Introduction
Gallbladder cancer (GBC) is the most common,
aggressive malignancy of the bile duct worldwide, showing an
increasing tendency in its incidence and mortality, and associated
with extremely poor prognosis (1,2). The
5-year survival rate has improved due to recent advances in
diagnostic and therapeutic approaches, but the prognosis is still
poor for the patients with an advanced stage, high local recurrence
and distant metastasis. Because of low surgical resection rate and
serious side effects caused by ineffective chemotherapy and
radiation therapy, the majority of patients now have unsatisfactory
results (3,4). Therefore, new effective strategies for
this lethal neoplasm is urgently needed.
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL), a member of the tumor necrosis factor (TNF) family,
has the ability to induce apoptosis in transformed, but not normal
cells, via extrinsic signaling pathways (5,6). TRAIL
initiates cell death upon binding to death receptor DR4 (TRAIL-R1)
and/or DR5 (TRAIL-R2) on the surface of cancer cells, which
subsequently causes the formation of DISC (death inducing signaling
complex) and caspase cascades (7).
Caspase-8 is activated in the death receptor signaling pathway due
to its death domain (8). The active
caspase-8 cleaves and directly activates downstream effector
caspase-3 and PARP, and finally initiates caspase-dependent
apoptosis (9). TRAIL therapy has
emerged as a promising cancer therapeutic strategy. However, some
tumor cells can downregulate the expression of TRAIL-receptors and
acquire resistance to TRAIL-induced apoptosis (10,11).
As such, TRAIL combination treatments that can upregulate
TRAIL-receptor expression or inhibit downregulation were proved to
be of therapeutic benefit (12–14).
The ubiquitin-proteasome system (UPS) plays a vital
role in the regulation of protein levels in eukaryotic cells, and
related to cell proliferation, survival, differentiation and
programmed cell death (15,16). The activity of UPS is closely
associated with the incidence and progression of many human
malignant tumors. Now, targeting the proteasome is evidenced to be
one of the most effective strategies for cancer treatment (17). MG132, a reversible peptide-aldehyde
of specific proteasome inhibitor (Fig.
1A), can effectively inhibit the activity of 26S proteasome and
cause accumulation of certain proteins harmful to the survival of
tumor cells, inducing cell cycle arrest and cell apoptosis
(18–20). Evidence has shown that MG132 could
inhibit the proliferation of tumor cells in a dose-dependent manner
in cell cycle transformation, and with the cell cycle protein
Cyclin A, Cyclin B and P27 accumulation, and cell cycle arrests in
G2/M phase (21). Likewise, it has
been reported that MG132 induced apoptosis of tumor cells by
caspase-related signal transduction pathway mediated by death
receptor (18). To date, the effect
of proteasome inhibitor MG132 on GBC still remains unclear, and
also the effect on TRAIL-induced apoptosis in GBC remains unknown.
Therefore, it may be more promising to uncover the mechanism that
links proteasome dysfunction caused by MG132 and TRAIL-induced
apoptosis to the treatment of GBC.
In this study, we investigated the effect of the
representative proteasome inhibitor MG132 on TRAIL-induced
apoptosis in GBC-SD cells in vitro and in vivo, and
further elucidated the mechanism of the cell apoptosis induction.
Our data evidenced that MG132 can inhibit the proliferation of
GBC-SD cells and induce apoptosis. The induction of apoptosis by
MG132 was mainly through the upregulation of DR5 and subsequent
caspase activation, which could promote TRAIL-induced apoptosis in
GBC-SD cells. Therefore, the combinatorial treatment of MG132 with
TRAIL could be a new and effective strategy for TRAIL-resistant
GBC.
Materials and methods
Reagents
The proteasome inhibitor MG132 (Z-Leu-Leu-Leucinal,
Sigma-Aldrich, St. Louis, MO, USA) was dissolved in dimethyl
sulfoxide (DMSO) as a 40-µM stock solution and stored at
−20°C. DMSO was purchased from the Beyotime Institute of
Biotechnology (Jiangsu, China). Antibodies against caspase-3, PARP,
caspase-8, DR5 and DR4 were obtained from Sigma-Aldrich. DMEM cell
culture medium and fetal bovine serum (FBS) were purchased from
Gibco Co. Ltd. (USA).
Cell line and mice
The human gallbladder cancer cell line (GBC-SD) was
purchased from the Committee on Type Culture Collection of Chinese
Academy of Sciences (Shanghai, China). Female BALB/c mice, 7 weeks
of age and weighing ~25 g, were obtained from the Science
Department of Experimental Animals of Fudan University in China.
All mice were housed in a specific pathogen-Free (SPF) level B
animal facility. The study was approved by the Review Board of
Fudan University Shanghai Cancer Center and Fudan Medical
College.
Cell culture and experimental
conditions
GBC-SD cells were cultured in DMEM medium
supplemented with 10% FBS and 1% penicillin-streptomycin in a
humidified incubator containing 5% CO2 at 37°C. Then,
the cells were seeded into 96-well plates at a density of 2,000
cells per well, incubated for 12 h, and further treated with
different concentrations of MG132 (2.5, 5, 10, 20, 40, 80 and 160
µM) for 24, 48 and 72 h. Then, 10 µl of Cell Counting
Kit-8 (CCK-8, Dojindo, Kumamoto, Japan) reagent was added to each
well of the plates and the plates were incubated for 1 h at 37°C.
After removal of the supernatant, 150 µl of DMSO was added
to each well. The optical density (OD) was measured at 450 nm using
a spectrophotometric plate reader (Bio-Rad Co., Hercules, CA, USA)
and the cell survival rate was determined according to the
absorbance relative to that of untreated controls. Data are
reported as the mean ± standard deviation of OD values obtained in
each group. The concentrations of MG132 in this study were
previously confirmed as being the most favorable for the following
experiment.
Flow cytometric analysis of
apoptosis
Apoptosis was performed with the Annexin V/PI
apoptosis kit according to the manufacturer's instructions
(Invitrogen, Carlsbad, CA, USA). GBC-SD cells were treated with
MG132 (10 µM), TRAIL (100 ng/ml), and the combination of
MG132 and TRAIL (10 µM + 100 ng/ml) for 48 h. After washing
twice with cold PBS, the cells were resuspended in 100 µl
binding buffer at a density of ×106 cells/ml. Then, 2.5
µl of Annexin V-FITC and 1 µl of PI working solution
(100 µg/ml) were added to these cells and incubated for 30
min in the dark. The samples were analyzed by a flow cytometer (BD
Biosciences, San Diego, CA, USA).
Western blot assay
GBC-SD cells were harvested, washed twice with cold
PBS, and lysed in RIPA buffer (Beyotime, Shanghai, China)
supplemented with protease inhibitor (Roche Applied Science,
Indianapolis, IN, USA) at 4°C for 5 min. Total protein of the
supernatant was determined by the bicinchoninic acid (BCA) assay
kit (Beyotime) with BSA as a standard. Equal amounts of protein (40
µg/lane) from each group were separated by SDS-PAGE and
transferred to nitrocellulose membranes (Millipore, Bedford, MA,
USA). The membranes were blocked with 5% skim milk, and incubated
with the primary antibodies against caspase-3, PARP, caspase-8,
DR5, DR4, and GAPDH at 4°C overnight. Then, the membranes were
incubated with a horseradish peroxidase-conjugated goat
anti-rabbit/anti-mouse secondary antibody (1:5,000; Abcam,
Cambridge, UK) for 1 h at room temperature, and visualized by a Gel
Doc 2000 (Bio-Rad).
Real-time PCR
Primers were synthesized and purchased from Beijing
Sunbiotech Co., Ltd. (Table I).
Total RNA of GBC-SD cells was extracted after 48 h of incubation
using the Purelink™ Micro-to-Midi purification system (Invitrogen
Co.). Then, the cDNA was synthesized using 1 µl of total RNA
with 0.5 µl AMV reverse transcriptase. PCR was carried out
using 2.5 µl cDNA, 0.1 µl Ex Taq HS, 0.1 µl
forward primer and 0.1 µl reverse primer. The PCR program
consisted of an initial 2-min step at 94°C, and 35 cycles of 15 sec
at 94°C, 40 sec at 60°C, and 1 min cycles at 72°C, followed by 72°C
for 5 min. The results were determined using a UV gel imaging
system and analyzed using Quantity One software (Bio-Rad Inc.), and
presented as the ratio of target genes to internal control
GAPDH.
 | Table IPrimer sequences for detection of
mRNA expression. |
Table I
Primer sequences for detection of
mRNA expression.
| Gene name | Sequence | Amplicons (bp) |
|---|
| Caspase-8 | F:
5′-CGACCTTTGGTAGGCCAATC-3′ | 356 |
| R:
5′-GCCAATTTGTATTGCCCAACTAT-3′ | |
| Caspase-3 | F:
5′-GGTTCATCCAGTCCCTTTGC-3′ | 278 |
| R:
5′-GCGAGTGAGAATGTGCATAAATTC-3′ | |
| PARP | F:
5′-ACGCACAATGCCTATGAC-3′ | 442 |
| R:
5′-CCAGCGGAACCTCTACAC-3′ | |
| DR4 | F:
5′-CTGAGCAACGCAGACTCGCTGTCCAC-3′ | 506 |
| R:
5′-TCCAAGGACACGGCAGAGCCTGTGCCAT-3′ | |
| DR5 | F:
5′-GCCTCATGGACAATGAGATAAAGGTGGCT-3′ | 502 |
| R:
5′-CCAAATCTCAAAGTACGCACAAACGG-3′ | |
| GAPDH | F:
5′-TGTGTCCGTCGTGGATCTGA-3′ | 346 |
| R:
5′-CCTGCTTCACCACCTTCTTGA-3′ | |
Xenograft tumor experiments
Subcutaneous injection of MG132 (10 µM),
TRAIL (100 ng/ml), and their combination were performed to evaluate
the growth of GBC-SD xenografts in athymic nude mice. All mice were
housed in the SPF level B animal facility. GBC-SD cells in
log-phase growth were subcutaneously injected into the right flank
of the mice. On day 10, the mice were randomly divided into four
groups (6 mice/group). The control group received an injection of
vehicle (10% DMSO and 90% PBS) intraperitoneally (i.p.) each day.
The other three groups were administered an i.p. injection of MG132
at 10 mg/kg, TRAIL at 80 µg/kg and the combination of MG132
and TRAIL, respectively, at 10 mg/kg and 80 µg/kg every day.
Visible subcutaneous tumor volumes were measured every 3 days with
calipers, and then calculated by the formula: volume = (length ×
width2) / 2, where length and width represent the length
and width of the tumor, respectively. After 3 weeks of the
treatment, all nude mice were sacrificed, and the tumor tissue was
removed and weighed.
Statistical analysis
All data are presented as mean ± SD. Statistical
analysis was conducted using SPSS 11.0 (SPSS Inc., Chicago, IL,
USA). Analysis of variance (ANOVA) was used to evaluate within
group data and one-way ANOVA was used to evaluate between group
data. Least squares difference was used for pairwise comparisons
between groups. The statistical significance was defined as
P<0.05.
Results
MG132 inhibits the proliferation of
GBC-SD cells and induces apoptosis
To investigate the effect of MG132 on the
proliferation of cells, GBC-SD cells were treated with increasing
concentrations of MG132 (2.5, 5, 10, 20, 40, 80 and 160 µM)
for 24, 48 and 72 h. We found that MG132 played a potent cytotoxic
role on GBC-SD cells in a time- and dose-dependent manner
(P<0.01) (Fig. 1B). Moreover,
this inhibitory rate in GBC-SD cells increased rapidly when the
increasing concentrations of MG132 were between 0 and 10 µM,
when the increasing inhibitory rate slowed down the concentrations
of MG132 were between 10 and 160 µM. Hence, MG132 has a more
sensitive killing effect on GBC-SD cells at the concentrations of
0–10 µM than at 10–160 µM, and we chose the low
concentrations of MG132 (2.5, 5 and 10 µM) at 48 h for the
following treatment. We further detected the hallmarks of apoptosis
in GBC-SD cells treated with MG132, and found that the protein
levels of cleavage of caspase-8, caspase-3 and PARP obviously
increased in a dose-dependent manner, which indicated that MG132
activated these caspases in the above cells (Fig. 1C). Therefore, MG132 clearly induces
apoptosis in GBC-SD cells, and has an important mechanism for its
tumor-killing effect.
MG132 induces apoptosis by upregulating the levels
of DR5 and DR4 in extrinsic apoptotic pathway in GBC-SD cells. The
data in a previous study (?) clearly exhibited that MG132
induced caspase-dependent apoptosis, especially activiated
caspase-8 which is an initiator caspase in the extrinsic apoptotic
pathway. To further determine the mechanism on the activation of
extrinsic apoptosis invovled in this experiment, we detected the
expression of DR5 and DR4 in the experiment, which are well-known
as TRAIL death receptors and can initiate extrinsic apoptotic
pathway. During the given concentration range of 2.5–10 µM,
MG132 obviously upregulated the protein levels of DR5 and DR4 in a
dose-dependent manner in GBC-SD cells, and the expression of DR5
was more obvious than that of DR4 (Fig.
2A and B). Consistent with the protein expression of DR5 and
DR4 in MG132-treated cells, the mRNA levels of DR5 and DR4 were
also substantially increased when treated with MG132 in GBC-SD
cells (Fig. 2C and D). These
results indicate that MG132 induces DR5 and DR4-dependent apoptosis
through extrinsic apoptotic pathway in GBC-SD cells.
MG132 potentiates TRAIL-induced apoptosis through
caspase-dependent pathway in GBC-SD cells. In the above
experiments, we confirmed that MG132 can enhance the expression of
DR5 and DR4 in GBC-SD cells, which have been proved to be major
death receptors for the death ligand TRAIL. Therefore, we
hypothesized that MG132 could enhance the apoptosis induced by
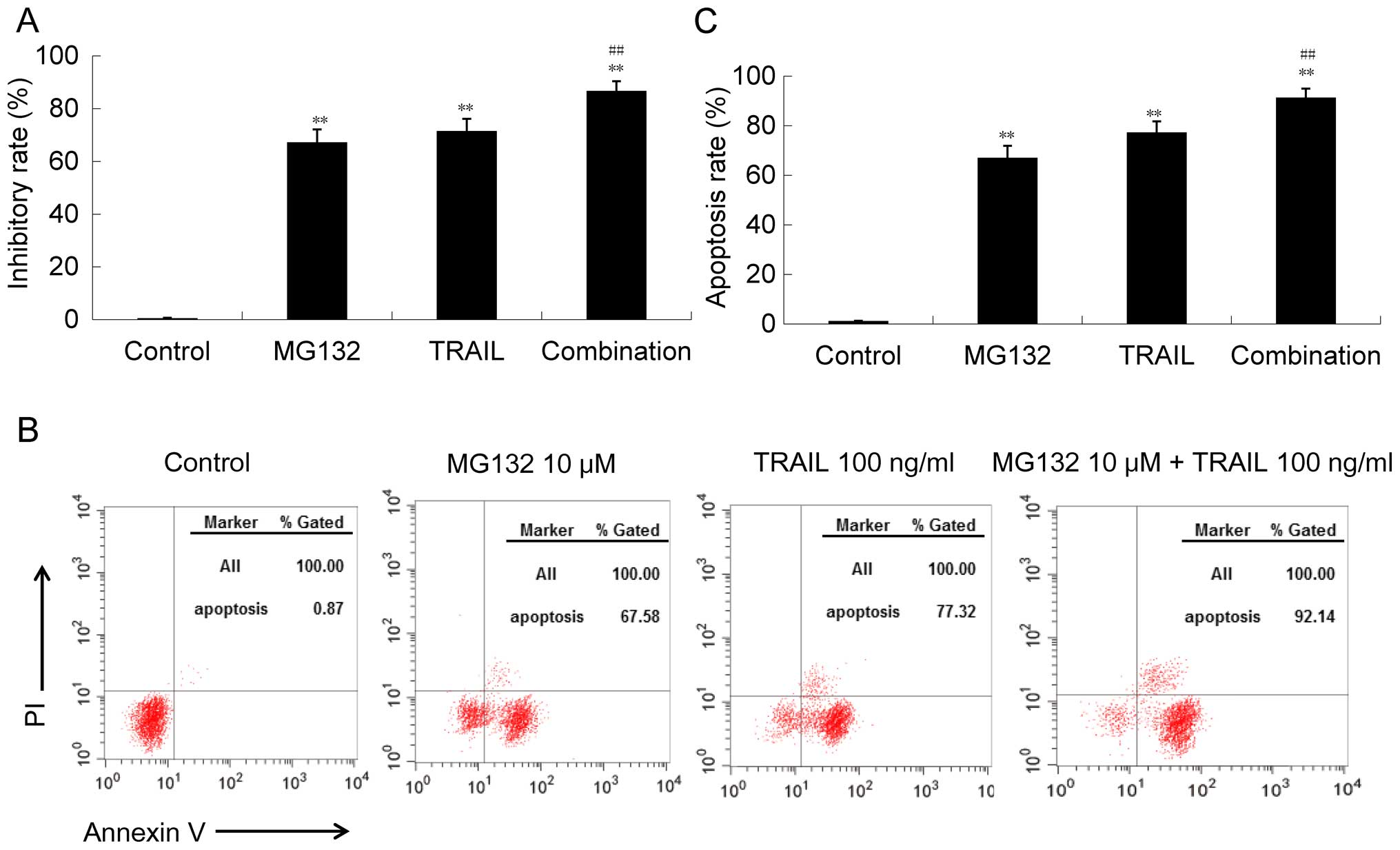
TRAIL. To confirm this hypothesis, we compared the cytotoxic effect
of MG132 alone, TRAIL alone, and their combination on GBC-SD cells.
We found that the given concentration of MG132 or TRAIL could
effectively inhibit the survival of GBC-SD cells, while the
combination could play a more effective role in the killing of
GBC-SD cells (Fig. 3A).
Consistently, the apoptosis induced by the combination of MG132 and
TRAIL was more potent than each single agent (Fig. 3B and C). Moreover, we determined
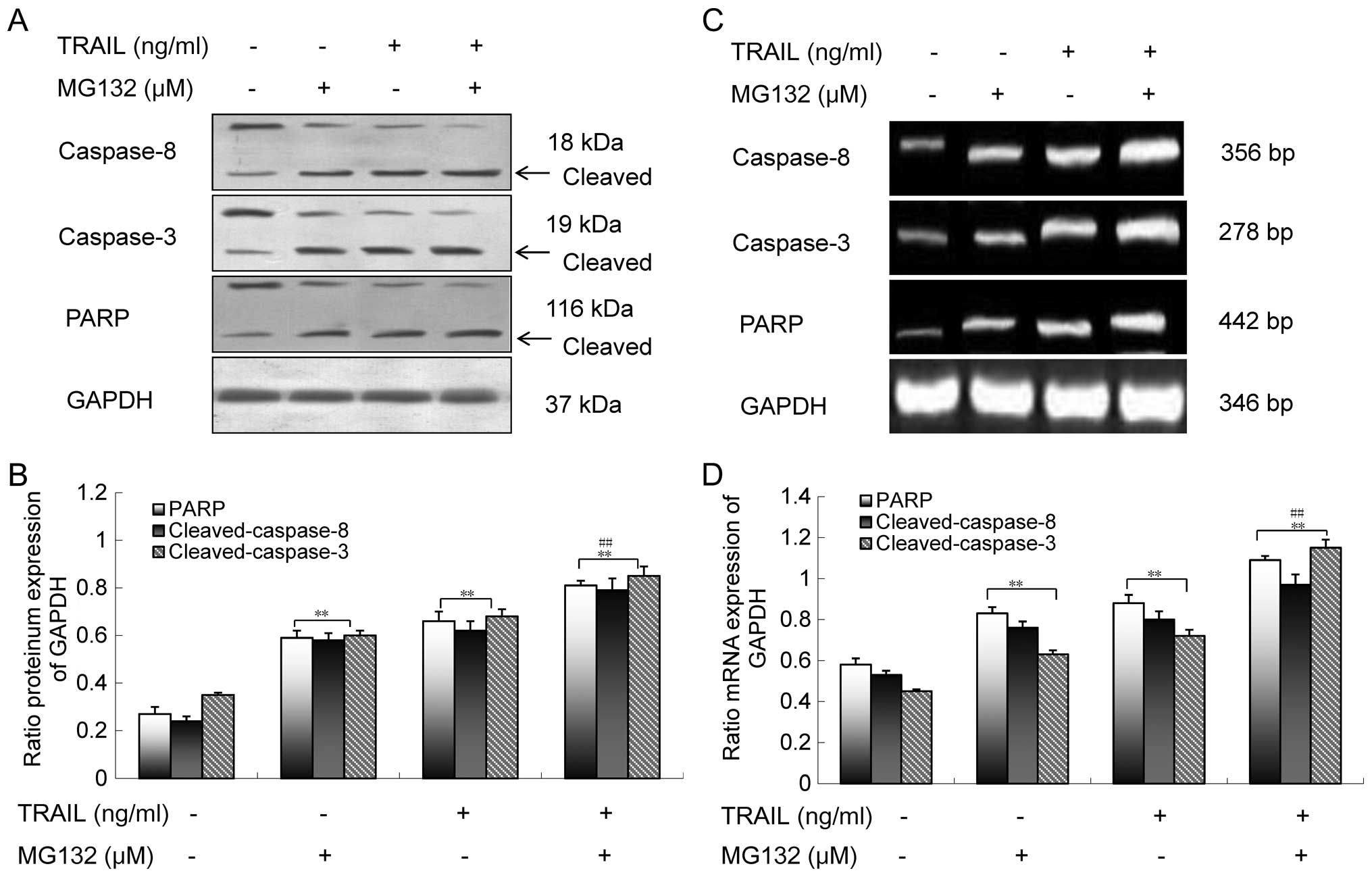
whether the combination of MG132 and TRAIL induces apoptosis by
activation of the caspase-dependent pathway. We compared the
effects of MG132 alone, TRAIL alone, and their combination in
inducing caspase-8, caspase-3 and PARP on GBC-SD cells, and found
that the tested protein and mRNA levels induced by the combination
were higher than those treated with either MG132 or TRAIL alone
(Fig. 4). Hence, these data further
support that MG132 enhances TRAIL-induced apoptosis through
caspase-dependent pathway in GBC-SD cells.
The combination of MG132 and TRAIL
augments the killing effect of gallbladder cancer cells in
vivo
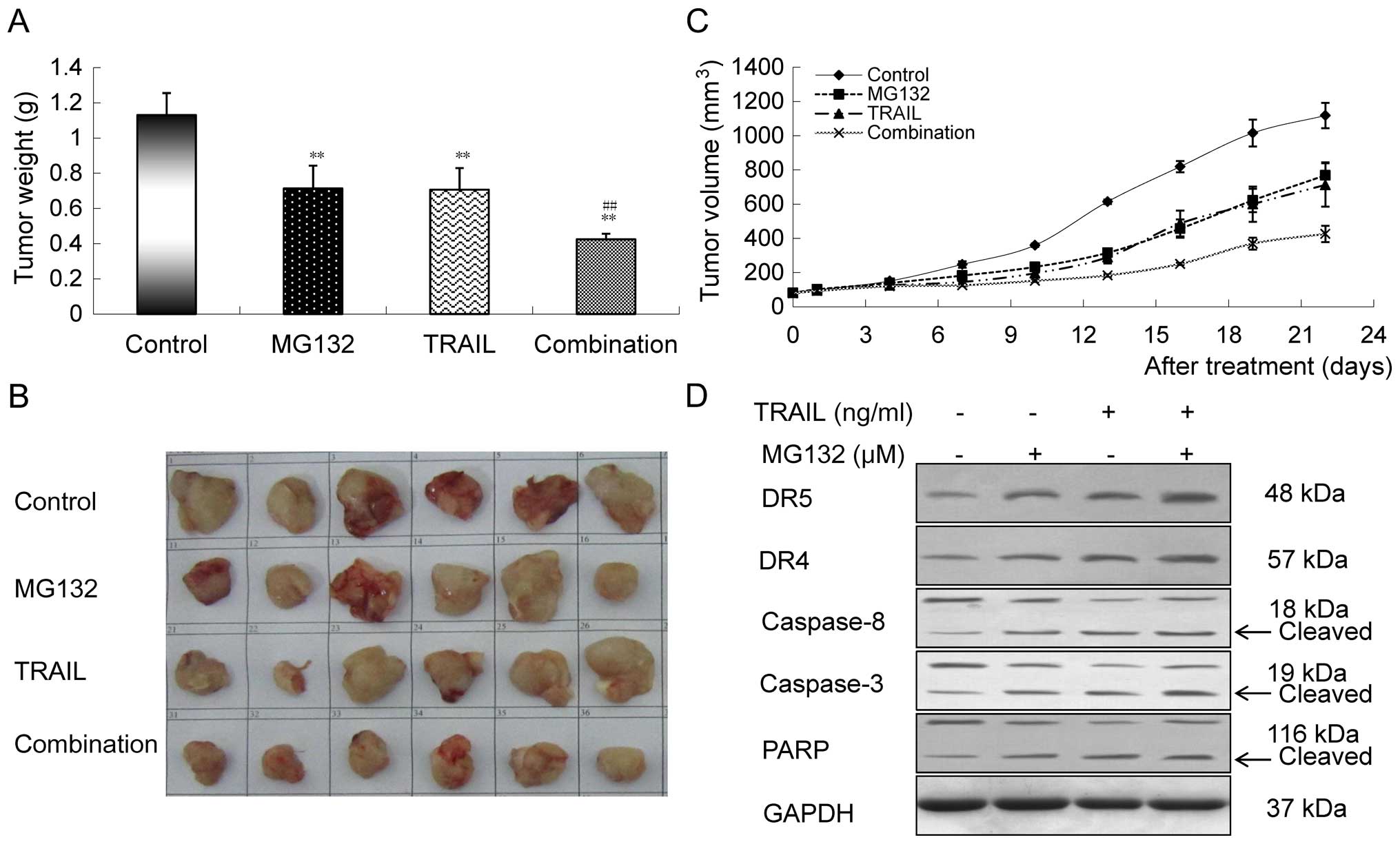
To observe whether MG132 could enhance the killing
effect of TRAIL on GBC-SD cells in vivo, we measured the
weight and volume of GBC-SD xenografts in this experiment (Fig. 5A and C). Tumors excised from the
mice are shown in Fig. 5B, and the
tumor weights after 3 weeks of treatment showed an decreasing
tendency from control to MG132 to TRAIL to combination groups
(Fig. 5A). The differences between
the groups were statistically significant (P<0.01). In addition,
a significant reduction was also detected in tumor volumes treated
with the combination compared to those treated with either MG132 or
TRAIL alone (Fig. 5C). To examine
whether the impact of MG132 and TRAIL on tumor growth inhibition
was related to extrinsic apoptotic pathway, we detected the protein
levels of DR5, DR4, caspase-8, caspase-3 and PARP in the
gallbladder tumor tissues by western blot analysis, and found that
the upregulation of DR5, DR4, cleaved caspase-3, caspase-8, and
cleaved PARP induced by the combination were more obvious than
those treated with either MG132 or TRAIL alone, which was in
agreement with the results of the in vitro experiment
(Fig. 5D).
Discussion
In this study, we demonstrated that the proteasome
inhibitor MG132 can effectively inhibit the proliferation of GBC-SD
cells and induce cell apoptosis in vitro and in vivo.
Apoptosis of GBC-SD cells induced by MG132 were mainly through the
activation of extrinsic apoptosis pathway. In addition, we
evidenced that MG132 can enhance TRAIL-induced apoptosis in GBC-SD
cells in vivo and in vitro. As far as we know, this
is the first study to indicate that MG132 induces apoptosis in
GBC-SD cells by activating the extrinsic apoptotic pathway.
Death receptor DR5 and DR4 are pro-apoptotic
receptors of TRAIL ligand, which can trigger the extrinsic
apoptotic signaling when interacting with TRAIL (7). To date, TRAIL-receptors, especially
DR5 and DR4, have emerged as key mediators of cell apoptosis
(12,22). In this study, we found that MG132
can upregulate the expression of DR5 and DR4 protein and mRNA in
GBC-SD cells in a dose-dependent manner, and the expression level
of DR5 was significantly stronger than that of DR4. Then it was
investigated whether upregulation of DR5 caused by MG132 could
potentiate TRAIL-induced apoptosis in GBC-SD cells.
The ubiquitin-proteasome system (UPS) is a key
regulator of protein degradation in cellular biological processes
including apoptosis (23). It has
been confirmed that death receptor-mediated apoptotic pathway is
regulated by UPS (24). In this
study, we found that proteasome inhibitor MG132 can increase the
expression level of DR5 protein in a dose-dependent manner. Thus,
we speculate that the upregulation of DR5 protein in tumor cells
may be attributed to the dysfunction of proteasome caused by MG132.
Furthermore, the expression of DR5 also increased at the
transcriptional level. In this study, we observed that MG132 can
enhance the expression level of DR5 mRNA in a dose-dependent
manner. Hence, the transcriptional regulation of DR5 expression is
another mechanism for the upregulation of DR5 expression. In
conclusion, the upregulation of DR5 expression caused by MG132 may
be due to enhanced protein stability and gene transcription, which
is consistent with previous studies (25–27).
At present, TRAIL has been proven to have
significant potential in cancer therapy for its good tumor
specificity and favorable safety (28). TRAIL-based therapies are being
increasingly explored also in clinical trials (29,30).
Unfortunately, many tumor cells are resistant to TRAIL-induced
apoptosis. Combination therapy has been evidenced to be an
effective way to overcome tumor resistance. Some studies have
demonstrated that treatment with proteasome inhibitors can
upregulate TRAIL-receptors expression on tumor cell surface, which
promoted sensitization to TRAIL-induced apoptosis (31,32).
In this study, we found that MG132 in vitro and in
vivo can enhance TRAIL-mediated apoptosis in GBC-SD cells, and
this process involves upregulation of DR5 and caspase-dependent
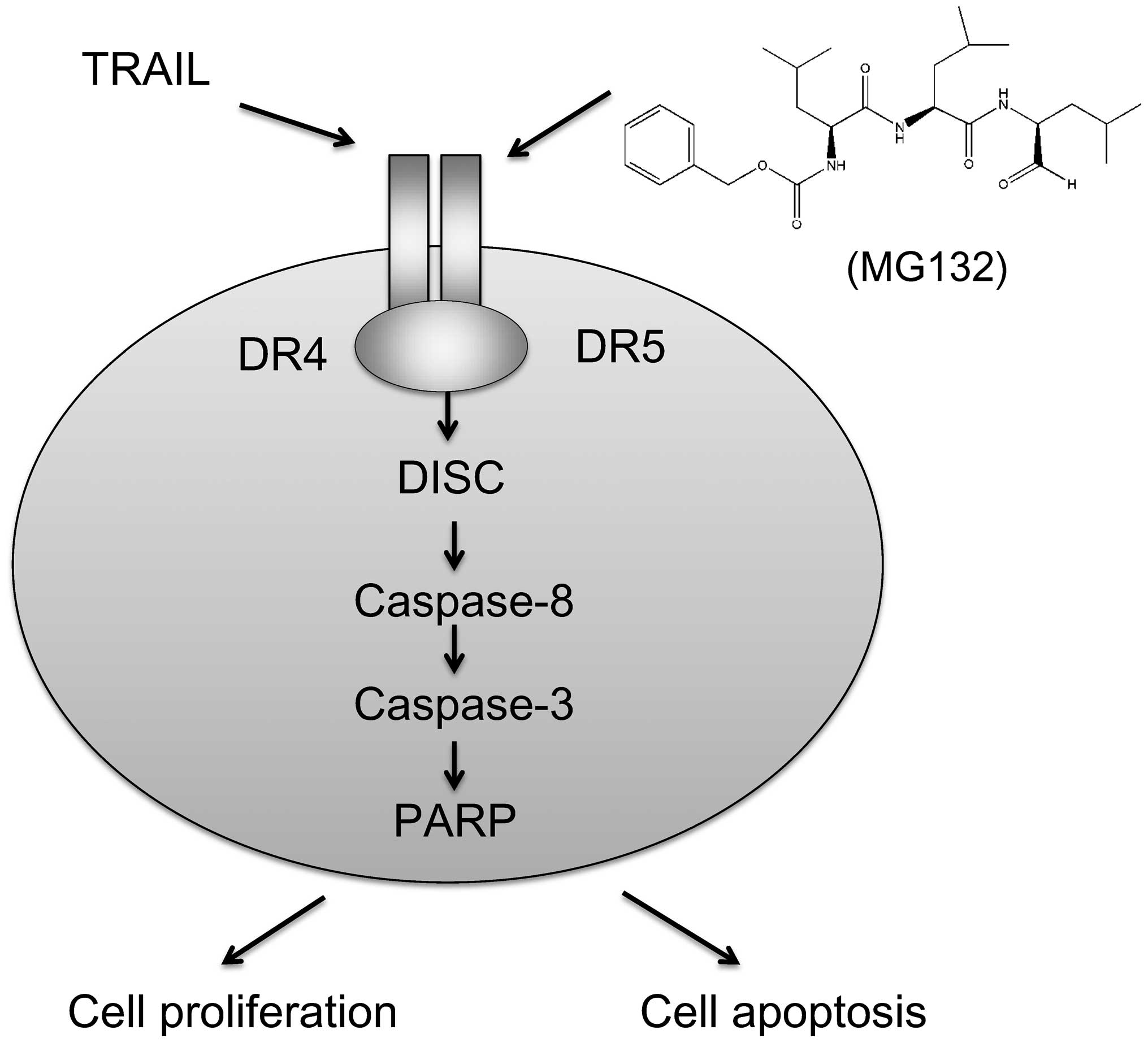
pathways (Fig. 6). Thus, we
speculate that MG132 can enhance TRAIL-induced apoptosis via
upregulating DR5 expression in GBC-SD cells, which may overcome
TRAIL resistance in cancer therapy.
In conclusion, we have established that proteasome
inhibitor MG132 can potentiate TRAIL-induced apoptosis both in
vitro and in vivo, and uncovered that MG132 induces
apoptosis by its effect on the upregulation of DR5. In this study,
we have provided experimental evidence demonstrating a possible
tumor suppressor role of MG132 in GBC-SD cells, and its increasing
effect on TRAIL-mediated activities.
Acknowledgments
The authors would like to thank Xin Wang for
technical support. This study was supported by the Minhang District
Natural Science Foundation of Shanghai (2012MHZ025), the Public
Health Bureau Youth Foundation of Shanghai (20134Y089), the Natural
Science Foundation of Shanghai (12nm0502202 and 114119a4700), the
Pudong New Area Science and Technology Development Fund
(PKJ2012-Y24), and the Pudong New Area Health System discipline
lead development program (PWRd2013-10).
Abbreviations:
|
GBC
|
gallbladder cancer
|
|
TNF
|
tumor necrosis factor
|
|
TRAIL
|
tumor necrosis factor-related
apoptosis-inducing ligand
|
|
DISC
|
death inducing signaling complex
|
|
UPS
|
ubiquitin-proteasome system
|
References
|
1
|
Bizama C, García P, Espinoza JA, Weber H,
Leal P, Nervi B and Roa JC: Targeting specific molecular pathways
holds promise for advanced gallbladder cancer therapy. Cancer Treat
Rev. 41:222–234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Misra S, Chaturvedi A, Misra NC and Sharma
ID: Carcinoma of the gallbladder. Lancet Oncol. 4:167–176. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Henley SJ, Weir HK, Jim MA, Watson M and
Richardson LC: Gallbladder cancer incidence and mortality, United
States 1999–2011. Cancer Epidemiol Biomarkers Prev. 24:1319–1326.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chan E and Berlin J: Biliary tract
cancers: Understudied and poorly understood. J Clin Oncol.
33:1845–1848. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Walczak H, Miller RE, Ariail K, Gliniak B,
Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, et al:
Tumoricidal activity of tumor necrosis factor-related
apoptosis-inducing ligand in vivo. Nat Med. 5:157–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nesterov A, Nikrad M, Johnson T and Kraft
AS: Oncogenic Ras sensitizes normal human cells to tumor necrosis
factor-alpha-related apoptosis-inducing ligand-induced apoptosis.
Cancer Res. 64:3922–3927. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan G, O'Rourke K, Chinnaiyan AM, Gentz R,
Ebner R, Ni J and Dixit VM: The receptor for the cytotoxic ligand
TRAIL. Science. 276:111–113. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bellail AC, Tse MC, Song JH, Phuphanich S,
Olson JJ, Sun SY and Hao C: DR5-mediated DISC controls caspase-8
cleavage and initiation of apoptosis in human glioblastomas. J Cell
Mol Med. 14A:1303–1317. 2010. View Article : Google Scholar
|
|
9
|
Fernald K and Kurokawa M: Evading
apoptosis in cancer. Trends Cell Biol. 23:620–633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shankar S and Srivastava RK: Enhancement
of therapeutic potential of TRAIL by cancer chemotherapy and
irradiation: mechanisms and clinical implications. Drug Resist
Updat. 7:139–156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shin EA, Sohn EJ, Won G, Choi JU, Jeong M,
Kim B, Kim MJ and Kim SH: Upregulation of microRNA135a-3p and death
receptor 5 plays a critical role in Tanshinone I sensitized
prostate cancer cells to TRAIL induced apoptosis. Oncotarget.
5:5624–5636. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stuckey DW and Shah K: TRAIL on trial:
Preclinical advances in cancer therapy. Trends Mol Med. 19:685–694.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sarhan D, Wennerberg E, D'Arcy P, Gurajada
D, Linder S and Lundqvist A: A novel inhibitor of proteasome
deubiquitinating activity renders tumor cells sensitive to
TRAIL-mediated apoptosis by natural killer cells and T cells.
Cancer Immunol Immunother. 62:1359–1368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dokouhaki P, Schuh NW, Joe B, Allen CA,
Der SD, Tsao MS and Zhang L: NKG2D regulates production of soluble
TRAIL by ex vivo expanded human γδ T cells. Eur J Immunol.
43:3175–3182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nalepa G, Rolfe M and Harper JW: Drug
discovery in the ubiquitin-proteasome system. Nat Rev Drug Discov.
5:596–613. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Adams J: The proteasome: A suitable
antineoplastic target. Nat Rev Cancer. 4:349–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johnson DE: The ubiquitin-proteasome
system: Opportunities for therapeutic intervention in solid tumors.
Endocr Relat Cancer. 22:T1–T17. 2015. View Article : Google Scholar
|
|
18
|
He Q, Huang Y and Sheikh MS: Proteasome
inhibitor MG132 upregulates death receptor 5 and cooperates with
Apo2L/TRAIL to induce apoptosis in Bax-proficient and -deficient
cells. Oncogene. 23:2554–2558. 2004. View Article : Google Scholar
|
|
19
|
Pandit B and Gartel AL: Proteasome
inhibitors induce p53-independent apoptosis in human cancer cells.
Am J Pathol. 178:355–360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ustundag Y, Bronk SF and Gores GJ:
Proteasome inhibition-induces endoplasmic reticulum dysfunction and
cell death of human cholangiocarcinoma cells. World J
Gastroenterol. 13:851–857. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim OH, Lim JH, Woo KJ, Kim YH, Jin IN,
Han ST, Park JW and Kwon TK: Influence of p53 and p21Waf1
expression on G2/M phase arrest of colorectal carcinoma HCT116
cells to proteasome inhibitors. Int J Oncol. 24:935–941.
2004.PubMed/NCBI
|
|
22
|
Watts TH: TNF/TNFR family members in
costimulation of T cell responses. Annu Rev Immunol. 23:23–68.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vucic D, Dixit VM and Wertz IE:
Ubiquitylation in apoptosis: A post-translational modification at
the edge of life and death. Nat Rev Mol Cell Biol. 12:439–452.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song JJ, Szczepanski MJ, Kim SY, Kim JH,
An JY, Kwon YT, Alcala MA Jr, Bartlett DL and Lee YJ:
c-Cbl-mediated degradation of TRAIL receptors is responsible for
the development of the early phase of TRAIL resistance. Cell
Signal. 22:553–563. 2010. View Article : Google Scholar :
|
|
25
|
Li X, Huang T, Jiang G, Gong W, Qian H and
Zou C: Proteasome inhibitor MG132 enhances TRAIL-induced apoptosis
and inhibits invasion of human osteosarcoma OS732 cells. Biochem
Biophys Res Commun. 439:179–186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han B, Yao W, Oh YT, Tong JS, Li S, Deng
J, Yue P, Khuri FR and Sun SY: The novel proteasome inhibitor
carfilzomib activates and enhances extrinsic apoptosis involving
stabilization of death receptor 5. Oncotarget. 6:17532–17542. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ko H, Jeong MH, Jeon H, Sung GJ, So Y, Kim
I, Son J, Lee SW, Yoon HG and Choi KC: Delphinidin sensitizes
prostate cancer cells to TRAIL-induced apoptosis, by inducing DR5
and causing caspase-mediated HDAC3 cleavage. Oncotarget.
6:9970–9984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kelley SK and Ashkenazi A: Targeting death
receptors in cancer with Apo2L/TRAIL. Curr Opin Pharmacol.
4:333–339. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Merchant MS, Geller JI, Baird K, Chou AJ,
Galli S, Charles A, Amaoko M, Rhee EH, Price A, Wexler LH, et al:
Phase I trial and pharmacokinetic study of lexatumumab in pediatric
patients with solid tumors. J Clin Oncol. 30:4141–4147. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jazirehi AR, Kurdistani SK and Economou
JS: Histone deacetylase inhibitor sensitizes apoptosis-resistant
melanomas to cytotoxic human T lymphocytes through regulation of
TRAIL/DR5 pathway. J Immunol. 192:3981–3989. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee YJ, Seol JW, Jeong JK, Moon MH and
Park SY: Inhibition of the ubiquitin-proteasome system sensitizes
TRAIL-resistant prostate cancer cells by up-regulation of death
receptor 5. Mol Med Rep. 4:1255–1259. 2011.PubMed/NCBI
|
|
32
|
de Wilt LH, Kroon J, Jansen G, de Jong S,
Peters GJ and Kruyt FA: Bortezomib and TRAIL: A perfect match for
apoptotic elimination of tumour cells? Crit Rev Oncol Hematol.
85:363–372. 2013. View Article : Google Scholar
|