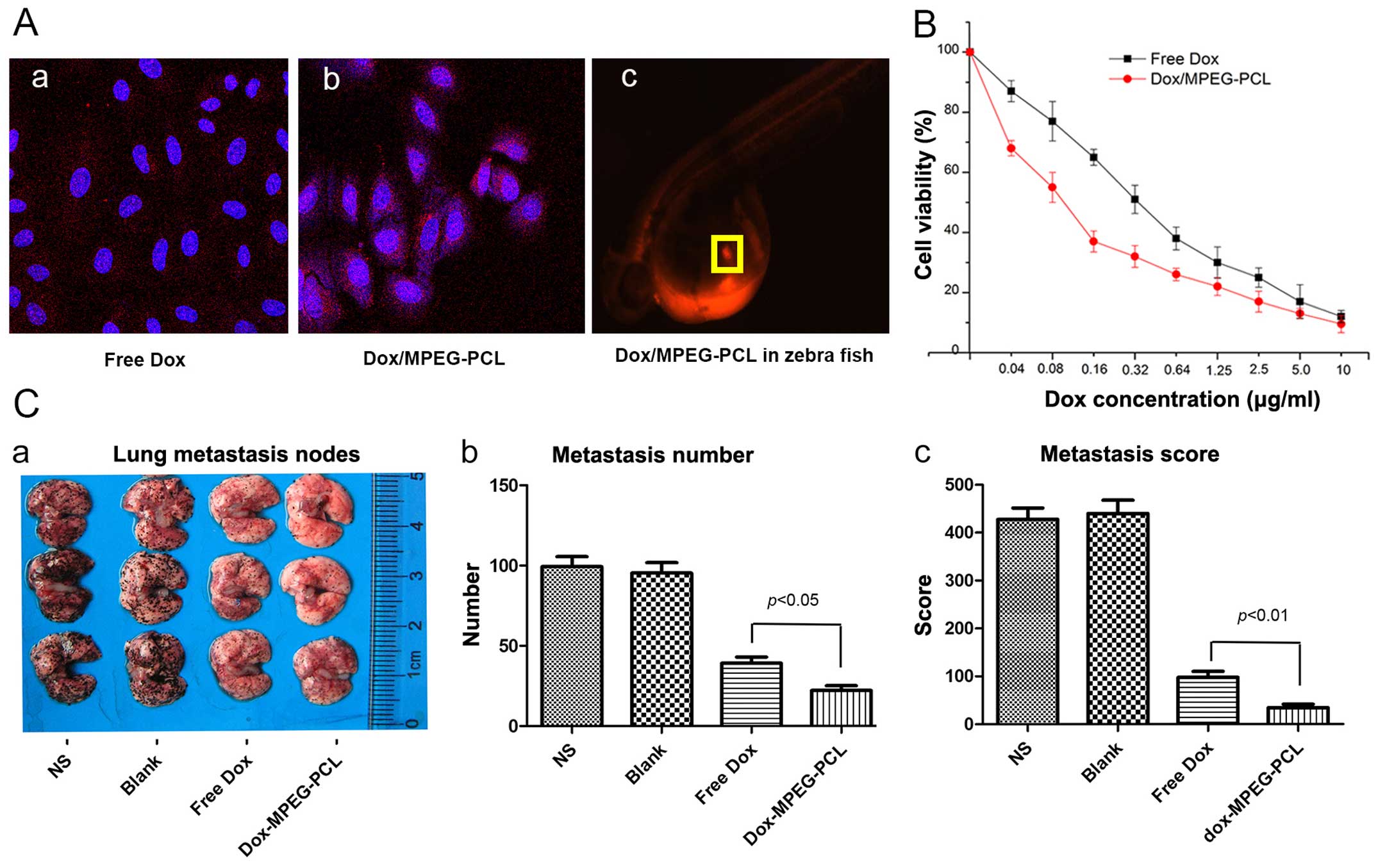
Increased cellular uptake
In order to compare the cellular uptake between free
Dox and Dox/MPEG-PCL, we treated B16-F10 cells with Dox and the
Dox/MPEG-PCL nanoparticles, respectively. Dox is a fluorescent
substance. Thus, to exhibit the intake and location of Dox, we used
fluorescence microscopy to capture the distribution of Dox. As
shown in Fig. 2A–a, in the free
Dox-treated cells, the red fluorescence of Dox was scattered around
the cells, without any obvious aggregation tendency around the
nucleus. In contrast, in the Dox/MPEG-PCL nanoparticle-treated
cells (2A–b), the red fluorescence of Dox was significantly
gathered around the nucleus, demonstrating a distinct intracellular
accumulation trend. In vivo, we also preliminarily observed
the relative enrichment of the Dox/MPEG-PCL nanoparticles at the
tumor cell injection site in the zebra fish model (2A–c). These
results indicated that the cellular uptake of the Dox/MPEG-PCL
nanoparticles was obvious more effective than that of free Dox.
Enhanced anticancer activity in
vitro
We further investigated the cell proliferation
inhibition of the Dox/MPEG-PCL nanoparticles using MTT assay.
Inhibition of the proliferation of B16-F10 cells by Dox and the
Dox/MPEG-PCL nanoparticles is shown in Fig. 2B. The results showed that treatment
with the Dox/MPEG-PCL nanoparticles resulted in a significantly
enhanced inhibition of tumor cell proliferation compared with free
Dox at equal equivalent concentrations of Dox. Notably, this
phenomenon was relatively more obvious at a low equivalent
concentration of Dox. When the equivalent concentration of Dox
increase above 5 µg/ml, the discrepancy was not obvious.
This result indicated that the Dox/MPEG-PCL nanoparticles could
significantly enhance the antitumor effect of Dox compared with its
free state in vitro.
Improved in vivo antitumor efficiency and
prolonged survival
To investigate the antitumor efficiency and
potential underlying mechanism of the Dox/MPEG-PCL nanoparticles, a
multiple pulmonary metastasis model of B16-F10 cells in C57BL/6
mice was established. The mice were then treated with an
intravenously injection of NS, blank MPEG-PCL, free Dox or
Dox/MPEG-PCL, respectively. As shown in Fig. 2C, although free Dox and Dox/MPEG-PCL
nanoparticle-treated mice both showed obviously suppressed tumor
growth compared with the mice treated with NS or blank MPEG-PCL
nanoparticles, the Dox/MPEG-PCL nanoparticle-treated mice exhibited
less and smaller metastatic nodules compared with that observed in
the mice treated with free Dox (Fig.
2C–a). To compare the difference, we counted the number of
metastatic nodules for each mouse and constructed a metastasis
index based on the number and diameter of the metastatic nodules
according to previous literature (11). As shown in Fig. 2C–b, the Dox/MPEG-PCL
nanoparticle-treated mice exhibited significantly reduced surface
metastatic nodules compared with the number of nodules in the mice
treated with free Dox (P<0.05). After we scored the metastatic
nodules by size (Fig. 2C–c), the
improved antitumor effect represented in the metastasis index was
even more significant (P<0.01). These findings indicated that
compared with its free state, Dox-loaded MPEG-PCL nanoparticles did
not only reduce tumor lung metastasis but also inhibited the growth
of lung metastases.
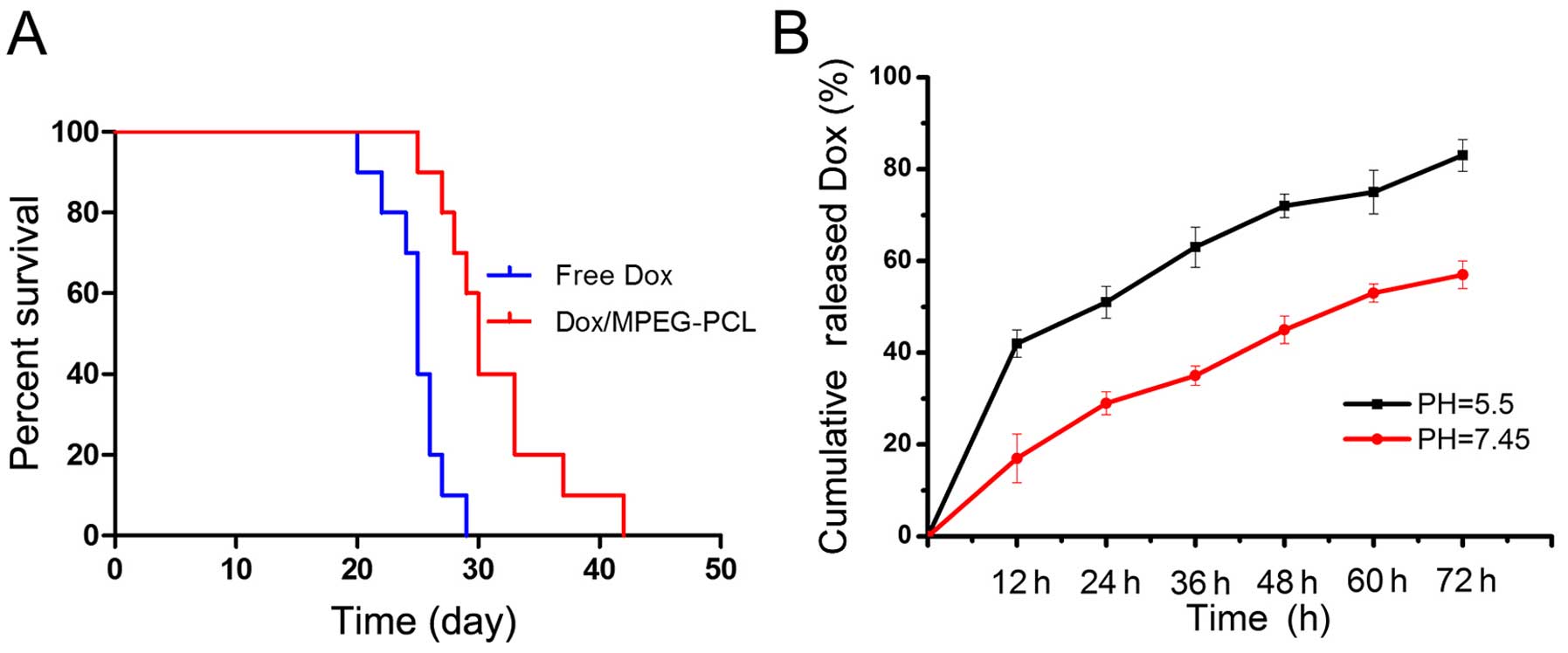
Survival analysis
In order to explore the potential benefit of
Dox/MPEG-PCL nanoparticles in terms of survival, a survival study
was carried out. The results indicated that, although the
Dox/MPEG-PCL nanoparticle- and free Dox-treated mice generally
lived obviously longer than the NS or blank MPEG-PCL-treated mice,
the mice treated with the Dox/MPEG-PCL nanoparticles exhibited
significantly prolonged survival time (P<0.05) when compared
with that of free Dox-treated mice as shown in the survival curve
in Fig. 3A. There was no
significant difference in survival between the NS-treated and blank
MPEG-PCL-treated mice (P>0.05). Therefore, this result indicated
that the encapsulation of Dox into MPEG-PCL nanoparticles did not
only improve inhibition of the growth of lung metastasis, but also
significantly prolonged the overall survival time when compared
with free Dox.
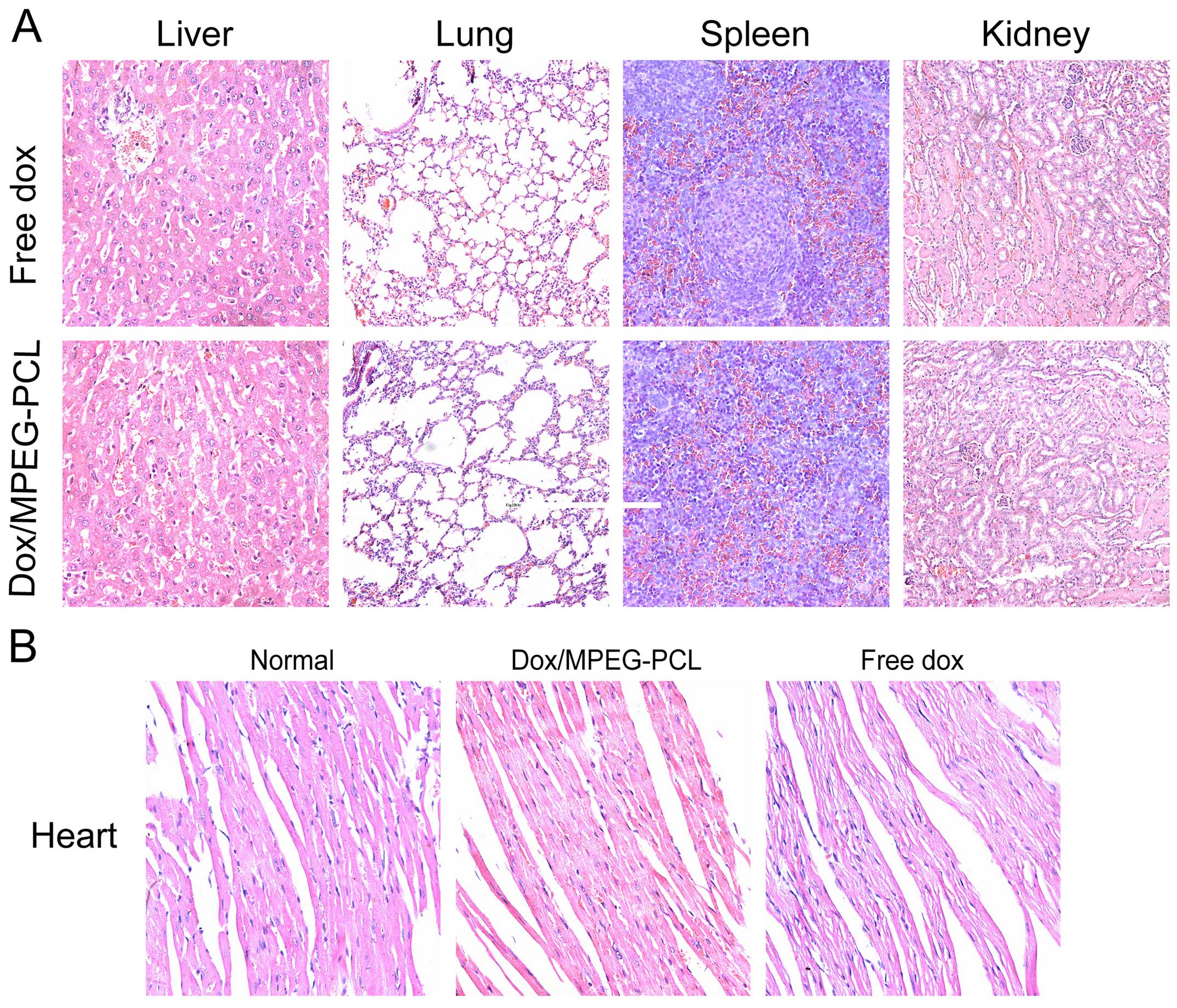
Comparison of the systemic toxicity
In order to appraise the toxicity of the
Dox/MPEG-PCL nanoparticles, we monitored the body weight and other
general conditions such as appetite, weight loss and behavior
change during the entire treatment period. Compared with the mice
treated with free Dox, the mice treated with the Dox/MPEG-PCL
nanoparticles showed an overall better general condition and
relative less weight loss, but there was no significant difference
(P>0.05). After the mice were sacrificed, their main organs were
harvested for H&E staining. As shown in Fig. 4A, there were no obvious
histopathologic difference in the main organs between the free Dox-
and Dox/MPEG-PCL nanoparticle-treated mice. This demonstrated that,
when compared with free Dox, Dox-loaded in MPEG-PCL nanoparticles
at least did not introduce additional acute systemic toxicity
during treatment.
Comparison of chronic myocardial
toxicity
Chronic myocardial toxicity is the dose-limiting
toxicity of Dox which is closely related to the accumulation dose.
It usually appears in a period of time after treatment. In order to
assess the chronic myocardial toxicity of Dox, we repeated the
experiment on mice without tumors and extended the observation time
to 10 weeks after the last time of administration. As shown in
Fig. 4B, the heart tissue sections
from free Dox-treated mice exhibited serious myocardial
pathological changes; the myocardial fibers became thin and loose,
and even presented varying degrees of rupture. However, the heart
tissue sections from the Dox/MPEG-PCL nanoparticle-treated mice
only showed slightly thin myocardial fibers compared with the
normal muscle fibers. In comarison with free Dox, this indicated
that Dox/MPEG-PCL nanoparticles significantly reduced the chronic
myocardial toxicity of Dox during the period of treatment.
Discussion
Although traditional chemotherapeutic agents have
made great progress in the treatment of cancer in recent decades,
further progress is limited due to inherent shortcomings, including
serious systemic toxicities and non-specific distribution (12). Thus, further research to modify
these agents in order to alleviate their toxicities and obtain
better treatment efficacy is urgently needed.
The clinical use of Dox, as one of the most widely
used antitumor drugs, is obstructed by its serious systemic
toxicities particularly cardiac toxicity and limited lifetime dose
(13). In order to overcome these
shortcomings, various drug delivery systems have been developed
such as liposome (14,15) and chitosan nanoparticles (16,17),
super paramagnetic iron oxide nanoparticles (SPIO) (18) and silica nanoparticles (19). However, their particle sizes usually
range from 150 to 800 nm, which are likely grasped by the spleen
and removed by phagocytes when injected intravenously (20). In addition, various types of these
nanoparticles are prepared using an emulsion solvent extract
method, which may introduce undesirable organic solvents and
surfactants to the products (21,22).
Furthermore, various inorganic nanoparticles are not biocompatible
and biodegradable thus may result in serious consequences even
death when intravenously used (23).
Recently, block copolymer nanoparticles have
emerging as a new promising drug delivery system and has attracted
much attention (24). In the
present study, we employed amphiphilic and biodegradable MPEG-PCL
diblock copolymer nanoparticles as a drug carrier and successfully
encapsulated Dox to prepare Dox-loaded MPEG-PCL nanoparticles.
Unlike the preparation of many other drug-loaded polymeric
nanoparticles, we prepared Dox/MPEG-PCL by a self-assembly method
rather than an emulsion solvent extract method which may produce
larger size nanoparticles than we wanted and introduce unwanted
organic solvents, surfactants difficult to remove. The prepared
nanoparticles had a desired average particle size of ~42 nm, and
superior solubility after freeze drying.
As a drug delivery system, the particle size is very
important (25). It has been
identified that particles larger than 200 nm usually are grasped by
the spleen and removed by phagocytes when intravenously injected.
On the contrary, particles <20 nm are quickly removed through
renal clearance (26,27). Based on the above findings, there is
a consensus that particle sizes ranging from 20 to 100 nm are ideal
for intravenous administration that may acquire extended half-life
in the bloodstream (28). Moreover,
new vessels in tumors are more leaky due to abnormal endothelial
cells with large spaces, ranging from 200 nm to 1.2 µm.
Particles within this range would easily extravasate from the new
vessels around tumor tissue, and thus could selectively increase
the drug accumulation at the tumor site through enhanced permeation
and retention (EPR) effect (29).
In the present study, we prepared the Dox/MPEG-PCL nanoparticles
with a desired particle size (~42 nm), and thus enhanced the
selective distribution of the drug at the tumor site by the EPR
effect.
In addition to particle size, zeta potential may be
another important factor that contributes to the passive targeting
effect of the Dox/MPEG-PCL nanoparticles. The Dox/MPEG-PCL
nanoparticles had a slightly negative zeta potential ~−5.6 mV. When
intravenously injected, the negative zeta potential rejected the
plasma protein with a negative charge thus increasing the plasmatic
circulation time and the probability for Dox to access the tumor
site (30).
When Dox/MPEG-PCL nanoparticles arrive at the tumor
site, low pH may change the zeta potential in a positive direction.
This may enhance its binding with the negative charge of the cell
membrane thus increasing the cellular uptake of Dox (31). In addition, pH is also a critical
factor that could affect Dox drug release. As known, the pH in
tumor tissues is usually lower due to hypoxia and accumulation of
lactic acid. Previous research and our data both showed that the
Dox release process from Dox/MPEG-PCL nanoparticles is a
pH-dependent process which is more rapid at pH 5.5 than at pH 7.45
(32) (Fig. 3B). When Dox/MPEG-PCL nanoparticles
arrive at the tumor site, low pH may trigger the quick release of
the Dox encapsulated in the MPEG-PCL micelles and facilitate the
passive accumulation of Dox at the tumor site, thus eventually
resulting in a higher local concentration than free Dox.
In the present study, we confirmed the significant
increase in cellular uptake and enhanced proliferation inhibition
of tumor cells treated with the Dox-encapsulated MPEG-PCL
nanoparticles in vitro. In vivo, the multiple
pulmonary metastasis model of melanoma further demonstrated the
improved antitumor effect and obviously decreased myocardial
toxicity of the Dox/MPEG-PCL nanoparticles than these parameters
noted for free DOX. Based on the main characteristics of the
Dox/MPEG-PCL nanoparticles and the above analysis, we may deduce
that the desired particle size, slightly negative zeta potential,
pH-dependent release and EPR effect all contribute to the superior
antitumor activity and attenuation of the toxicity of the
Dox/MPEG-PCL nanoparticles. Considering the advantages and safety,
Dox/MPEG-PCL nanoparticles may serve as a new approach for tumor
treatment, and encapsulation of drugs in MPEG-PCL nanoparticles may
represent a promising strategy for traditional drug
modification.
Acknowledgments
This study was supported by Science Foundation of
Chengdu Municipal Bureau of Science and Technology, Grant No.
2015-HM01-00254-SF.
References
|
1
|
Azim HA, Santoro L, Bociek RG, Gandini S,
Malek RA and Azim HA Jr: High dose intensity doxorubicin in
aggressive non-Hodgkin's lymphoma: A literature-based
meta-analysis. Ann Oncol. 21:1064–1071. 2010. View Article : Google Scholar
|
|
2
|
Sauter C: Doxorubicin adjuvant
combinations for breast cancer. Lancet. 342:1550–1551. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Staropoli N, Ciliberto D, Botta C,
Fiorillo L, Grimaldi A, Lama S, Caraglia M, Salvino A, Tassone P
and Tagliaferri P: Pegylated liposomal doxorubicin in the
management of ovarian cancer: A systematic review and metaanalysis
of randomized trials. Cancer Biol Ther. 15:707–720. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grenader T, Goldberg A, Hadas-Halperin I
and Gabizon A: Long-term response to pegylated liposomal
doxorubicin in patients with metastatic soft tissue sarcomas.
Anticancer Drugs. 20:15–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heger Z, Cernei N, Kudr J, Gumulec J,
Blazkova I, Zitka O, Eckschlager T, Stiborova M, Adam V and Kizek
R: A novel insight into the cardiotoxicity of antineoplastic drug
doxorubicin. Int J Mol Sci. 14:21629–21646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chatterjee K, Zhang J, Honbo N and
Karliner JS: Doxorubicin cardiomyopathy. Cardiology. 115:155–162.
2010. View Article : Google Scholar :
|
|
7
|
Wright J: Nanotechnology: Deliver on a
promise. Nature. 509:S58–S59. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gou M, Zheng X, Men K, Zhang J, Wang B, Lv
L, Wang X, Zhao Y, Luo F, Chen L, et al: Self-assembled hydrophobic
honokiol loaded MPEG-PCL diblock copolymer micelles. Pharm Res.
26:2164–2173. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng L, Gou M, Zhou S, Yi T, Zhong Q, Li
Z, He X, Chen X, Zhou L, Wei Y, et al: Antitumor activity of
monomethoxy poly(ethylene glycol)-poly(ε-caprolactone)
micelle-encapsulated doxorubicin against mouse melanoma. Oncol Rep.
25:1557–1564. 2011.PubMed/NCBI
|
|
10
|
van Spriel AB, van Ojik HH, Bakker A,
Jansen MJ and van de Winkel JG: Mac-1 (CD11b/CD18) is crucial for
effective Fc receptor-mediated immunity to melanoma. Blood.
101:253–258. 2003. View Article : Google Scholar
|
|
11
|
Tsai NM, Chen BM, Wei SL, Wu CW and
Roffler SR: Anti-tumor immunoglobulin M increases lung metastasis
in an experimental model of malignant melanoma. Clin Exp
Metastasis. 20:103–109. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bourzac K: Nanotechnology: Carrying drugs.
Nature. 491:S58–S60. 2012. View
Article : Google Scholar
|
|
13
|
Aiken MJ, Suhag V, Garcia CA, Acio E,
Moreau S, Priebat DA, Chennupati SP and Van Nostrand D:
Doxorubicin-induced cardiac toxicity and cardiac rest gated blood
pool imaging. Clin Nucl Med. 34:762–767. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Fang J, Kim YJ, Wong MK and Wang P:
Codelivery of doxorubicin and paclitaxel by cross-linked
multilamellar liposome enables synergistic antitumor activity. Mol
Pharm. 11:1651–1661. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Green AE and Rose PG: Pegylated liposomal
doxorubicin in ovarian cancer. Int J Nanomedicine. 1:229–239.
2006.
|
|
16
|
Unsoy G, Khodadust R, Yalcin S, Mutlu P
and Gunduz U: Synthesis of Doxorubicin loaded magnetic chitosan
nanoparticles for pH responsive targeted drug delivery. Eur J Pharm
Sci. 62:243–250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sanyakamdhorn S, Agudelo D and
Tajmir-Riahi HA: Encapsulation of antitumor drug Doxorubicin and
its analogue by chitosan nanoparticles. Biomacromolecules.
14:557–563. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang X, Grailer JJ, Rowland IJ, Javadi A,
Hurley SA, Steeber DA and Gong S: Multifunctional SPIO/DOX-loaded
wormlike polymer vesicles for cancer therapy and MR imaging.
Biomaterials. 31:9065–9073. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meng H, Liong M, Xia T, Li Z, Ji Z, Zink
JI and Nel AE: Engineered design of mesoporous silica nanoparticles
to deliver doxorubicin and P-glycoprotein siRNA to overcome drug
resistance in a cancer cell line. ACS Nano. 4:4539–4550. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jia Y, Yuan M, Yuan H, Huang X, Sui X, Cui
X, Tang F, Peng J, Chen J, Lu S, et al: Co-encapsulation of
magnetic Fe3O4 nanoparticles and doxorubicin
into biodegradable PLGA nanocarriers for intratumoral drug
delivery. Int J Nanomedicine. 7:1697–1708. 2012. View Article : Google Scholar :
|
|
21
|
Gaucher G, Dufresne MH, Sant VP, Kang N,
Maysinger D and Leroux JC: Block copolymer micelles: Preparation,
characterization and application in drug delivery. J Control
Release. 109:169–188. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Farokhzad OC, Jon S, Khademhosseini A,
Tran TN, Lavan DA and Langer R: Nanoparticle-aptamer bioconjugates:
A new approach for targeting prostate cancer cells. Cancer Res.
64:7668–7672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hudson SP, Padera RF, Langer R and Kohane
DS: The biocompatibility of mesoporous silicates. Biomaterials.
29:4045–4055. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chow EK, Zhang XQ, Chen M, Lam R, Robinson
E, Huang H, Schaffer D, Osawa E, Goga A and Ho D: Nanodiamond
therapeutic delivery agents mediate enhanced chemoresistant tumor
treatment. Sci Transl Med. 3:73ra212011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Canelas DA, Herlihy KP and DeSimone JM:
Top-down particle fabrication: Control of size and shape for
diagnostic imaging and drug delivery. Wiley Interdiscip Rev Nanomed
Nanobiotechnol. 1:391–404. 2009. View
Article : Google Scholar
|
|
26
|
Gupta AK and Gupta M: Synthesis and
surface engineering of iron oxide nanoparticles for biomedical
applications. Biomaterials. 26:3995–4021. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gupta AK and Wells S: Surface-modified
superparamagnetic nanoparticles for drug delivery: Preparation,
characterization, and cytotoxicity studies. IEEE Trans
Nanobioscience. 3:66–73. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Agarwal A, Mackey MA, El-Sayed MA and
Bellamkonda RV: Remote triggered release of doxorubicin in tumors
by synergistic application of thermosensitive liposomes and gold
nanorods. ACS Nano. 5:4919–4926. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matsumura Y and Maeda H: A new concept for
macromolecular therapeutics in cancer chemotherapy: Mechanism of
tumoritropic accumulation of proteins and the antitumor agent
smancs. Cancer Res. 46:6387–6392. 1986.PubMed/NCBI
|
|
30
|
Sant S, Poulin S and Hildgen P: Effect of
polymer architecture on surface properties, plasma protein
adsorption, and cellular interactions of pegylated nanoparticles. J
Biomed Mater Res A. 87:885–895. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu J, Jia H, Guo L, Zhang G, Cao Y, Yan H
and Liu K: Zwitterionic polymeric micelles that undergo a
pH-triggered positive charge for enhanced cellular uptake. Eur
Polym J. 66:376–385. 2015. View Article : Google Scholar
|
|
32
|
Gou M, Zheng X, Men K, Zhang J, Zheng L,
Wang X, Luo F, Zhao Y, Zhao X, Wei Y, et al:
Poly(epsilon-caprolactone)/poly(ethylene
glycol)/poly(epsilon-caprolactone) nanoparticles: Preparation,
characterization, and application in doxorubicin delivery. J Phys
Chem B. 113:12928–12933. 2009. View Article : Google Scholar : PubMed/NCBI
|