Introduction
Hypopharyngeal squamous cell carcinoma (HSCC) is a
common type of malignant tumor among head and neck squamous cell
carcinomas (HNSCCs) (1–3). In China, the morbidities of lip,
oropharynx, hypopharynx and larynx carcinomas were reported as
0.04–0.14, 1–2, 0.15–0.8 and 3–5/100,000, respectively (4–6).
Tobacco and alcohol are the predominant risk factors in patients
with HNSCC. However, human papillomavirus (HPV) type 16 DNA is
found in up to 30% of these cancers, and such cases of HNSCC are
often found in individuals without the risk factors of alcohol and
tobacco use (7–10). HPV-positive cases of HNSCC have a
much better disease outcome compared to HNSCC cases lacking HPV
(11–13). Differences in microRNA (miRNA)
expression may affect their clinical outcomes (14).
miRNAs are small, non-coding RNAs ~18–24 nucleotides
in length that negatively regulate gene expression at the
post-transcriptional and/or translational level by binding to
complimentary sequences in the 3′-untranslated regions (3′-UTRs) of
target mRNAs (15). miRNAs regulate
up to 30% of human protein coding genes (16) and can function as oncogenes or
tumor-suppressor genes by modulating the expression of their
targets in many types of cancers (17).
Recent studies indicate that miR-15a is
downregulated in chronic lymphocytic leukemia (18), prostate cancer (19), osteosarcoma (20), keratocystic odontogenic tumors
(21) and breast cancer (22). Overexpression of miR-15a was found
to downregulate BCL-2 and induce the apoptosis of MCF-7 breast
cancer cells (23). However, the
mechanism by which miR-15a contributes to HPV-positive HSCC
tumorigenesis remains unclear.
Our previous study (24) showed that miR-15a is highly
expressed in HPV-positive tissues and cells. It may be speculated
that HPV-positive tumors have a better prognosis. In the present
study, a synthetic miR-15a inhibitor and miR-15a mimics were
transfected into HPV-positive and HPV-negative cell lines,
respectively, to examine the effects of miR-15a on these cells.
The aim of the present study was to investigate the
mechanism by which miR-15a induces HPV-positive HSCC apoptosis. The
novel tumor-suppressive miR-15a-mediated cancer pathways identified
herein provide new insights into the potential mechanisms of
HPV-positive HSCC and apoptosis and suggest potential therapeutic
targets for the treatment of HPV-positive HSCC.
Materials and methods
Patients and tumor samples
Tumor samples were collected from patients with
pharyngeal cancer who had undergone surgery at the Department of
Otolaryngology-Head and Neck Surgery, The First Affiliated Hospital
of Zhengzhou University (Zhengzhou, China). Patients recruited to
the present study had not undergone chemotherapy, radiotherapy or
immunotherapy. Collected tumor samples were frozen in liquid
nitrogen and stored at −80°C until required. The present study was
approved by the Ethics Committee of Zhengzhou University and
informed consent was obtained from each patient.
Cell lines and culture conditions
FaDu cells were purchased from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China).
HPV-positive HSCC were established using lentiviral vectors
expressing the E6 and E7 proteins of HPV-16. Positive clones were
identified according to the expression of enhanced green
fluorescent protein (EGFP). Cells were cultured in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% fetal bovine
serum (FBS) (both from Gibco-BRL, Co., Ltd., Carlsbad, CA, USA) and
grown in a 37°C/5% CO2 incubator.
miR-15a transfection
miR-15a mimics, miR-15a inhibitors and their
negative controls were obtained from GenePharma Co., Ltd.
(Shanghai, China). For transfections, cells (2×106) were
added into each well of a 6-well plate and cultured with DMEM
without serum and antibiotics. When the density of cells reached
50–60%, mimics and inhibitor were transfected into FaDu cells
(HPV-negative) and HPV-positive HSCC cells using the Lipofectamine
transfection reagent (Invitrogen, Carlsbad, CA, USA). The
mimics/inhibitor and Lipofectamine transfection reagent were each
diluted in 500 μl DMEM at a ratio of 1 μg:3 μl
and incubated for 5 min at room temperature (RT). The two mixtures
were then gently combined and incubated for a further 30 min at RT.
Subsequently, 1,000 μl of the complexes were added to each
well. After 4–6 h of incubation, the medium was replaced by DMEM
with 10% FBS. Cells were incubated at 37°C in a CO2
incubator for 48 h before further testing.
Quantitative reverse-transcription
polymerase chain reaction (qRT-PCR)
Total RNA was extracted from cells using the TRIzol
reagent (Invitrogen) according to the manufacturer's protocol. RNA
concentration was spectrophotometrically determined. RNA quality
was confirmed using a NanoDrop 1000 Spectrophotometer (Thermo
Fisher Scientific, Wilmington, DE, USA). Conversion to cDNA was
performed using ReverTra Ace® qPCR RT kit (Toyobo,
Osaka, Japan). qRT-PCR was carried out using the Maxima SYBR-Green
qPCR kit (Thermo Fisher Scientific) on a 7500 Fast Real-Time PCR
system (Applied Biosystems, Foster City, CA, USA). U6 was used as
an internal normalized reference for miRNA expression, and GAPDH
was used as an endogenous control for mRNA expression. PCR was
performed with primers specific for miR-15a, U6, BCL2, BCL2L2 and
GAPDH. The primer sequences are shown in Table I. The PCR parameters for relative
quantification were as follows: 5 min at 94°C, followed by 30
cycles of 30 sec at 94°C, 45 sec at 55°C and 45 sec at 72°C. Each
sample was tested in triplicate. The fold-change of target
miRNA/mRNA expression was calculated based on the threshold cycle
(Ct) with the 2−ΔΔCt method (25).
 | Table ImiR-15a, U6, BCL2, BCL2L2 and GAPDH
reverse transcription (RT) primers. |
Table I
miR-15a, U6, BCL2, BCL2L2 and GAPDH
reverse transcription (RT) primers.
| Name | RT primers | | PCR primers |
|---|
| miR-15a |
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACATCGTCG-3′ | F |
5′-TCCGAGTGTTTGGTAATACA-3′ |
| R |
5′-GTGCAGGGTCCGAGGT-3′ |
| U6 nRNA |
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAATA-3′ | F |
5′-TCCGATCGTGAAGCGTTC-3′ |
| R |
5′-GTGCAGGGTCCGAGGT-3′ |
| BCL-2 |
Oligo(dT)18 primer | F |
5′-ACGACTTCTCCCGCCGCTA-3′ |
| R |
5′-CATCTCCCGGTTGACGCTCT-3′ |
| BCL2L2 |
Oligo(dT)18 primer | F |
5′-TGAGTTCGAGACCCGCTTC-3′ |
| R |
5′-AAAAGTTCATCGGAGACCTG-3′ |
| GAPDH |
Oligo(dT)18 primer | F |
5′-CCACCCATGGCAAATTCCATGGCA-3′ |
| R |
5′-TCTAGACGGCAGGTCAGGTCCCC-3′ |
Western blot analysis
FaDu cells and HPV-positive HSCC were lysed and
total proteins were isolated. Total cell protein concentrations
were determined using the bicinchoninic acid protein assay kit
(Pierce Biotechnology, Inc., Rockford, IL, USA). Equal amounts of
protein (10 μg) from the cell lysates were separated by 12%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Electrophoresed proteins
were transferred to nitrocellulose membranes (GE Healthcare, Logan,
UT, USA), which were subsequently blocked with 5% (w/v) non-fat
milk and incubated overnight at 4°C with antibodies against BCL2
(diluted 1:1,000; Cell Signaling Technology, Danvers, MA, USA) and
BCL2L2 (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA).
The membranes were then incubated with the appropriate horseradish
peroxidase-conjugated secondary antibody (1:2,000; Santa Cruz
Biotechnology). Protein band density was quantified using a
Molecular Dynamics densitometer (Molecular Dynamics, Sunnyvale, CA,
USA). Glyceraldehyde 3-phosphate dehydrogenase was used as an
internal reference.
Analysis of apoptosis by Annexin
V-APC/7-aminoactinomycin D (7-AAD) staining
The Annexin V-APC apoptosis detection kit (Abcam,
Cambridge, MA, USA) was used to detect and quantify apoptosis by
flow cytometry. miR-15a mimics, miR-15a inhibitor, and their
negative control groups of adherent cells were harvested and
incubated with Annexin V incubation reagent (prepared by combining
10 μl of 10X binding buffer, 10 μl of 7-AAD, 1
μl Annexin V-APC and 79 μl of deionized, distilled
H2O) at 105–106 cells/100
μl for 15 min at RT in the dark. All samples were processed
by flow cytometry (FACSCanto™ II; BD Biosciences). FACS analyses
were performed at least three times with reproducible results.
Hoechst 33342 and propidium iodide (PI)
staining
Hoechst 33342 and PI staining was used to evaluate
the morphological changes in apoptotic cells. The cells were
stained with 10 μg/ml Hoechst 33342 and 10 μg/ml PI
for 30 min at 37°C. Following two successive washes with
phosphate-buffered saline (PBS), images of the cells were captured
with a digital camera attached to a fluorescence microscope (IX70;
Olympus Corporation, Tokyo, Japan).
Caspase-3/-9 activity assay
The activity of caspase-3/-9 was determined using
the Colorimetric Assay kit (KeyGen Biotech Co., Ltd., Nanjing,
Jiangsu, China). Cell lysates were prepared and incubated with
reaction buffer containing caspase-3/-9 substrate after treatments
as indicated. Assays were performed on 96-well plates by incubating
10 ml of cell lysate/sample in 80 ml of reaction buffer containing
10 ml caspase-3/-9 substrate at 37°C for 2 h according to the
manufacturer's protocol. Cell fluorescence intensity at 465 nm was
measured by ELISA tablet counter for quantitative assessment.
Statistical analysis
All statistical analyses were performed using SPSS
17.0 (SPSS, Inc., Chicago, IL, USA) software. Multiple comparisons
between parental and control groups were made using Tukey's honest
significant difference test. The expression levels of miRNAs in
cells were analyzed using the Wilcoxon signed-rank test. Values are
presented as the mean ± SD. A p-value <0.05 was considered to
indicate a statistically significant result.
Results
miR-15a is overexpressed in HPV-positive
HSCC samples
miR-15a expression was significantly higher in
samples from HPV-positive than in those from HPV-negative HSCC
patients (Fig. 1).
Overexpression of HPV-16 E6-E7 in
HPV-positive hypopharyngeal squamous cells
HPV-16 E6-E7-positive FaDu cells were established by
recombinant lentivirus infection and termed HPV-positive
hypopharyngeal squamous cell carcinoma. PCR was used to identify
the E6 and E7 genes. Analysis by 1.5% agarose gel electrophoresis
showed a bright band at ~750 bp consistent with the theoretical
value of the HPV-16 E6-E7 gene sequence. Positive clones were
identified based on the expression of EGFP (Fig. 2).
Overexpression of miR-15a in HPV-positive
HSCC
Before transfection, the expression of miR-15a was
detected in FaDu cells (HPV-negative) and HPV-16 E6-E7-infected
FaDu cells (HPV-positive) by real-time PCR. The results confirmed
that miR-15a was overexpressed in the HPV-positive FaDu cells
compared with the levels in normal FaDu cells (Table II and Fig. 3).
 | Table IIRelative amounts of miR-15a from
three independent experiments. |
Table II
Relative amounts of miR-15a from
three independent experiments.
| Group | Ct miR-15a | Ct U6 | ΔCt |
2−ΔCt |
|---|
| HPV-E6-E7
(HPV-positive) | 22.53±0.62 | 24.58±0.63 | −2.05±0.47 | 4.15±0.13a |
| Empty vector
control | 28.37±0.32 | 26.09±0.40 | 2.08±0.08 | 0.20±0.03 |
| FaDu cells
(HPV-negative) | 26.82±0.45 | 24.61±0.47 | 2.21±0.03 | 0.21±0.02 |
miR-15a is upregulated in FaDu cells and
is downregulated in HPV-positive HSCC after transfection
To upregulate or downregulate miR-15a, cells were
transfected with miR-15a mimics or inhibitor, which consisted of
chemically modified sense or antisense oligonucleotides designed to
specifically target mature miR-15a. The efficient overexpression
and downregulation of miR-15a in cells is shown in Fig. 4. Cellular miR-15a levels were
increased ~15-fold when FaDu cells were transfected with miR-15a
mimics, while these levels decreased when HPV-positive HSCC were
treated with miR-15a inhibitor.
miR-15a expression is negatively
correlated with BCL2L2 and BCL2 expression
To assess the correlation between miR-15a and BCL2L2
or BCL2, BCL2L2 or BCL2 expression was evaluated in cells
transfected with miR-15a mimics or inhibitor. Overexpression of
miR-15a significantly suppressed BCL2L2 or BCL2 mRNA levels in the
HPV-positive FaDu cells, whereas miR-15a knockdown had the opposite
effect on BCL-2 and BCL2L2 expression in the HPV-positive cells
(Fig. 5A and D). The downstream
proteins of BCL2L2 and BCL2 were also analyzed. To determine the
regulatory levels at which miR-15a modulates BCL2L2 and BCL2
protein expression, we repeated the above experiments and examined
protein levels after transfections (Fig. 5B, C, E and F). The results of
western blotting were consistent with those of real-time PCR and
showed that overexpression or knockdown of miR-15a suppressed or
increased BCL2L2 and BCL2 protein levels in the cells.
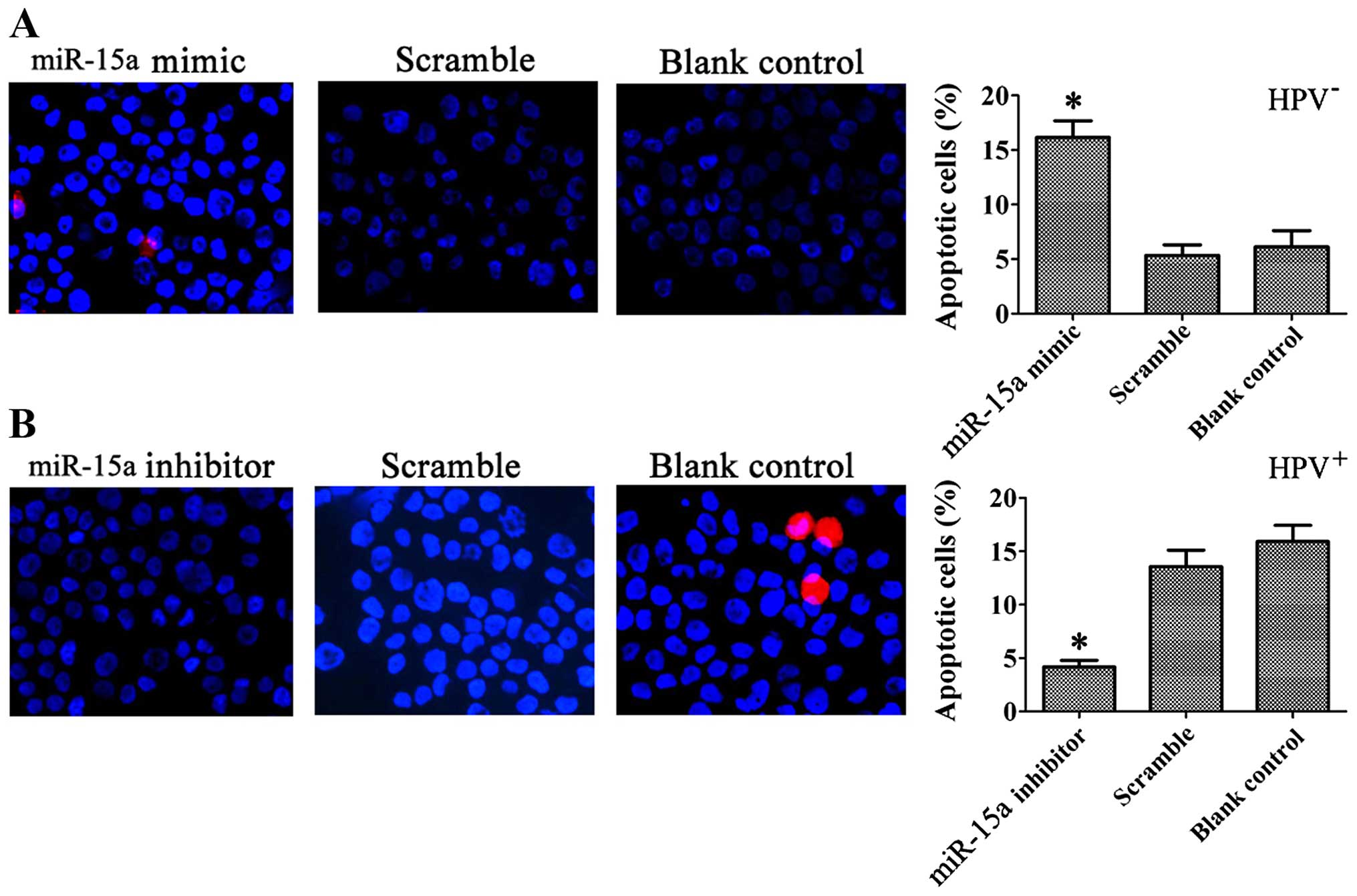
miR-15a overexpression induces
apoptosis
We next analyzed the biological consequences of the
miR-15a-driven repression of BCL2L2 and BCL2 expression in
HPV-positive HSCC. Since BCL2L2 and BCL2 are involved in the
regulation of cell apoptosis, we evaluated the effects of miR-15a
on the apoptosis of HPV-positive HSCC using Annexin V-APC/7-AAD
staining. Statistically significant (p<0.05) increases in
Annexin V apoptotic cells were observed in the miR-15a
mimic-treated FaDu cells (14.30±1.24%) compared to the scramble
control (4.33±2.89%) and blank control (2.80±0.71%) cells. The
apoptosis rate in the miR-15a inhibitor-treated HPV-positive HSCC
was decreased (2.30±0.61%; p<0.05) compared to the scramble
control (9.03±1.35%) and blank control cells (7.90±0.89%) (Fig. 6). Typical apoptotic changes, such as
nuclear fragmentation, were observed in the cells transfected with
the miR-15a mimics, and the percentage of apoptotic cells was
significantly greater than that of cells transfected with the
miR-15a inhibitor, as shown by Hoechst 33342/PI staining at 48 h
after transfection (Fig. 7).
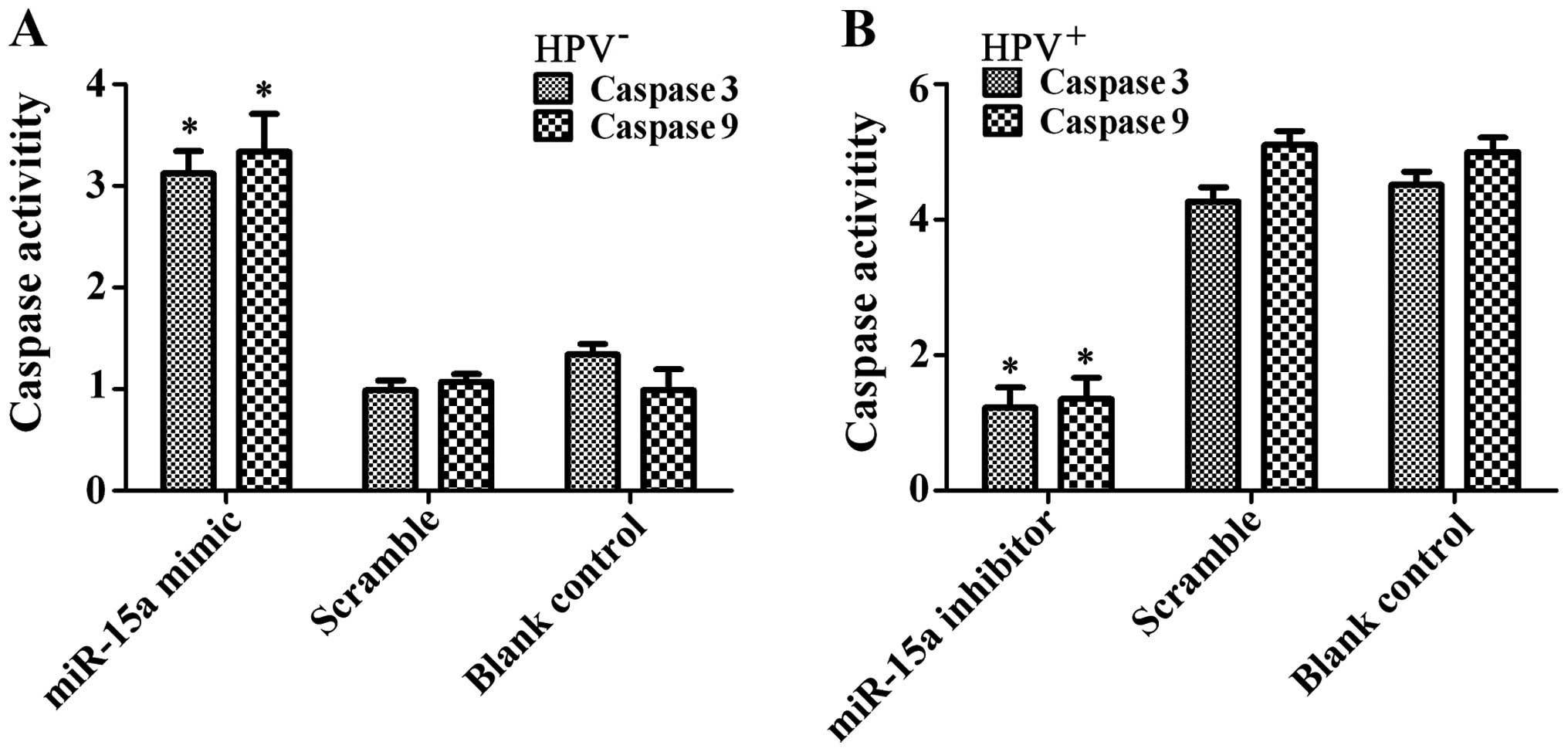
Analysis of caspase-3/-9 activity showed significantly increased
enzyme activity in the FaDu cells after miR-15a mimic transfection
(p<0.01) (Fig. 8). The results
showed that miR-15a promoted FaDu cell apoptosis in
vitro.
Discussion
HPV-associated hypopharyngeal squamous cell
carcinoma (HSCC) is a distinct clinical entity with better
prognosis than that of the classical tobacco and alcohol-associated
cancer (25). HPV-positive HNSCC
harbors wild-type p53, while the classical smoking- and
alcohol-induced cancers have mutated p53 (26). In addition, several cellular miRNAs
are differentially expressed in HPV-positive HNSCC cell lines and
in HPV-negative HNSCC cell lines (27). In our previous study (24), we detected the expression of miR-15a
in HNSCC tissues, and found that miR-15a expression was
significantly higher in HPV-positive than in HPV-negative HNSCC.
These results suggested that miR-15a plays an important role in
HPV-positive HNSCC and acts as an anti-oncogene.
In the present study, we examined the expression
levels of miR-15a in HPV-positive HNSCC and HPV-negative HNSCC.
miR-15a was overexpressed in FaDu cells by transfection with
miR-15a mimics. This led to a significant induction of apoptosis as
determined by Annexin V-APC/7-AAD staining, Hoechst 33342/PI
staining, and caspase-3/-9 expression. Transfection of a miR-15a
inhibitor into HPV-positive HSCC had the opposite effects. Taken
together, these data indicate that miR-15a may be a novel tumor
suppressor in HPV-positive HNSCC.
BCL2L2, also known as BCL-W, is a prosurvival member
of the BCL2 protein family and functions as an oncogene.
Upregulation of BCL2L2 was reported in various human malignancies,
such as gastric, colon and cervical cancers (28). In the present study, BCL2L2 and BCL2
were negatively regulated directly by miR-15a in HPV-positive HSCC
as shown by western blotting. miR-15a had a significant effect on
BCL2L2 and BCL2 mRNA levels as detected by qRT-PCR, suggesting that
miR-15a negatively regulates BCL2L2 and BCL2 expression at the
post-transcriptional level. These results indicate that miR-15a may
induce apoptosis by targeting BCL2L2 and BCL2 in HPV-positive
HSCC.
In conclusion, we found that miR-15a was
significantly upregulated in HPV-positive HSCC. miR-15a
overexpression induced apoptosis by targeting BCL2L2 and BCL2.
Further study is needed to dissect the molecular mechanisms by
which miR-15a, BCL2L2 and BCL2 play a role in HPV-positive HSCC to
design optimal prevention and treatment strategies.
Acknowledgments
We are grateful to Professor G.Z. for the helpful
comments and suggestions during all stages of the project. The
present study was partially supported by a grant from the National
Natural Science Foundation of China (no. 81503677).
References
|
1
|
Mendenhall WM and Logan HL: Human
papillomavirus and head and neck cancer. Am J Clin Oncol.
32:535–539. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dayyani F, Etzel CJ, Liu M, Ho CH, Lippman
SM and Tsao AS: Meta-analysis of the impact of human papillomavirus
(HPV) on cancer risk and overall survival in head and neck squamous
cell carcinomas (HNSCC). Head Neck Oncol. 2:152010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fakhry C, Westra WH, Li S, Cmelak A, Ridge
JA, Pinto H, Forastiere A and Gillison ml: Improved survival of
patients with human papillomavirus-positive head and neck squamous
cell carcinoma in a prospective clinical trial. J Natl Cancer Inst.
100:261–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sinha P, Logan HL and Mendenhall WM: Human
papillomavirus, smoking, and head and neck cancer. Am J
Otolaryngol. 33:130–136. 2012. View Article : Google Scholar
|
|
5
|
Nagadia R, Pandit P, Coman WB,
Cooper-White J and Punyadeera C: miRNAs in head and neck cancer
revisited. Cell Oncol. 36:1–7. 2013. View Article : Google Scholar
|
|
6
|
Michaud DS, Langevin SM, Eliot M, Nelson
HH, Pawlita M, McClean MD and Kelsey KT: High-risk HPV types and
head and neck cancer. Int J Cancer. 135:1653–1661. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mendelsohn AH, Lai CK, Shintaku IP,
Elashoff DA, Dubinett SM, Abemayor E and St John MA:
Histopathologic findings of HPV and p16 positive HNSCC.
Laryngoscope. 120:1788–1794. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Benson E, Li R, Eisele D and Fakhry C: The
clinical impact of HPV tumor status upon head and neck squamous
cell carcinomas. Oral Oncol. 50:565–574. 2014. View Article : Google Scholar
|
|
9
|
Dok R and Nuyts S: HPV positive head and
neck cancers: Molecular pathogenesis and evolving treatment
strategies. Cancers. 8:E412016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grandis J: HER2 and HER3 in
HPV+ and HPV− HNSCC-Response. Clin Cancer
Res. 22:18262016. View Article : Google Scholar
|
|
11
|
Antonsson A, Neale RE, Boros S, Lampe G,
Coman WB, Pryor DI, Porceddu SV and Whiteman DC: Human
papillomavirus status and p16INK4A expression in
patients with mucosal squamous cell carcinoma of the head and neck
in Queensland, Australia. Cancer Epidemiol. 39:174–181. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang J, Wang Z, Chen L, Huang G and Hu X:
Gossypol acetate induced apoptosis of pituitary tumor cells by
targeting the BCL-2 via the upregulated microRNA miR-15a. Int J
Clin Exp Med. 8:9079–9085. 2015.PubMed/NCBI
|
|
13
|
Lin K, Farahani M, Yang Y, Johnson GG,
Oates M, Atherton M, Douglas A, Kalakonda N and Pettitt AR: Loss of
MIR15A and MIR16-1 at 13q14 is associated with increased TP53 mRNA,
de-repression of BCL2 and adverse outcome in chronic lymphocytic
leukaemia. Br J Haematol. 167:346–355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ricieri Brito JA, Gomes CC, Santos Pimenta
FJ, Barbosa AA, Prado MA, Prado VF, Gomez MV and Gomez RS: Reduced
expression of mir15a in the blood of patients with oral squamous
cell carcinoma is associated with tumor staging. Exp Ther Med.
1:217–221. 2010.PubMed/NCBI
|
|
15
|
Begum S, Cao D, Gillison M, Zahurak M and
Westra WH: Tissue distribution of human papillomavirus 16 DNA
integration in patients with tonsillar carcinoma. Clin Cancer Res.
11:5694–5699. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sivars L, Näsman A, Tertipis N, Vlastos A,
Ramqvist T, Dalianis T, Munck-Wikland E and Nordemar S: Human
papillomavirus and p53 expression in cancer of unknown primary in
the head and neck region in relation to clinical outcome. Cancer
Med. 3:376–384. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jakscha J, Zlobec I, Storck C, Obermann
EC, Tornillo L, Terracciano LM and Fischer CA: The clinical impact
of p16 status in fine-needle aspirates of cervical lymph node
metastasis of head and neck squamous cell carcinomas. Eur Arch
Otorhinolaryngol. 270:661–667. 2013. View Article : Google Scholar
|
|
18
|
Keller LM, Galloway TJ, Holdbrook T, Ruth
K, Yang D, Dubyk C, Flieder D, Lango MN, Mehra R, Burtness B, et
al: p16 status, pathologic and clinical characteristics,
biomolecular signature, and long-term outcomes in head and neck
squamous cell carcinomas of unknown primary. Head Neck.
36:1677–1684. 2014. View Article : Google Scholar :
|
|
19
|
Tribius S, Hoffmann AS, Bastrop S, Görögh
T, Haag J, Röcken C, Clauditz T, Grob T, Wilczak W, Tennstedt P, et
al: HPV status in patients with head and neck of carcinoma of
unknown primary site: HPV, tobacco smoking, and outcome. Oral
Oncol. 48:1178–1184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fotopoulos G and Pavlidis N: The role of
human papilloma virus and p16 in occult primary of the head and
neck: A comprehensive review of the literature. Oral Oncol.
51:119–123. 2015. View Article : Google Scholar
|
|
21
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rautava J and Syrjänen S: Biology of human
papillomavirus infections in head and neck carcinogenesis. Head
Neck Pathol. 6(Suppl 1): S3–S15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jensen DH, Hedback N, Specht L, Høgdall E,
Andersen E, Therkildsen MH, Friis-Hansen L, Norrild B and von
Buchwald C: Human papillomavirus in head and neck squamous cell
carcinoma of unknown primary is a common event and a strong
predictor of survival. PLoS One. 9:e1104562014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu W, Feng L, Li P, Wang Y, Du Y, Chen X,
Wu S, Zhao G and Lou W: Effects of HPV-16 infection on
hypopharyngeal squamous cell carcinoma and FaDu cells. Oncol Rep.
35:99–106. 2016.
|
|
25
|
Westra WH: Detection of human
papillomavirus (HPV) in clinical samples: Evolving methods and
strategies for the accurate determination of HPV status of head and
neck carcinomas. Oral Oncol. 50:771–779. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee HW, Lee SS, Lee SJ and Um HD: Bcl-w is
expressed in a majority of infiltrative gastric adenocarcinomas and
suppresses the cancer cell death by blocking stress-activated
protein kinase/c-Jun NH2-terminal kinase activation. Cancer Res.
63:1093–1100. 2003.PubMed/NCBI
|
|
27
|
Qu J, Zhao L, Zhang P, Wang J, Xu N, Mi W,
Jiang X, Zhang C and Qu J: MicroRNA-195 chemosensitizes colon
cancer cells to the chemotherapeutic drug doxorubicin by targeting
the first binding site of BCL2L2 mRNA. J Cell Physiol. 230:535–545.
2015. View Article : Google Scholar
|
|
28
|
Wang F, Liu M, Li X and Tang H: MiR-214
reduces cell survival and enhances cisplatin-induced cytotoxicity
via down-regulation of Bcl2l2 in cervical cancer cells. FEBS Lett.
587:488–495. 2013. View Article : Google Scholar : PubMed/NCBI
|