Introduction
Myelodysplastic syndrome (MDS) is a heterogeneous
group of clonal hematopoietic stem cell malignancies characterized
by bone marrow failure, morphologic dysplasia of bone marrow cells,
pancytopenia in the peripheral blood and a high risk of acute
myeloid leukemia (1,2). MDS tends to occur in the elderly
(median age at diagnosis, 71–76 years) (3,4) who
cannot afford the complications following hematopoietic stem cell
transplantation (HSCT) and high intensity chemotherapy (5). Low doses of arsenic trioxide (ATO), a
common agent used for the treatment of patients with acute
promyelocytic leukemia (APL), can induce complete remission without
myelosuppression and causes only few adverse effects (6,7).
Consequently, it has similarly been applied to treat MDS patients
alone or in combination with another agent in clinical trials
(8–10). Unfortunately, the hematological
improvement rates of MDS patients were only 20–30% (11). Additionally, high doses of ATO cause
intolerable toxicity (12).
Furthermore, MDS, as a pre-leukemia status, exhibits the
characteristics of leukemia transformation and relapse, which may
be closely associated with pre-leukemic stem cells (pre-LSCs) and
leukemic stem cells (LSCs) (13,14).
Effective killing of MDS cells, as well as pre-LSCs and LSCs, by
combining ATO with a chemosensitizer may be a potential strategy to
improve the response rate of MDS patients to ATO treatment, block
leukemic transformation and prevent MDS relapse. Previous studies
have reported the combination of ATO with another agent, including
thalidomide, ascorbic acid and cytarabine, in vitro and
in vivo (11,15,16);
however, few studies have been conducted to assess the combination
of ATO with a chemosensitizer, particularly curcumin (CUR).
CUR, a type of polyphenol plant derived from the
rhizome of turmeric, is widely used as chemopreventive and
chemosensitization agent and has extensively been studied in
various types of cancers, including leukemia, colon, breast, liver
and lung cancer (17). Accumulating
research has revealed that CUR sensitizes neoplasms to diverse
chemotherapeutic drugs in vivo and in vitro,
including vincristine, melphalan, butyrate, cisplatin, 5-FU,
vinorelbine, gemcitabine and oxaliplatin (18–22).
CUR interferes with diverse processes in cancer cells, including
the cell cycle, apoptosis, proliferation, survival, invasion,
metastasis and inflammation (23),
which may be associated with its sensitizing effect. It
downregulated various growth regulatory pathways and targets
including NF-κB, STAT3, COX2, Akt, apoptosis-related proteins,
growth factor receptors and multidrug-resistance proteins (17). More importantly, CUR is readily
available and safe, thus representing an ideal chemosensitizer.
The SKM-1 cell line, which was established from
leukemia cells from a 76-year-old Japanese male patient with overt
mono-blastic leukemia following MDS (24–26),
is an established MDS cell model for investigating MDS in
vitro. KG1a cells demonstrate characteristics of LSCs,
including self-renewal potential, resistance to chemotherapy and
immunotherapy, and a CD34+CD38- cell
phenotype (27,28). KG1a cells are thus considered to be
leukemia stem-like cells, and they provide an ideal cell model for
investigating LSCs in vitro.
In the present study, we explored the ability of CUR
to sensitize SKM-1 and KG1a cells to ATO by investigating the
cytotoxic efficiency and molecular mechanisms of CUR and ATO alone
and in combination in SKM-1 and KG1a cells in vitro.
Materials and methods
Reagents
RPMI-1640 medium (11875093), fetal bovine serum
(FBS) (16000-044) (both from Gibco, Grand Island, NY, USA)
penicillin and streptomycin (P11-010; PAA Laboratories, Dartmouth,
MA, USA), dimethyl sulfoxide (DMSO) (A3009; AppliChem GmbH,
Darmstadt, Germany), CUR (458-37-7; Sigma, St. Louis, MO, USA), ATO
(ShuangLu Corp., Beijing, China),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Seebio Biotech, Inc., Shanghai, China), hydroxypropyl
methylcellulose (MP Biomedicals, Santa Ana, CA, USA), the FITC
Annexin V apoptosis detection kit I, anti-PARP (1:500) (both from
BD Biosciences, San Jose, CA, USA) anti-caspase-3 (1:5,000) [Cell
Signaling Technology (CST) Danvers, MA, USA] anti-survivin
(1:5,000; BD Biosciences), the Human Apoptosis Antibody Array kit
(RayBio, Norcross, GA, USA), electrophoresis apparatus trophoresis
(EPS200; Tanon Science and Technology Co., Ltd., Shanghai, China),
and the LI-COR Odyssey scanner (LI-COR, Lincoln, NE, USA) were
used.
Cell lines and culture
SKM-1 cells were purchased from Jennio Biotech Co.
(Guangzhou, China), and KG1a cells were provided by She et
al (28). Cells were cultured
in RPMI-1640 medium with 10% inactivated FBS, penicillin and
streptomycin at 37°C with 5% CO2.
Cell viability assays
Cell viability was detected using the MTT assay.
SKM-1 and KG1a cells in logarithmic phase were seeded into 96-well
plates at 5×105 cells/ml in the presence or absence of
the indicated test samples in a final volume of 0.2 ml for 24 or 48
h at 37°C with 5% CO2. Next, 20 μl of MTT
solution [5 mg/ml in phosphate-buffered saline (PBS)] was added to
each well and incubated for 4 h at 37°C, followed by the addition
of 200 μl of DMSO. Finally, the plates were gently shaken
and analyzed at 490 nm using a microplate reader (Multiskan MK3;
Shanghai). Each experiment was performed in triplicate. The cell
viabilities in the two cell lines were calculated as follows:
Inhibition (%) =1 − (OD value of experimental samples/OD value of
control samples) × 100%.
Methylcellulose colony formation
test
Approximately 500 untreated or treated cells/well
were cultured in RPMI-1640 medium supplemented with 0.9%
methylcellulose and 20% FBS in a final volume of 2 ml of 24-well
plate at 37°C with 5% CO2. The number of colonies formed
(>50 cells) was counted under a light microscope after 14 days
of incubation. The experiments were performed in triplicate.
Analysis of apoptosis using Annexin
V/PI
The apoptotic cells were examined by Annexin V
binding assays according to the manufacturer's instructions (WinMDI
2.9 software; BD Corporation). Briefly, ~1.0×106 cells
in 6-well plates were treated with various concentrations of the
indicated test samples at 37°C with 5% CO2 for 48 h. The
cells were then harvested for subsequent experiments. The cells
were washed three times with cold PBS and then re-suspended in 1X
binding buffer at a concentration of 1×106 cells/ml, and
100 μl of the solution (1×105 cells) was
transferred to a 5-ml culture tube, followed by the addition of 5
μl of FITC Annexin V and 5 μl PI and incubation for
15 min at room temperature (25°C) in the dark. Finally, 400
μl of 1X binding buffer was added to each tube, and the
cells were analyzed by flow cytometry.
Western blot analysis
Total cellular proteins of SKM-1 and KG1a cells were
isolated using lysis buffer (RIPA). Equal amounts of protein were
subjected to 10 or 15% polyacrylamide gel electrophoresis and
transferred to polyvinylidene difluoride (PVDF) membranes. The
membranes were blocked with 5% skim-milk and incubated with primary
antibodies (anti-PARP, anti-caspase-3 and anti-survivin) overnight
at 4°C, followed by horseradish peroxidase-conjugated anti-mouse
secondary antibody at room temperature (25°C) for 2 h. The protein
bands were imaged using chemiluminescence reagent (CTB; USA), and
the band density values were analyzed using ImageJ software.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH; HC301; 1:5,000)
served as the internal reference.
Short interfering RNA (siRNA)
transfection of surviving
SKM-1 and KG1a cells in logarithmic phase were
moderately (106/ml) inoculated into 6-well plates for 24
h before transfection. Control scrambled siRNA was synthesized and
purchased from GenePharma, Co., Ltd. (Shanghai, China). siRNA
survivin (10 μM): 5′-GAGCCAAGAACAAAATTGC-3′ (29) or control scramble sequences were
transfected using Lipofectamine 2000 reagent (Invitrogen) strictly
according to the manufacturer's protocol. Briefly, 5 μl of
Lipofectamine 2000 was diluted in 250 μl of Opti-MEM medium
(Invitrogen) in each well. The mixture was gently added to a
solution containing siRNA in 250 μl of Opti-MEM medium,
incubated for 20 min, and then added to the plates. After
transfection with siRNA for 24 h, the cells were harvested for
subsequent assays.
Analysis of apoptosis-related proteins by
RayBio arrays
The expressions of 43 apoptosis-related proteins
were analyzed using a Human Apoptosis Antibody Array kit (RayBio,
Norcross, GA, USA). Briefly, according to instructions (available
from the RayBiotech Corp. official website (http://www.raybiotech.com/), each of the capture
antibodies was printed on the membranes, followed by the addition
of the treated or untreated cell lysate. After extensive washing,
the membranes were incubated with a cocktail of biotin-conjugated
anti-apoptotic protein antibodies. After incubation with the
infrared fluorescent agent-streptavidin, the fluorescence signals
were visualized using a LI-COR Odyssey scanner.
Statistical analysis
Data are represented as the mean ± standard
deviation (SD) and analyzed using SPSS 13.0 and GraphPad Prism 5
software. Means of different groups were compared using one-way
ANOVA followed by Bonferroni's multiple comparisons to evaluate the
differences between two groups under multiple conditions. When the
data failed the normality test, the Kruskal-Wallis one-way ANOVA on
ranks was used for data that failed the normality test. A value of
p<0.05 was considered statistically significant. CompuSyn
software was used to evaluate the synergistic effects of drug
combinations. The combination index (CI) was generated by CompuSyn
software, where CI<1, CI=1 and CI>1 indicated synergism,
additive effect and antagonism, respectively.
Results
CUR inhibits cell growth and induces cell
apoptosis in SKM-1 and KG1a cells
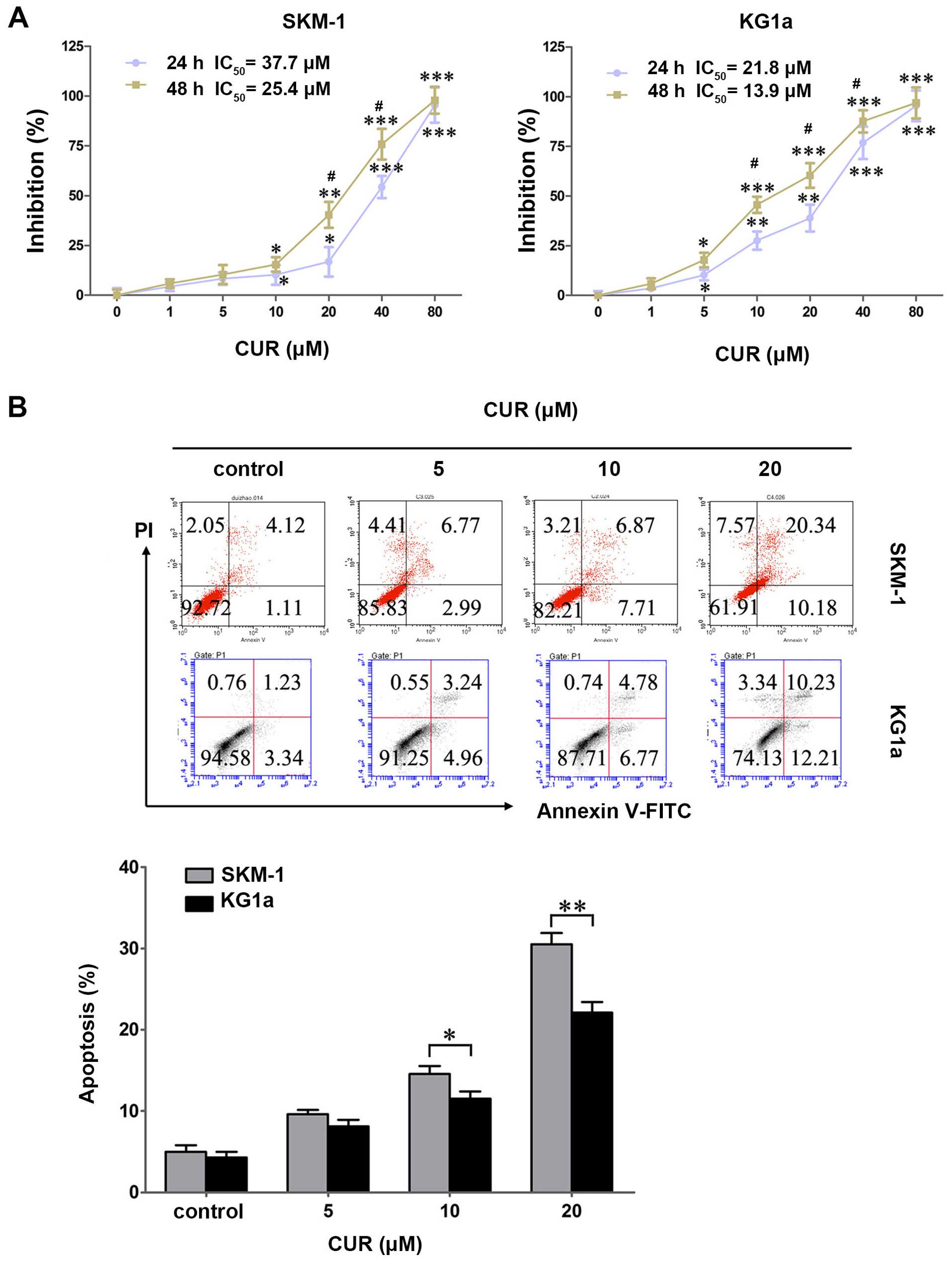
SKM-1 and KG1a cells were treated with various
concentrations of CUR (0–80 μM) for 24 and 48 h, and the
cytotoxic effects were detected by MTT assays. CUR exhibited a
growth inhibitory effect dose- and time-dependently in the two cell
lines (Fig. 1A). The
IC50 values in SKM-1 cells at 24 and 48 h were 37.7 and
25.4 μM, respectively, and those in KG1a cells were 21.8 and
13.9 μM, respectively.
To explore whether CUR induced apoptosis in SKM-1
and KG1a cells, the two cell lines were exposed to CUR for 48 h
followed by detection by Annexin V/PI. CUR induced early and late
apoptosis in a dose-dependent manner in the two cell lines
(Fig. 1B). By contrast, CUR induced
significantly more apoptosis in SKM-1 cells than in the KG1a cells
at an equivalent concentration (Fig.
1B), indicating that SKM-1 cells were more sensitive to CUR
when compared with KG1a cells in terms of apoptosis-induction.
These results were contrary to cytotoxic effects that the growth
inhibitory effect of CUR is higher for KG1a cells than in the SKM-1
cells. This finding demonstrated apoptosis may not be the
predominant mode of cell death, and it may also occur through
alternate pathways.
ATO inhibits cell growth and induces cell
apoptosis in SKM-1 and KG1a cells
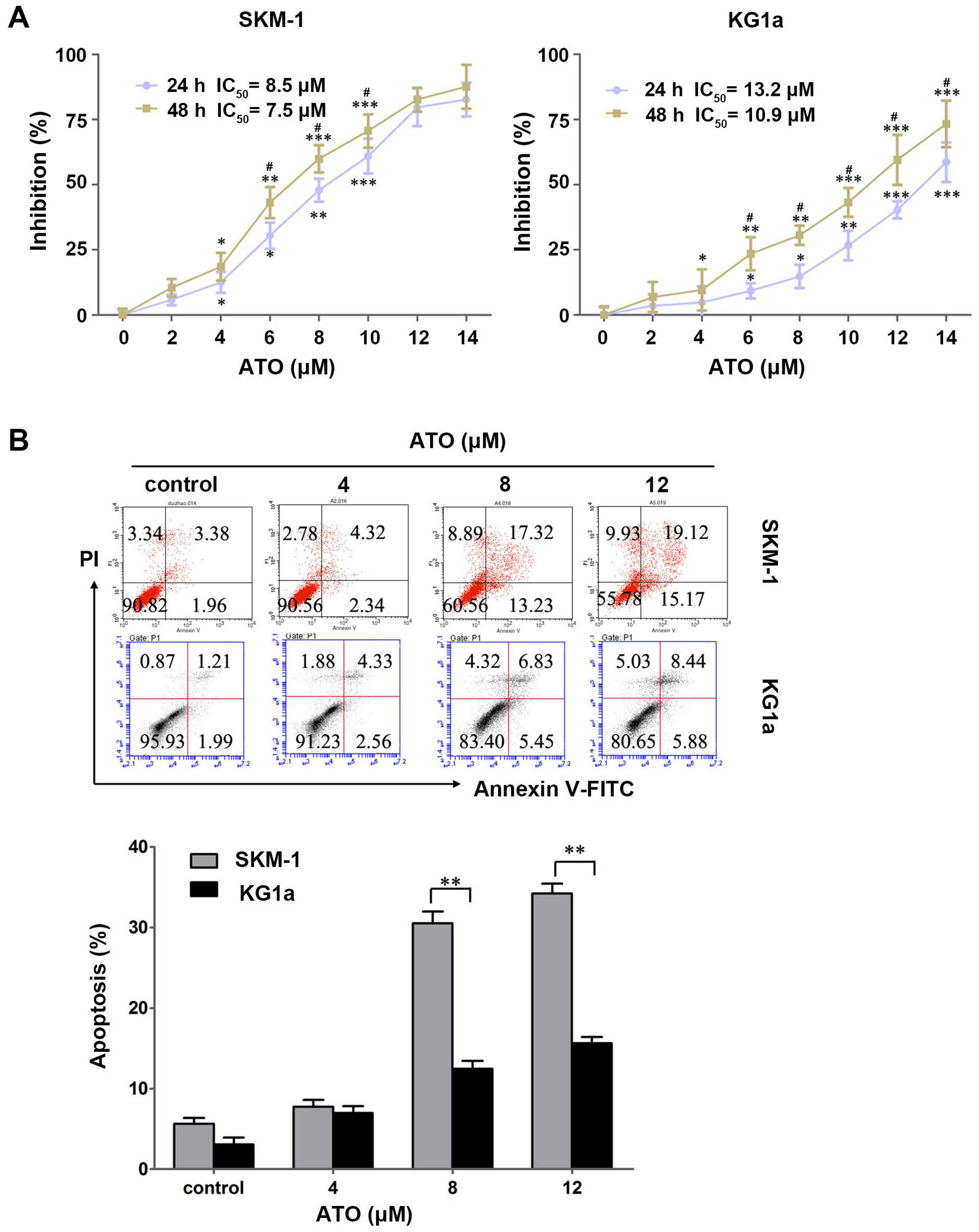
To evaluate the cytotoxic effects of ATO on the two
cell lines, SKM-1 and KG1a cells were treated with various
concentrations of ATO (0–14 μM) for 24 and 48 h, and the
cytotoxic effects were detected by MTT assays. ATO exhibited growth
inhibitory effects dose- and time-dependently in the two cell lines
(Fig. 2A). The IC50
values in SKM-1 cells at 24 and 48 h were 8.5 and 7.5 μM,
respectively, and those in KG1a cells were 13.2 and 10.9 μM,
respectively.
To clarity whether ATO induced apoptosis in SKM-1
and KG1a cells, the two cell lines were exposed to ATO for 48 h
followed by detection with Annexin V/PI. ATO induced early and late
apoptosis in a dose-dependent manner in the two cell lines
(Fig. 2B). Similar to the results
obtained for CUR, ATO induced significantly more apoptosis in SKM-1
cells than in KG1a cells at an equivalent concentration (Fig. 2B), indicating SKM-1 cells were more
sensitive to ATO compared with KG1a cells.
CUR increases ATO-induced apoptosis by
upregulating cleaved caspase-3 followed by PARP degradation in
SKM-1 and KG1a cells
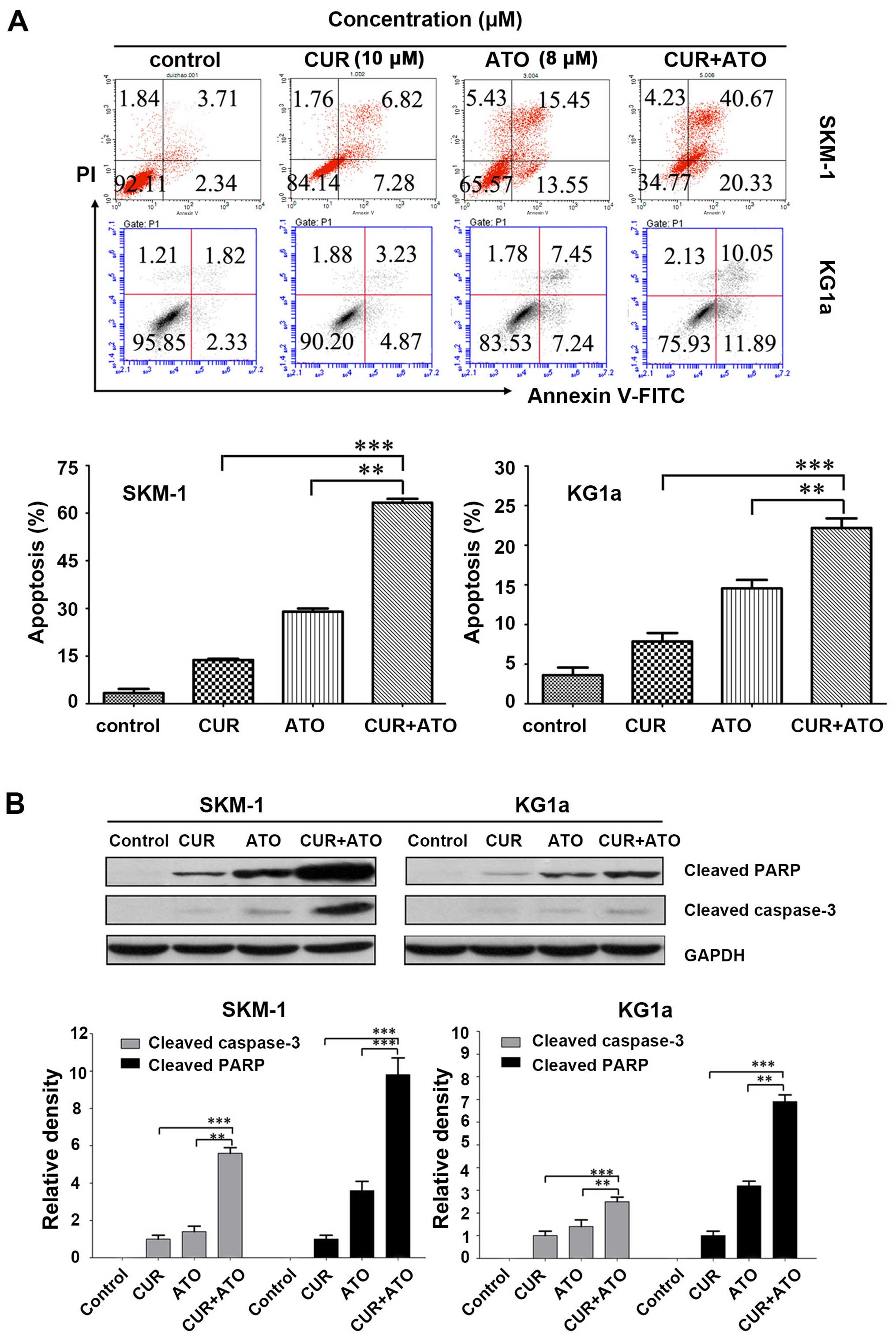
We determined whether CUR could increase ATO-induced
apoptosis in SKM-1 and KG1a cells by evaluating the pro-apoptotic
effects of CUR (10 μM) and ATO (8 μM) alone and in
combination (CUR + ATO) using Annexin V/PI. Apoptosis of the two
cell lines increased significantly in the CUR + ATO group compared
with the CUR or ATO alone groups (Fig.
3A), particularly in SKM-1 cells. For example, apoptotic SKM-1
cells in response to CUR and ATO alone and in combination were
13.7±0.4, 28.9±0.9 and 63.2±1.3%, respectively. Western blot
analysis further revealed that co-treatment with CUR and ATO
significantly induced caspase-3 activation and PARP cleavage
(Fig. 3B), two hallmarks of
apoptosis, in both SKM-1 and KG1a cells, which was consistent with
the results obtained by Annexin V/PI. These results strongly
indicated that CUR was able to enhance ATO-induced apoptosis and
sensitize SKM-1 and KG1a cells to ATO.
CUR synergistically enhances the
cytotoxic effects of ATO in SKM-1 and KG1a cells
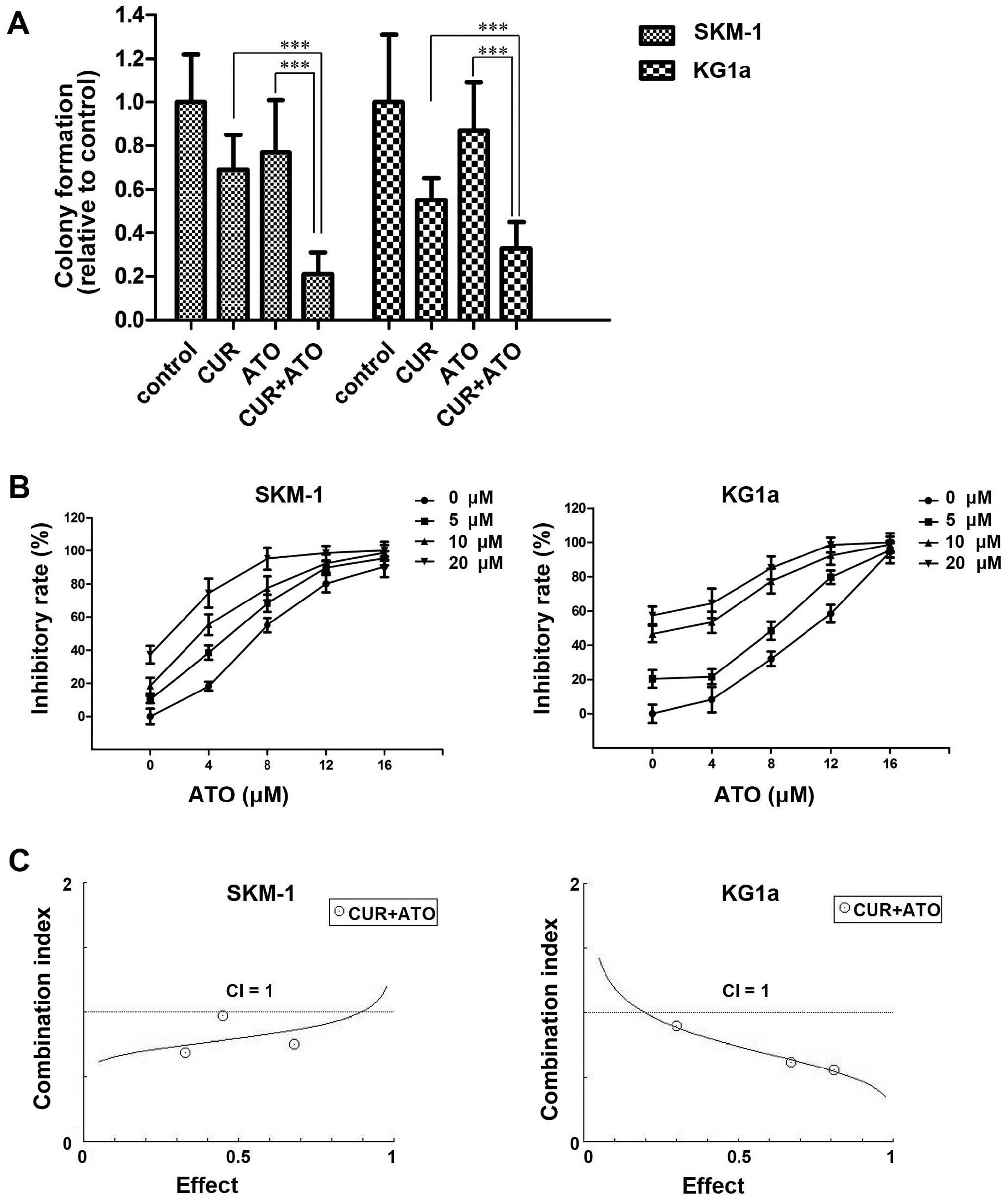
To evaluate the ability of CUR to enhance the
cytotoxic effects of ATO, we assessed the inhibition of the
clonogenicity of CUR and ATO alone or in combination in SKM-1 and
KG1a cells. Cells were treated with CUR (10 μM) and ATO (8
μM) alone or in combination for 48 h, followed by
inoculation in a methylcellulose for 14 days, and observation and
counting under a light microscope. Colony formation was
significantly reduced in the combination groups compared with the
CUR or ATO alone groups (Fig. 4A),
which indicated that CUR enhanced the ATO-induced inhibition of
colony formation.
Furthermore, we detected the inhibition of survival
in response to CUR and ATO alone or in combination in SKM-1 and
KG1a cells. Cells were exposed to a range of concentrations of ATO
(0–16 μM) and CUR (0–20 μM) for 48 h. The cell
viability in each sample was measured using MTT assays. The
dose-response curve of ATO was shifted to the left by CUR (Fig. 4B). Accordingly, CUR enhanced the
cytotoxic effects of ATO on SKM-1 and KG1a cells. To evaluate the
synergism of CUR and ATO in the two cell lines, cells were treated
with combinations of the two drugs at different doses but at a
constant ratio (CUR to ATO: 5-4, 10-8 and 20-16 μM,
respectively) for 48 h. Synergistic effects were estimated using
CompuSyn software. The CI values were <1 in both cell lines
(Fig. 4C), demonstrating the
synergism of CUR and ATO in combination.
In summary, these results demonstrated that CUR
synergistically enhanced the cytotoxic effects of ATO and
sensitized SKM-1 and KG1a cells to ATO.
Effects of CUR and ATO alone or in
combination on the expression of apoptosis-related proteins in
SKM-1 and KG1a cells
To explore the mechanisms and potential targets by
which CUR increased ATO-induced apoptosis, we detected 43
apoptosis-related proteins using apoptosis antibody array assays in
SKM-1 and KG1a cells treated with CUR (10 mM) and ATO (8 mM) alone
or in combination for 48 h. Fold-changes ≤0.667 or ≥1.5 usually
indicated that the protein expression level was modulated. As shown
in Table I, 27 proteins were
upregulated and four proteins were downregulated in SKM-1 cells in
the drug combination group (Table
I). In addition, five proteins were upregulated and seven
proteins were downregulated in KG1a cells in the drug combination
group (Table I). Caspase-3 was
upregulated significantly in both SKM-1 and KG1a cells, in
accordance with the results of the western blot analysis (Fig. 3B). The protein expression level of
survivin was significantly upregulated in the ATO group, but
significantly downregulated in the CUR and drug combination groups
in both SKM-1 and KG1a cells (Table
I). Thus, we inferred that survivin may be a potential target
of sensitizing SKM-1 and KG1a cells to ATO.
 | Table IExpression of apoptosis-related
proteins in the treated groups. |
Table I
Expression of apoptosis-related
proteins in the treated groups.
| Apoptosis-related
proteins | SKM-1
| KG1a
|
|---|
| CUR/control
(fold-change) | ATO/control
(fold-change) | CUR+ATO/control
(fold-change) | CUR/control
(fold-change) | ATO/control
(fold-change) | CUR+ATO/control
(fold-change) |
|---|
| bad | 1.141 | 1.741 | 2.044a | 1.224 | 0.951 | 0.854 |
| bax | 1.036 | 1.321 | 1.375 | 0.989 | 1.586 | 1.970a |
| bcl-2 | 0.996 | 2.094 | 2.304a | 0.775 | 1.895 | 0.428b |
| bcl-w | 1.083 | 1.892 | 2.098a | 0.852 | 1.907 | 0.667b |
| BID | 0.739 | 0.272 | 0.543b | 0.934 | 1.254 | 1.138 |
| BIM | 1.146 | 2.071 | 2.389a | 0.883 | 0.962 | 0.843 |
| caspase3 | 1.172 | 2.280 | 2.616a | 1.125 | 1.967 | 2.812a |
| caspase8 | 1.118 | 2.952 | 3.118a | 1.157 | 1.075 | 1.152 |
| CD40 | 0.930 | 1.534 | 1.634a | 0.789 | 0.870 | 0.771 |
| CD40L | 1.081 | 1.803 | 1.879a | 0.805 | 0.867 | 0.816 |
| cIAP-2 | 0.934 | 0.491 | 0.528b | 0.664 | 1.320 | 0.345b |
| cytoC | 1.011 | 2.156 | 2.918a | 1.314 | 1.916 | 2.848a |
| DR6 | 1.063 | 1.448 | 1.588a | 0.792 | 0.924 | 0.822 |
| Fas | 1.272 | 2.407 | 2.468a | 0.878 | 0.851 | 0.917 |
| FasL | 1.080 | 1.601 | 1.694a | 0.841 | 1.026 | 0.858 |
| HSP27 | 0.894 | 1.424 | 1.297 | 0.881 | 1.112 | 0.905 |
| HSP60 | 1.084 | 1.604 | 1.721a | 0.858 | 0.972 | 0.899 |
| HSP70 | 1.350 | 2.763 | 2.968a | 1.244 | 1.059 | 1.171 |
| HTRA | 0.664 | 2.784 | 2.288a | 2.088 | 1.250 | 2.874a |
| IGF-I | 0.891 | 1.187 | 1.163 | 0.794 | 1.068 | 0.720 |
| IGF-II | 1.123 | 2.707 | 2.948a | 1.009 | 1.334 | 1.070 |
| IGFBP-1 | 0.933 | 1.241 | 1.304 | 0.701 | 0.912 | 0.599b |
| IGFBP-2 | 0.971 | 1.223 | 1.253 | 0.794 | 0.978 | 0.479b |
| IGFBP-3 | 1.119 | 1.769 | 1.888a | 0.749 | 0.891 | 0.878 |
| IGFBP-4 | 0.974 | 1.980 | 2.012a | 0.782 | 0.961 | 0.805 |
| IGFBP-5 | 1.002 | 4.794 | 4.849a | 0.810 | 0.952 | 0.860 |
| IGFBP-6 | 1.102 | 0.811 | 0.905 | 0.829 | 1.042 | 0.867 |
| IGF-1sR | 0.929 | 1.320 | 1.340 | 0.745 | 0.965 | 0.880 |
| livin | 1.139 | 43.971 | 47.444a | 0.913 | 1.102 | 0.910 |
| p21 | 1.044 | 1.664 | 2.177a | 0.921 | 0.980 | 0.960 |
| p27 | 1.200 | 2.269 | 2.624a | 0.918 | 1.057 | 0.950 |
| p53 | 1.097 | 2.970 | 3.364a | 0.946 | 1.081 | 1.005 |
| SMAC | 1.025 | 0.979 | 1.069 | 1.032 | 1.062 | 1.523a |
| Survivin | 0.805 | 2.118 | 0.360b | 0.607 | 1.805 | 0.215b |
| sTNF-R1 | 1.051 | 1.492 | 1.323 | 0.836 | 1.152 | 0.772 |
| sTNF-R2 | 0.819 | 1.124 | 1.042 | 0.693 | 0.909 | 0.733 |
| TNF-α | 0.828 | 1.263 | 1.393 | 0.671 | 1.012 | 0.723 |
| TNF-β | 0.945 | 1.376 | 1.531a | 0.701 | 0.902 | 0.820 |
| TRAILR-1 | 0.942 | 0.907 | 0.996 | 0.790 | 1.104 | 0.855 |
| TRAILR-2 | 1.008 | 1.633 | 1.785a | 0.843 | 1.055 | 0.872 |
| TRAILR-3 | 0.955 | 1.421 | 1.546a | 0.799 | 1.047 | 0.848 |
| TRAILR-4 | 1.074 | 1.620 | 1.794a | 0.845 | 1.050 | 0.909 |
| XIAP | 0.896 | 1.340 | 0.550b | 0.741 | 1.155 | 0.403b |
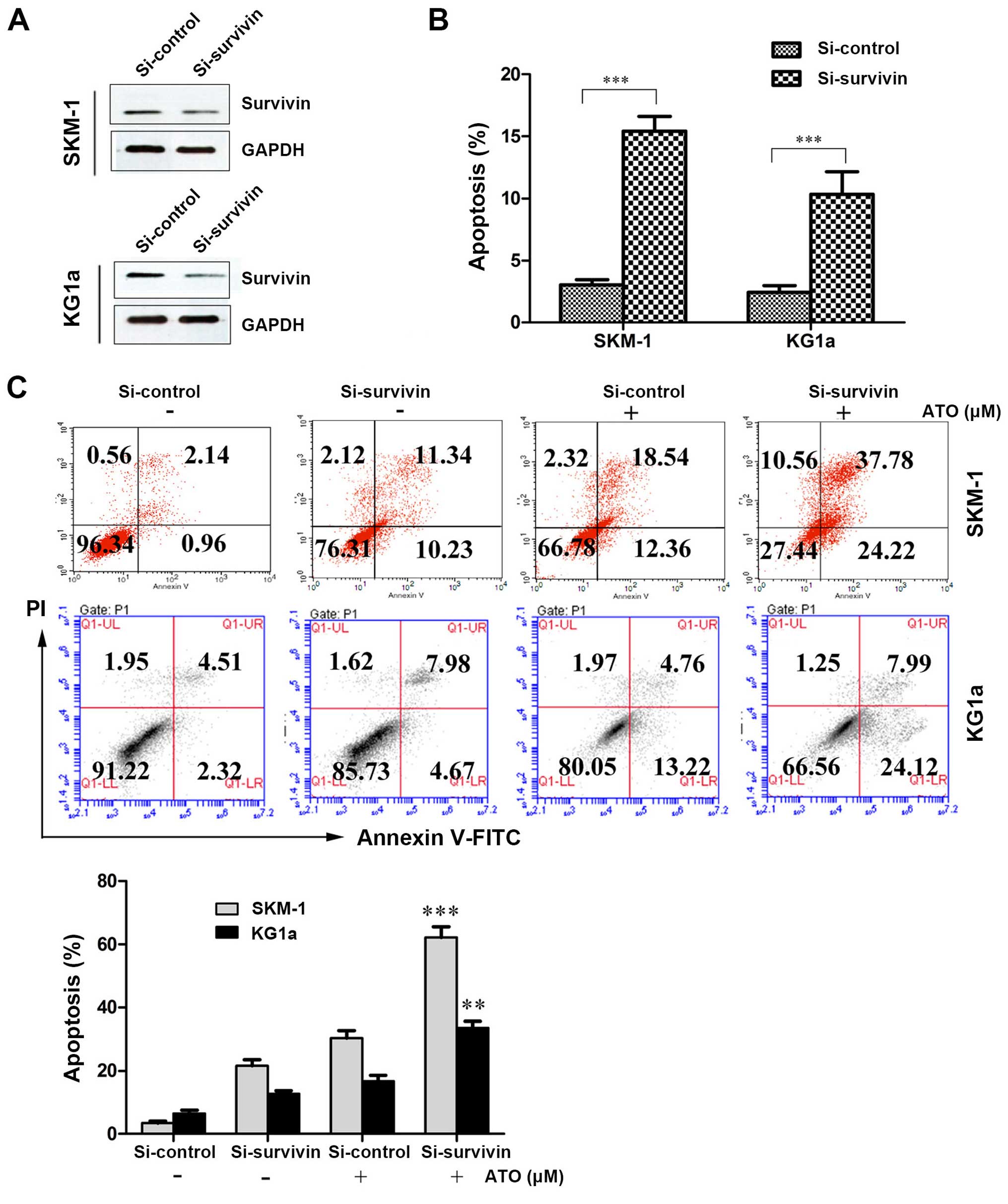
Suppression of survivin with siRNA
induces apoptosis and increases susceptibility to ATO in SKM-1 and
KG1a cells
We determined the role of CUR-induced downregulation
of survivin in the sensitization of SKM-1 and KG1a cells to ATO by
interfering with survivin expression using siRNA and evaluating the
effect on apoptosis using Annexin V/PI assays. After treatment with
siRNA-survivin for 24 h, the protein expression level of survivin
was significantly downregulated and apoptosis was significantly
increased (similar to the CUR-treated groups) compared with the
si-control groups in the two cell lines (Fig. 5A and B). As shown in Fig. 5C, the suppression of survivin by
siRNA increased the susceptibility of SKM-1 and KG1a cells to
ATO-induced apoptosis (62.00% in SKM-1 cells and 32.11% in KG1a
cells) compared with ATO alone (30.90% in SKM-1 cells and 17.98% in
KG1a cells). These results demonstrated that suppression of
survivin expression could increase ATO-induced apoptosis in the two
cell lines.
Discussion
MDS possesses the characteristics of a poor response
to traditional chemotherapy (11),
leukemic transformation (30), and
easy relapse, which are associated with the age of the patient
(tend to occur in the elderly) and LSCs or pre-LSCs (31). LSCs, which are characterized by
self-renewal, chemoresistance and immune-resistance, are thus
responsible for the origin, drug resistance and relapse of leukemia
and leukemia-related disease (28,32).
Only by enhancing the sensitivity of MDS cells and LSCs or pre-LSCs
to chemotherapeutic drugs can we effectively circumvent the above
barriers. In the present study, we investigated the combination of
CUR and ATO on MDS-SKM-1 cells and leukemia stem-like KG1a cells to
assess whether CUR could increase the susceptibility of these cells
to ATO.
The anticancer activities of CUR have been
extensively investigated and reported in various types of cancers,
including leukemia, lymphoma, gastrointestinal, genitourinary,
breast and ovarian cancer, head and neck squamous cell carcinoma,
lung cancer, melanoma and sarcoma (23). However, little research has been
conducted to assess the anticancer potential of CUR in MDS cells
and LSCs. In the present study, CUR exhibited growth inhibition and
apoptosis induction time- and dose-dependently in both MDS-SKM-1
cells and leukemia stem-like KG1a cells. Thus, we considered CUR
may be a potential sensitization agent to ATO in SKM-1 and KG1a
cells.
ATO has received extensive attention due to its
anticancer activities in various cancers by affecting cellular
functions via different molecular targets. For instance, ATO can
induce apoptosis by activating the caspase cascade, decreasing the
mitochondrial membrane potential, and increasing the production of
reactive oxygen species (33,34).
The most successful application of ATO for the treatment of cancer
is currently its use to treat patients with APL by targeting the
PML-RARa fusion protein (35),
achieving complete remission with only few adverse effects
(6). Similarly, ATO has been
applied to treat MDS patients but without promising results
(11) due to a low response and
subsequent relapse. In the present study, ATO could indeed inhibit
cell growth and induce partial apoptosis in SKM-1 and KG1a cells
in vitro, but high-dose concentrations were needed. In
addition, KG1a cells exhibited reduced sensitivity to ATO compared
with SKM-1 cells (Fig. 2B), in
accordance with the characteristics of LSCs, which may provide an
explanation for MDS relapse after treatment with ATO alone.
To solve the problems associated with a low response
and easy relapse after treatment with ATO alone, the sensitivity of
MDS cells and LSCs to ATO must be enhanced. Thus, we adopted the
strategy of combining CUR with ATO to treat SKM-1 and KG1a cells
in vitro and explored their synergistic effect. We found
that CUR could significantly increase ATO-induced apoptosis and had
a synergistic cytotoxic effect with ATO on both SKM-1 and KG1a
cells. Previous studies have reported that ATO combined with other
agents, including ascorbic acid (11), thalidomide, retinoid acid (10), and low-dose cytarabine (15), can enhance the treatment efficacy
in vivo and in vitro. Sánchez et al
demonstrated that the addition of CUR increased the efficacy of ATO
as an antitumor drug in U937, HL60 and K562 cells (36). However, we reported the first
demonstration of the combination of CUR and ATO to treat MDS and
leukemia stem-like cells in vitro. These results provide a
strong basis for the treatment of MDS by combining CUR with ATO
in vivo.
We also explored the mechanisms and searched for the
target by which CUR enhanced ATO-induced apoptosis by detecting 43
apoptosis-related proteins using protein array assays in SKM-1 and
KG1a cells following treatment with CUR and ATO alone or in
combination for 48 h. Thirty-one proteins were modulated
(upregulation or downregulation) in SKM-1 cells, whereas 12
proteins were modulated (upregulation or downregulation) in KG1a
cells in the combination groups (Table
I). These data indicated that co-treatment of these cells with
CUR and ATO could affect various targets and pathways of apoptosis,
particularly in SKM-1 cells.
Apoptotic signal transduction can proceed via two
main signaling pathways, including the death receptor (extrinsic)
and the mitochondrial (intrinsic) pathways (37). Caspase-8, which directly cleaves
caspase-3, is considered to be the initiator and hallmark of the
extrinsic pathway (38). In the
present study, caspase-8 was significantly upregulated in SKM-1
cells in response to co-treatment with CUR and ATO, but no change
was detected in KG1a cells. These results indicated that
co-treatment with CUR and ATO induced SKM-1 cell apoptosis by both
extrinsic and intrinsic pathways, leading to a higher sensitivity
of SKM-1 cells to ATO compared with KG1a cells. The upregulation of
death receptors (TRAILR-2/3/4, Fas) in SKM-1 cells further
supported these findings.
Survivin is a member of the inhibitor of apoptosis
proteins (IAPs) that is expressed in the vast majority of neoplasms
but not in differentiated normal tissue (39). In the present study, survivin
protein overexpression was downregulated in both SKM-1 and KG1a
cells in the CUR groups and drug combination groups. Some previous
reports have shown that the suppression of survivin can lead to
apoptosis of cancer cells and can enhance the chemotherapeutic
sensitivity of drugs, including cisplatin and doxorubicin, in lung
cancer and breast cancer cells (40,41).
Notably, we confirmed that suppressing the expression of survivin
by siRNA indeed enhanced the sensitivity of SKM-1 and KG1a cells to
ATO. These results strongly indicated that survivin may be a
potential target of CUR and ATO co-treatment in SKM-1 and KG1a
cells. X-linked IAP (XIAP), another member of the IAPs, inhibits
the caspase-dependent apoptotic pathway by forming a survivin-XIAP
complex (42). The XIAP stability
may be disrupted via the suppression of survivin. In the present
study, XIAP was significantly downregulated in both cell lines by
co-treatment with CUR and ATO, representing another potential
mechanism underlying the enhancement of apoptosis in the drug
combination groups in these two cell lines.
In summary, we demonstrated that CUR could enhance
the sensitivity of MDS-SKM-1 cells and leukemia stem-like KG1a
cells to ATO by downregulating a potential target survivin protein.
Thus, the barriers associated with a poor response and frequent MDS
relapse following treatment with ATO alone may be solved by
combining CUR with ATO.
Acknowledgments
The present study was supported by the Science and
Technology Project Foundation of Jiangmen City (grant no.
2013019).
References
|
1
|
Nimer SD: Myelodysplastic syndromes.
Blood. 111:4841–4851. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cogle CR, Craig BM, Rollison DE and List
AF: Incidence of the myelodysplastic syndromes using a novel
claims-based algorithm: High number of uncaptured cases by cancer
registries. Blood. 117:7121–7125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sekeres MA, Schoonen WM, Kantarjian H,
List A, Fryzek J, Paquette R and Maciejewski JP: Characteristics of
US patients with myelodysplastic syndromes: Results of six
cross-sectional physician surveys. J Natl Cancer Inst.
100:1542–1551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma X, Does M, Raza A and Mayne ST:
Myelodysplastic syndromes: Incidence and survival in the United
States. Cancer. 109:1536–1542. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zeidan AM, Linhares Y and Gore SD: Current
therapy of myelodysplastic syndromes. Blood Rev. 27:243–259. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lengfelder E, Hofmann WK and Nowak D:
Impact of arsenic trioxide in the treatment of acute promyelocytic
leukemia. Leukemia. 26:433–442. 2012. View Article : Google Scholar
|
|
7
|
Mayorga J, Richardson-Hardin C and Dicke
KA: Arsenic trioxide as effective therapy for relapsed acute
promyelocytic leukemia. Clin J Oncol Nurs. 6:341–346. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vey N, Bosly A, Guerci A, Feremans W,
Dombret H, Dreyfus F, Bowen D, Burnett A, Dennis M, Ribrag V, et
al: Arsenic trioxide in patients with myelodysplastic syndromes: A
phase II multicenter study. J Clin Oncol. 24:2465–2471. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schiller GJ, Slack J, Hainsworth JD, Mason
J, Saleh M, Rizzieri D, Douer D and List AF: Phase II multicenter
study of arsenic trioxide in patients with myelodysplastic
syndromes. J Clin Oncol. 24:2456–2464. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wei W, Zhou F, Zhang Y, Guo L, Shi H and
Hou J: A combination of thalidomide and arsenic trioxide is
effective and well tolerated in patients with myelodysplastic
syndromes. Leuk Res. 36:715–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Galimberti S, Guerrini F, Salvi F, Petrini
I, Gioia D, Messa E, Palumbo GA, Cilloni D, Petrini M and Levis A:
Arsenic trioxide and ascorbic acid interfere with the BCL2 family
genes in patients with myelodysplastic syndromes: An ex-vivo study.
J Hematol Oncol. 5:532012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo Q, Li Y, Deng J and Zhang Z: PARP-1
inhibitor sensitizes arsenic trioxide in hepatocellular carcinoma
cells via abrogation of G2/M checkpoint and suppression of DNA
damage repair. Chem Biol Interact. 226:12–22. 2015. View Article : Google Scholar
|
|
13
|
Tao JL, Li LJ, Fu R, Wang HQ, Jiang HJ,
Yue LZ, Zhang W, Liu H, Ruan EB, Qu W, et al: Elevated TIM3+
hematopoietic stem cells in untreated myelodysplastic syndrome
displayed aberrant differentiation, overproliferation and decreased
apoptosis. Leuk Res. 38:714–721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pandolfi A, Barreyro L and Steidl U:
Concise review: Preleukemic stem cells: Molecular biology and
clinical implications of the precursors to leukemia stem cells.
Stem Cells Transl Med. 2:143–150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roboz GJ, Ritchie EK, Curcio T, Samuel M,
Provenzano J, Segovia J, Christos PJ, Mathew S, Allen-Bard S and
Feldman EJ: Arsenic trioxide and low-dose cytarabine for patients
with intermediate-2 and high-risk myelodysplastic syndrome. Leuk
Res. 35:522–525. 2011. View Article : Google Scholar
|
|
16
|
Zheng WL, Zhang GS, Xu YX, Shen JK, Dai CW
and Pei MF: Arsenic trioxide, thalidomide and retinoid acid
combination therapy in higher risk myelodysplastic syndrome
patients. Leuk Res. 32:251–254. 2008. View Article : Google Scholar
|
|
17
|
Goel A and Aggarwal BB: Curcumin, the
golden spice from Indian saffron, is a chemosensitizer and
radiosensitizer for tumors and chemoprotector and radioprotector
for normal organs. Nutr Cancer. 62:919–930. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Du B, Jiang L, Xia Q and Zhong L:
Synergistic inhibitory effects of curcumin and 5-fluorouracil on
the growth of the human colon cancer cell line HT-29. Chemotherapy.
52:23–28. 2006. View Article : Google Scholar
|
|
19
|
Everett PC, Meyers JA, Makkinje A, Rabbi M
and Lerner A: Preclinical assessment of curcumin as a potential
therapy for B-CLL. Am J Hematol. 82:23–30. 2007. View Article : Google Scholar
|
|
20
|
Kamat AM, Sethi G and Aggarwal BB:
Curcumin potentiates the apoptotic effects of chemotherapeutic
agents and cytokines through down-regulation of nuclear
factor-kappaB and nuclear factor-kappaB-regulated gene products in
IFN-alpha-sensitive and IFN-alpha-resistant human bladder cancer
cells. Mol Cancer Ther. 6:1022–1030. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bharti AC, Donato N, Singh S and Aggarwal
BB: Curcumin (diferuloylmethane) down-regulates the constitutive
activation of nuclear factor-kappa B and IkappaBalpha kinase in
human multiple myeloma cells, leading to suppression of
proliferation and induction of apoptosis. Blood. 101:1053–1062.
2003. View Article : Google Scholar
|
|
22
|
Li L, Ahmed B, Mehta K and Kurzrock R:
Liposomal curcumin with and without oxaliplatin: Effects on cell
growth, apoptosis, and angiogenesis in colorectal cancer. Mol
Cancer Ther. 6:1276–1282. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Anand P, Sundaram C, Jhurani S,
Kunnumakkara AB and Aggarwal BB: Curcumin and cancer: An ̔old-age̓
disease with an ̔age-old̓ solution. Cancer Lett. 267:133–164. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matozaki S, Nakagawa T, Kawaguchi R,
Aozaki R, Tsutsumi M, Murayama T, Koizumi T, Nishimura R, Isobe T
and Chihara K: Establishment of a myeloid leukaemic cell line
(SKNO-1) from a patient with t(8;21) who acquired monosomy 17
during disease progression. Br J Haematol. 89:805–811. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakagawa T, Matozaki S, Murayama T,
Nishimura R, Tsutsumi M, Kawaguchi R, Yokoyama Y, Hikiji K, Isobe T
and Chihara K: Establishment of a leukaemic cell line from a
patient with acquisition of chromosomal abnormalities during
disease progression in myelodysplastic syndrome. Br J Haematol.
85:469–476. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zou J, Hong Y, Tong Y, Wei J, Qin Y, Shao
S, Wang C and Zhou K: Sonic hedgehog produced by bone
marrow-derived mesenchymal stromal cells supports cell survival in
myelodysplastic syndrome. Stem Cells Int. 2015:9575022015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fuchs D, Daniel V, Sadeghi M, Opelz G and
Naujokat C: Salinomycin overcomes ABC transporter-mediated
multidrug and apoptosis resistance in human leukemia stem cell-like
KG-1a cells. Biochem Biophys Res Commun. 394:1098–1104. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
She M, Niu X, Chen X, Li J, Zhou M, He Y,
Le Y and Guo K: Resistance of leukemic stem-like cells in AML cell
line KG1a to natural killer cell-mediated cytotoxicity. Cancer
Lett. 318:173–179. 2012. View Article : Google Scholar
|
|
29
|
Uchida H, Tanaka T, Sasaki K, Kato K,
Dehari H, Ito Y, Kobune M, Miyagishi M, Taira K, Tahara H, et al:
Adenovirus-mediated transfer of siRNA against survivin induced
apoptosis and attenuated tumor cell growth in vitro and in vivo.
Mol Ther. 10:162–171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tefferi A and Vardiman JW: Myelodysplastic
syndromes. N Engl J Med. 361:1872–1885. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yue LZ, Fu R, Wang HQ, Li LJ, Hu HR, Fu L
and Shao ZH: Expression of CD123 and CD114 on the bone marrow cells
of patients with myelodysplastic syndrome. Chin Med J.
123:2034–2037. 2010.PubMed/NCBI
|
|
32
|
Becker MW and Jordan CT: Leukemia stem
cells in 2010: Current understanding and future directions. Blood
Rev. 25:75–81. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rojewski MT, Körper S and Schrezenmeier H:
Arsenic trioxide therapy in acute promyelocytic leukemia and
beyond: From bench to bedside. Leuk Lymphoma. 45:2387–2401. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qian W, Liu J, Jin J, Ni W and Xu W:
Arsenic trioxide induces not only apoptosis but also autophagic
cell death in leukemia cell lines via up-regulation of Beclin-1.
Leuk Res. 31:329–339. 2007. View Article : Google Scholar
|
|
35
|
Emadi A and Gore SD: Arsenic trioxide - An
old drug rediscovered. Blood Rev. 24:191–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sánchez Y, Simón GP, Calviño E, de Blas E
and Aller P: Curcumin stimulates reactive oxygen species production
and potentiates apoptosis induction by the antitumor drugs arsenic
trioxide and lonidamine in human myeloid leukemia cell lines. J
Pharmacol Exp Ther. 335:114–123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fulda S: Targeting extrinsic apoptosis in
cancer: Challenges and opportunities. Semin Cell Dev Biol.
39:20–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Waligórska-Stachura J, Jankowska A, Waśko
R, Liebert W, Biczysko M, Czarnywojtek A, Baszko-Błaszyk D, Shimek
V and Ruchała M: Survivin - prognostic tumor biomarker in human
neoplasms - review. Ginekol Pol. 83:537–540. 2012.
|
|
40
|
Yu DD, Wang CT, Shi HS, Li ZY, Pan L, Yuan
QZ, Leng F, Wen Y, Chen X and Wei YQ: Enhancement of cisplatin
sensitivity in Lewis lung carcinoma by liposome-mediated delivery
of a survivin mutant. J Exp Clin Cancer Res. 29:462010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xu Y, Zheng W, Wang T, Wang P, Zhu L and
Ma X: Genetic protein TmSm(T34A) enhances sensitivity of
chemotherapy to breast cancer cell lines as a synergistic drug to
doxorubicin. Biomed Pharmacother. 66:368–372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Athanasoula KC, Gogas H, Polonifi K,
Vaiopoulos AG, Polyzos A and Mantzourani M: Survivin beyond
physiology: Orchestration of multistep carcinogenesis and
therapeutic potentials. Cancer Lett. 347:175–182. 2014. View Article : Google Scholar : PubMed/NCBI
|