Introduction
Whole tumor cell vaccines have been investigated for
a long time in clinical and basic studies (1–3). Whole
tumor cell vaccines have obvious advantages in cancer immunotherapy
(4). First of all, whole tumor cell
vaccines offer a wide spectrum of tumor antigens (5) and unknown tumor associated antigen to
elicit tumor-specific immunity (6).
Second, whole tumor cell vaccines facilitate a high-efficiency
antitumor response (7). Whole tumor
cell vaccines were processed and presented all tumor antigen
peptides to induce a robust polyclonal T cell immune response by
MHC class I and class II of dendritic cells. The CD4+ Th
cells stimulated by whole tumor cell vaccines could also promote
CD8+ T cells to generate a stronger antitumor effect and
long-term memory (2). Numerous
phase I and II clinical trials have shown that whole tumor cell
vaccines had significant clinical benefits. However, phase III
clinical trials of whole tumor cell vaccines usually fail to
produce significant clinical effect (8–10).
Many studies have shown that the lack of tumor antigen expression
in cancer evolution and immunosuppression exploited by the tumor
itself may be the main determinant of the limited efficiency
(11,12). Therefore, in order to improve the
antitumor effect of whole tumor cell vaccines, exploiting other
antitumor approach is required.
The α-Gal is a carbohydrate epitope, which is
generated in pigs and New World monkeys by the α-1,
3-galactosyltransferase (α-1, 3-GT) (13–15).
α-Gal epitope does not exist in humans and old world monkeys
because their α-1,3-GT genes have become silenced during biological
evolution (16–18). As humans do not have α-Gal epitopes,
they produce a lot of anti-α-Gal antibody in response to
stimulation of gastrointestinal bacteria (19–21).
α-Gal on cell surface could trigger rapid humoral immune response
and strong cellular immune response. In addition, α-Gal also
promote T cell division and secretion of TNF-α, and IFN-γ (22,23).
These results suggest α-Gal is a potential adjuvant for cancer
immunotherapy.
We modified genetically MDA-MB-231 cell vaccines by
replication defective retroviral expressing α-1, 3-GT genes. Our
results demonstrated that the whole tumor cell vaccines expressing
α-Gal produced high efficient protection and anti-tumor immunity.
In conclusion, our data showed that whole tumor cell vaccines
expressing α-Gal enhanced therapeutic antitumor effect.
Materials and methods
Cells and animals
The human breast cancer cell line MDA-MB-231 cells
were purchased from American Type Culture Collection (ATCC, VA,
USA). MDA-MB-231 cells were cultured in complete DMEM media (Gibco,
CA, USA) with 10% fetal bovine serum (FBS) and 100 U/ml
penicillin/streptomycin. MDA-MB-231Gal+ cells expressing
α-1, 3-GT and control cells were obtained by GenScript (Nanjing,
China) and maintained in DMEM cultured with 10% FBS, 100 U/ml
penicillin/streptomycin and 0.35 µg/ml puromycin.
NOD.CB17-Prkdcscid/NcrCrlVr (NOD-scid) mice 3–4
weeks of age were obtained from Vital River Lab Animal Technology
Co. (Beijing, China). They were housed in a specific pathogen-free
facility in microisolator cages, given autoclaved food and water.
All experiments were carried out according to the Federation of
European Laboratory Animal Science Association guidelines and all
protocols were approved by the Institutional Animal Care and Use
Committees of Guangxi Medical University, Nanning, Guangxi,
China.
Construction of α-1, 3-GT genes and
lentiviral transduction of MDA-MB-231 cells
Lentiviral containing α-1, 3-GT genes were
constructed as previously described. The cDNAs expressing α-1, 3-GT
was cloned into a lentiviral vector pLVX- Puro. The reconstructed
lentiviral plasmid or empty lentiviral plasmid vector were
co-transfected into the 293T cells to produce virus stock.
MDA-MB-231 cells were infected by lentiviral stably expressing α-1,
3-GT-His and then cultured in complete DMEM media and 0.35
µg/ml puromycin. Cells were selected with puromycin and were
named MDA-MB-231Gal+ cells. Similarly, the control cell
were named MDA-MB-231Gal− cells.
RT-PCR
RT-PCR was performed as previously described
(24). mRNA was obtained from
MDA-MB-231Gal+ cells, MDA-MB-231Gal− cells by
use of the RNeasy kit (Qiagen). cDNA was synthesized from total
RNA. Sequences of primers were for α-1, 3-GT (forward,
TGCTTGTCTCAACTGTAATG; reverse, TCTTCTTCTTCGTGGTAACT), β-actin
(forward, ACCACACCTTCTACAATGA; reverse, ATAGCACAGCCTGGATAG)
designed by primer premier 5.0. RT-PCR was carried out in a Applied
Biosystems 7300 real-time PCR system. Results were analysed by use
of Light Cycler Software version 3.
Western blotting
MDA-MB-231Gal−, MDA-MB-231Gal+
tumor cells were washed at 4°C and lysed in RIPA buffer on ice.
Then, cell lysates were centrifuged at 13,000 g at 4°C for 20 min.
Total protein concentration in the cell lysate was measured by BCA
Protein Quantification kit (Beyotime Biotechnology). Cell lysates
were separated by SDS-PAGE and transferred onto a nitrocellulose
membrane. The nitrocellulose membrane was incubated with primary
His antibody at 4°C overnight. After washing the nitrocellulose
membrane with TBS-T three times for 10 min each at room
temperature, the nitrocellulose membrane was incubated with
horseradish peroxidase (HRP)-conjugated anti-Igg Abs (ZSGB-Bio,
Beijing, China). Protein band intensities were quantified by use of
the diaminobenzidine (DAB) kit (SolarBio, Beijing, China). β-actin
was used as internal control.
Immune and tumor models
Human PBMCs and serum were obtained from fresh
peripheral blood of healthy volunteers, with signed informed
consent for use of blood in accordance with the Declaration of
Helsinki. PBMCs were isolated by Ficoll Hypaque density-gradient
centrifugation. PBMCs were suspended at a density of
1×108 cells/ml by RPMI-1640. PBMCs suspension (100
µl) and 100 µl serum were injected into NOD-SCID
mice. NOD-SCID mice with reconstituted intact human immune system
were named Hu-NOD-SCID mice.
To study protective effect of these vaccines, the
Hu-NOD-SCID mice were inoculated with 2×106 irradiated
MDA-MB-231Gal+, MDA-MB-231Gal− tumor cell
vaccines, or phosphate-buffered saline (PBS) intraperitoneally on
days 0, 7, 14 and 21. After the completion of immunization,
Hu-NOD-SCID mice were implanted with 1×106 live
MDA-MB-231 tumor cells into the right side. Animal experiments were
approved by the Institutional Animal Care and Use Committees of
Guangxi Medical University, Nanning, Guangxi, China.
To study the antitumor effect of these vaccines on
tumor-bearing Hu-NOD-SCID mice, the tumor-bearing Hu-NOD-SCID mice
were injected 1×106 irradiated
MDA-MB-231Gal+, MDA-MB-231Gal− tumor cell
vaccines or PBS on days 7, 14 and 21 after Hu-NOD-SCID mice were
implanted with 1×106 live MDA-MB-231 tumor cells into
the right side on day 0.
Tumor volume was measured every 5 days, according to
the formula: width2 × length × 0.52. Mice were monitored
every day until they died, the date of death were recorded.
Flow cytometry
A total of 5×105
MDA-MB-231Gal+, MDA-MB-231Gal− cells were
incubated with Isolectin Griffonia
simplicifolia-IB4 (Invitrogen, CA, USA) at 4°C for 1
h. The α-Gal reacted with Isolectin GS-IB4 was detected by flow
cytometry (Beckman Coulter Epics XL-MCL, MA, USA) (25).
To determine whether the immune cells change in the
tumor of mice immunized by tumor cell vaccines. The tumor cell
suspensions were obtained from tumor-bearing mice. Tumor cell
suspensions were incubated with FITC-conjugated anti-CD8 antibody
and PE-conjugated anti-CD3 antibody at 4°C for 30 min for
extracellular staining. The fluorescently stained cells were
detected by flow cytometry (Beckman Coulter Epics XL-MCL), and the
data were analyzed using FlowJo Software 7.6.2 (OR, USA).
ELISA
The levels of INF-γ, IL-12, IL-10, TGF-β in serum of
mice were quantified by ELISA according to the manufacturer's
instructions. Optical densities were texted by use of a microplate
reader at 450-nm wavelength. The data are presented as the means of
triplicate wells.
Immunohistochemistry
Cell proliferation and apoptosis in tumor xenografts
of mice were evaluated by immunohistochemistry. Tumor tissues were
cut to 0.4-mm sections, deparaffinized with xylene and graded
alcohols. Endogenous peroxidase inactivation and antigen retrieval
of tumor tissues were performed by standard procedure.
Proliferation was evaluated by Ki67 immunostaining. TdT-mediated
dUTP-biotin nick end labeling (TUNEL) was used to detect apoptotic
cells in FFPE cell blocks.
Statistical analysis
Data were analysed by using SPSS 17.0. Data are
expressed as mean ± SD. The significance of the results of
experiments was analysed by one-way ANOVA. Survival curves were
analyzed by Kaplan-Meier method and the log-rank test. Three
replicates were done for each experiment. *P<0.05,
**P<0.01, ***P<0.001,
****P<0.0001 compared with PBS control (CON).
Results
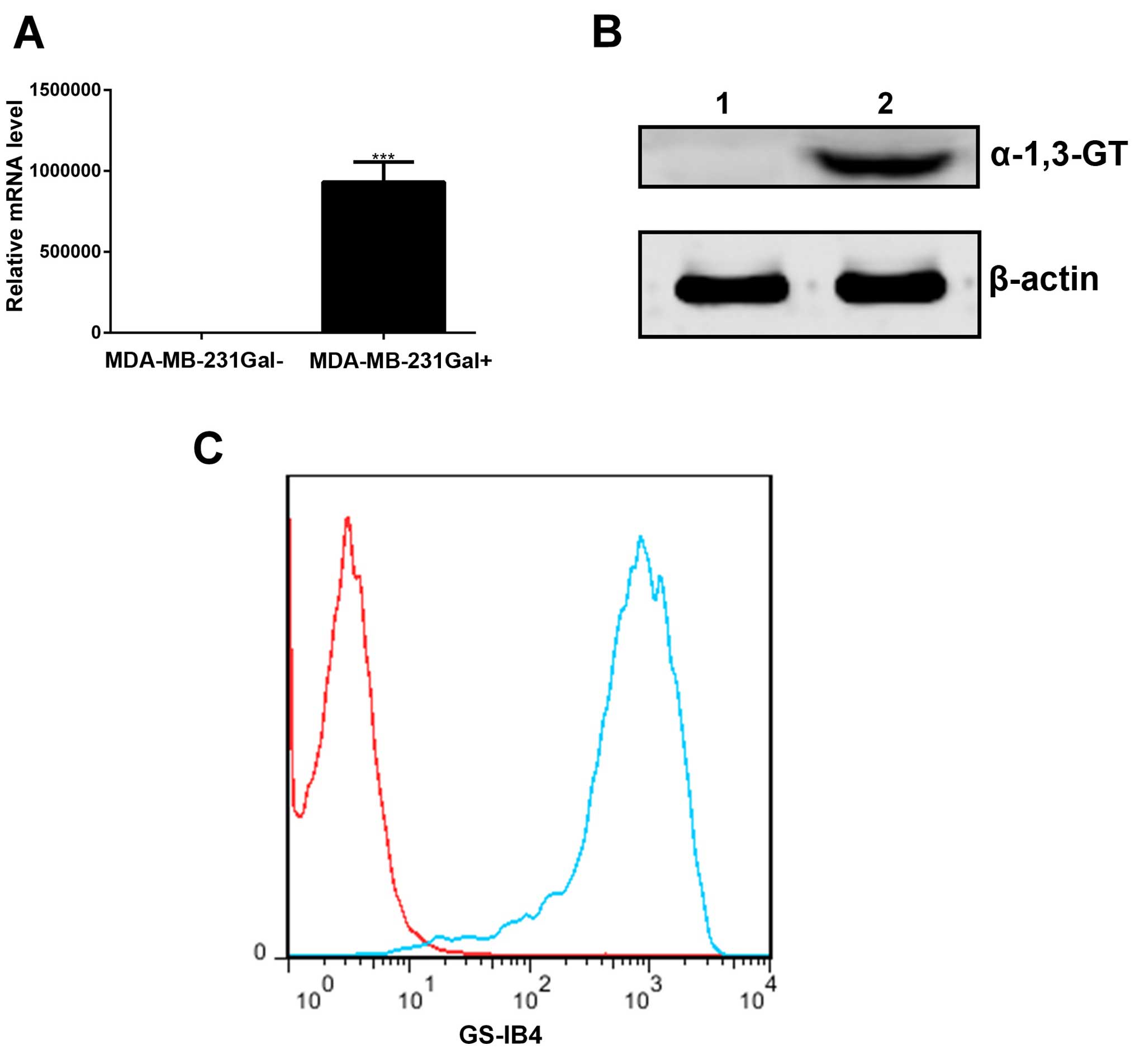
MDA-MB-231Gal+ tumor cells can
express α-1, 3-GT in vitro
MDA-MB-231Gal+ tumor cells were
transfected with the α-1, 3-GT expression lentiviral or empty
lentiviral as described in Materials and methods. Total RNA and
protein samples were obtained from MDA-MB-231Gal+ and
MDA-MB-231Gal− tumor cells, and then analyzed via RT-PCR
and western blotting using antibody. As shown in Fig. 1A and B, the α-1, 3-GT RNA and α-1,
3-GT were detected in the MDA-MB-231Gal+ cells, but not
in MDA-MB-231Gal− cells.
To assess α-Gal generated by α-1, 3-GT,
MDA-MB-231Gal+ cells were incubated with Isolectin
GS-IB4 and then detected by flow cytometry (25). As shown in Fig. 1C, the results showed higher levels
of α-Gal in the MDA-MB-231Gal+ tumor cells than in the
MDA-MB-231Gal− cells. These results demonstrated that
the MDA-MB-231Gal+ cells expressed active α-1, 3-GT and
produced α-Gal epitope in vitro.
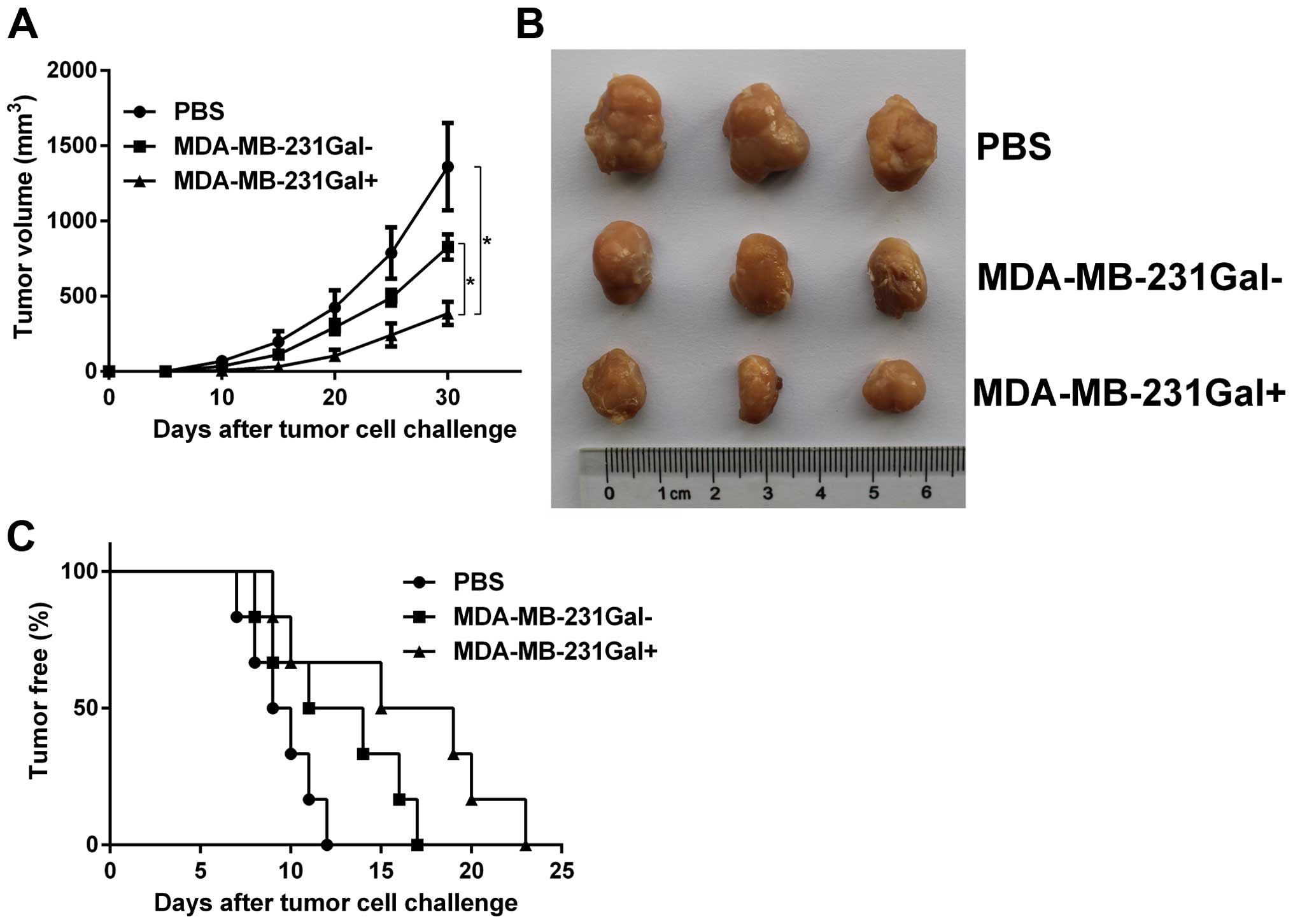
MDA-MB-231Gal+ cell vaccines
inhibit tumor growth and tumorigenesis in immunized mice
In order to study whether the irradiated
MDA-MB-231Gal+ cell vaccines induced protective
antitumor immunity, we evaluated the formation and growth of tumors
in immunized mice. Hu-NOD-SCID mice were immunized with irradiated
tumor cell vaccines and challenged with live MDA-MB-231 tumor cells
as previously described. As shown in Fig. 2, tumorigenesis rates in mice
immunized with MDA-MB-231Gal+ cell vaccines were lower
than in mice immunized with MDA-MB-231Gal− cells or PBS.
Tumor growth curves showed that the tumor growth in mice immunized
with MDA-MB-231Gal+ cell vaccines was slower than other
groups.
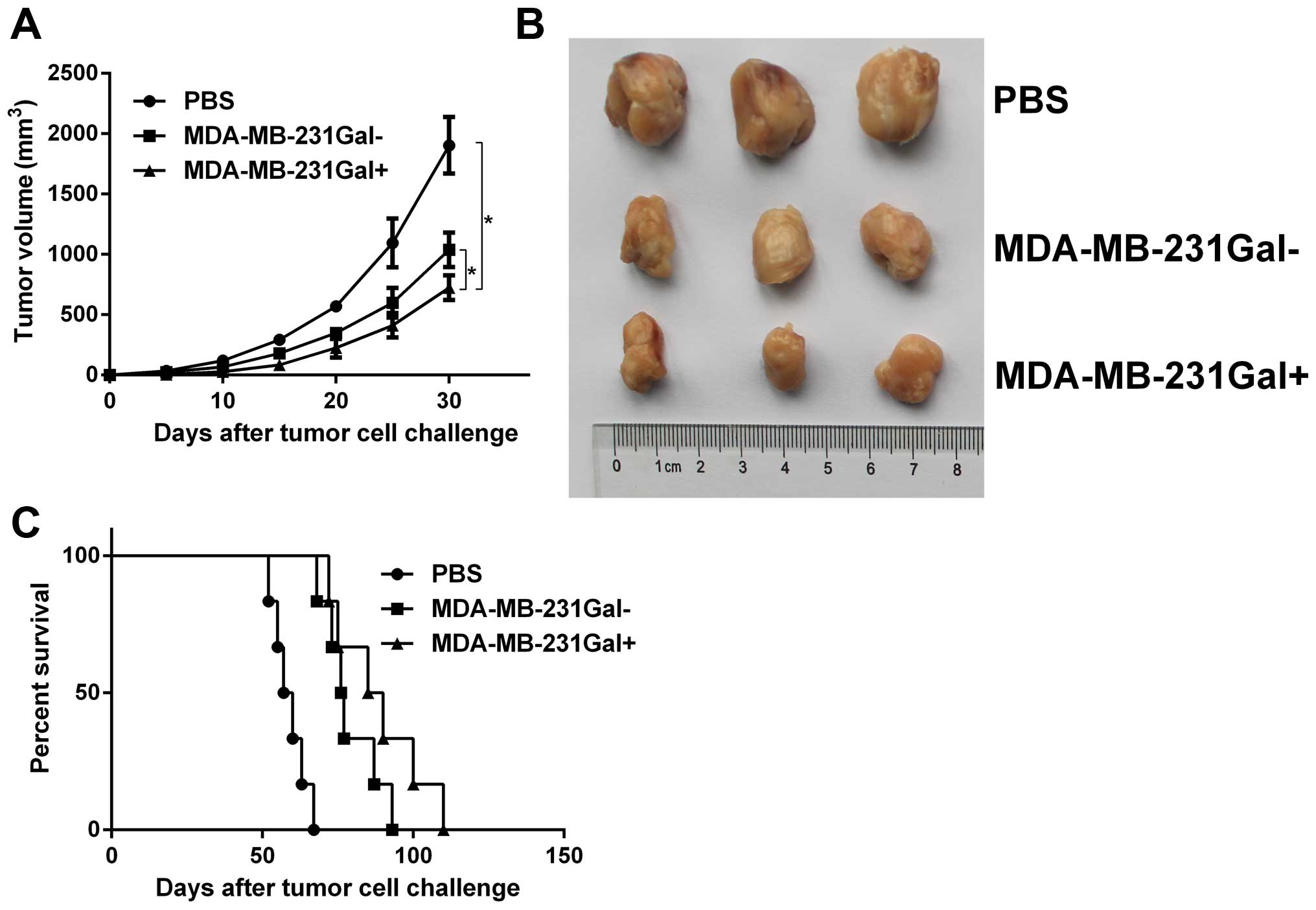
MDA-MB-231Gal+ cell vaccines
inhibit tumor growth in mammary tumor-bearing mice
The antitumor efficacy of MDA-MB-231Gal+
cell vaccines was evaluated in mammary tumor models. Tumor-bearing
mice were injected with the tumor cell vaccines three times. As
shown in Fig. 3, tumor growth in
the MDA-MB-231Gal+ tumor cell vaccines treated group was
significantly slower and the lifespan in the
MDA-MB-231Gal+ tumor cell vaccines treated group was
markedly prolonged compared with the other control groups. These
data demonstrated that MDA-MB-231Gal+ tumor cell
vaccines were more effective than the control groups in eliciting
anti-tumor effect in tumor-bearing mice.
MDA-MB-231Gal+ tumor cell
vaccines increase apoptosis and inhibit proliferation in tumor
xenografts of mice
To measure apoptotic cells in tumor xenografts of
mice, we performed TdT-mediated duTP-biotin nick end labeling
(TUNEL). As shown in Fig. 4,
MDA-MB-231Gal+ tumor cell vaccines significantly
increased the number of apoptotic cells compared with the other
control groups. To evaluate the effect of MDA-MB-231Gal+
cell vaccines on proliferation of tumor, it was assessed by
counting Ki67-positive tumor cells. MDA-MB-231Gal+ tumor
cell vaccines significantly inhibited proliferation of tumor
compared with the other control groups. In summary, these data
indicated that MDA-MB-231Gal+ tumor cell vaccines
significantly inhibited growth of breast cancer.
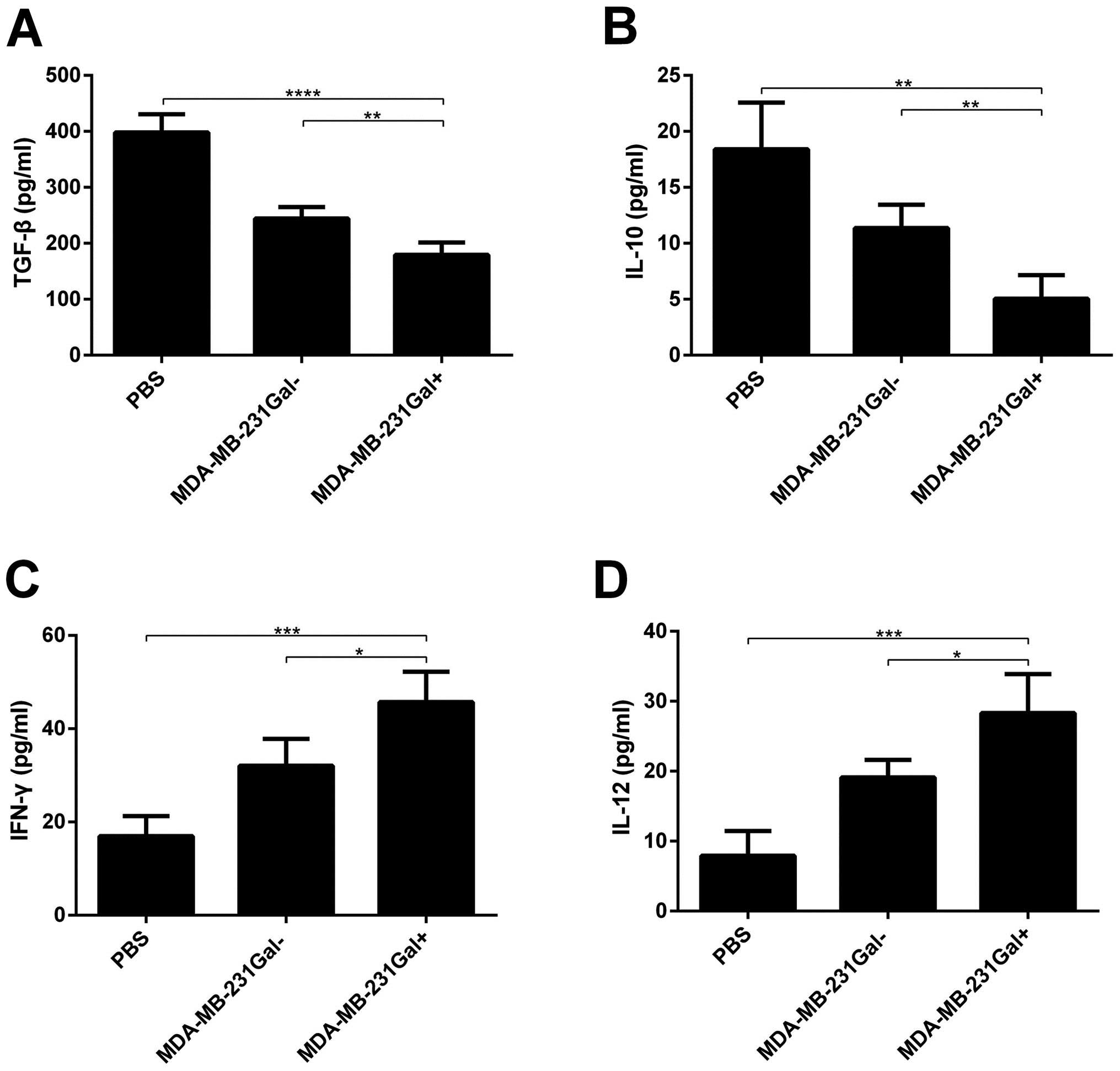
The levels of cytokine secretion in the
serum of mice treated with tumor cell vaccines
Cytokine secretion was analyzed to determine
immunity activation. Mice were treated at indicated time and dose,
expression of IL-12p70, INF-γ, IL-10, TGF-β in the serum of mice
treated with tumor cell vaccines was measured. As shown in Fig. 5, the levels of IL-12p70, INF-γ in
the serum of mice treated with MDA-MB-231Gal+ tumor cell
vaccines were significantly higher than mice treated with
MDA-MB-231Gal− tumor cells or PBS, whereas the levels of
IL-10, TGF-β were contrary.
MDA-MB-231Gal+ tumor cell
vaccines enhance CD8+ T cells recruitment in the tumor
microenvironment
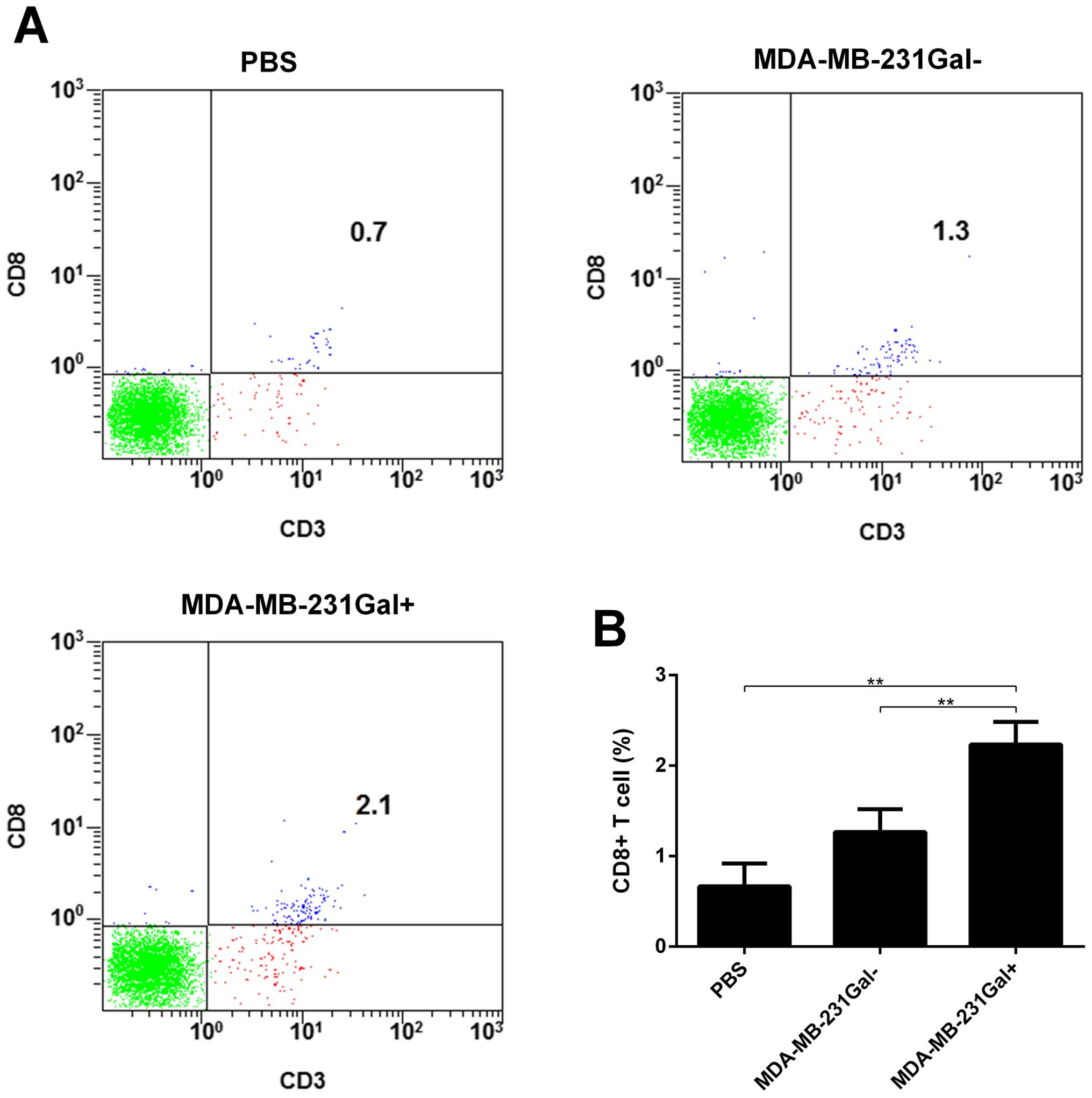
To determine CD8+ T cells in the solid
tumors, we performed flow cytometry to identify CD8+ T
cells in the solid tumor cell suspension. As shown in Fig. 6, MDA-MB-231Gal+ cell
vaccines significantly increased the percentage of CD8+
T cells in the solid tumor cell suspension. These results showed
that MDA-MB-231Gal+ tumor cell vaccines promoted the
recruitment of antitumor immune effective cells in the tumor
microenvironment.
Discussion
Many studies have indicated that tumor cell vaccines
have higher efficiency when treated in combination with immunologic
adjuvant, chemotherapy and radiotherapy, cytokines (5,6). In
addition, genetically modified tumor cell vaccines can also induce
highly effective antitumor effect. Different genetically modified
tumor cell vaccines have been developed, most of which were
transfected with genes encoding proteins such as IL-12, IL-4, IL-7,
IL-2, IL-6/sIL-6R, GM-CSF, TNF, IFN-γ, HLA molecules (HLA-B7),
co-stimulatory molecules (B-7,1) (3,26,27).
With advances in genetically modified technology, tumor cell
vaccines will be introduced into cancer immunotherapy in clinical
practice.
In our studies, MDA-MB-231Gal+ cells were
transfected with lentiviral recombined α-1, 3-GT genes and
expressed bioactive α-1, 3-GT in vitro. In this assay, we
used live MDA-MB-231 tumor cells to generate tumor models to
evaluate the antitumor effects of MDA-MB-231Gal+ cell
vaccines. Our data showed that MDA-MB-231Gal+ cell
vaccines inhibited tumor growth and tumorigenesis and proliferation
in tumor xenografts of mice. In addition, MDA-MB-231Gal+
cell vaccines could promote the levels of cytokine secretion and
the recruitment of antitumor immune effective cells and apoptosis
in tumor xenografts of mice. Our result implied that
MDA-MB-231Gal+ tumor cell vaccines could produce strong
protective and antitumor immune effect. The result showed that
genetically modified MDA-MB-231Gal+ tumor cell vaccines
represent promising strategies for highly effective antitumor
therapies.
An efficient antitumor immune response induced by
tumor antigen was not expressed, or expressed weakly on normal
cells. The ideal tumor antigen should be expressed in all cancer
and immunogenic cells (3,28). Unfortunately, some of normal cells
may also express tumor antigens. So tumor cells are not immunogenic
enough to elicit immune response (28). However, studies have shown that the
α-Gal epitope increased the immunogenicity of viral vaccines,
including the human immunodeficiency virus (HIV) vaccine and flu
vaccine (29). The α-Gal epitope
expressing on vaccines specifically binds to anti-Gal antibody
(30). The anti-Gal antibody also
binds with the antigen presenting cells (APCs), which can promote
the highly effective uptake of α-Gal epitope on the vaccine by APCs
(31–33). In addition, then antigen-specific
CD8+ T cells are activated by MHC class I and class II
molecules expressed on APCs. The antitumor immune response is
mainly mediated by antigen-specific CD8+ T cells
(34). In our studies,
MDA-MB-231Gal+ cell vaccines achieve high antitumor
effects, which may owe to α-Gal epitope. However, further studies
are required to determine the molecular mechanisms.
Studies have shown that tumor cells escape from
immune effectors attack via complementary immunosuppressive
mechanisms which make immunologic effector cell anergy. Tumor cells
can improve secretion of immunosuppressive molecules and cytokines
such as IL-10 and TGF-β (35),
which inhibit antitumor immunity responses and enhance Treg cell
functions. TGF-β could also inhibit dendritic cell differentiation
(36,37) and NK cell immunologic functions
(38). As shown in Fig. 5A and B, an decrease of TGF-β, IL-10
were observed in MDA-MB-231Gal+ cell vaccinated mice.
Therefore, MDA-MB-231Gal+ cell vaccines can reduce
immunosuppressive cytokine production.
As shown in Fig. 5C and
D, increase of IFN-γ and IL-12p70 were observed in
MDA-MB-231Gal+ cell vaccinated mice. IFN-γ is shown to
have antitumorigenic immune effects (39) and activate NK cells, CD8+
T cells, and macrophages. IL-12 can stimulate NK cells and Th1
cells. Studies have shown that IL-12 plays a main role in antitumor
immunity (5,40) and protect prophylactically against
live tumor cell challenge (26,41).
In a mouse model, IL-12 can increase infiltrating CD3+ T
cells (42). Therefore, this can
account for MDA-MB-231Gal+ cell vaccines promoting
antitumor immunity.
Taken together, immunotherapy has been the focus of
tumor therapy study (43). Our
studies are the first report showing that MDA-MB-231Gal+
cell vaccines induced powerful protective and antitumor effect.
Notably, MDA-MB-231Gal+ cell vaccines directly enhanced
the recruitment of effector T cells and promoted the levels of
cytokine secretion and cell apoptosis in tumor-bearing mice. These
results implicate genetically modified tumor cells as vaccines can
be exploited as a powerful therapeutic strategy for cancer, but
more studies are necessary before performing clinical trials.
Acknowledgments
This study was supported, in part, by grants from
Programs for Changjiang Scholars and Innovative Research Team in
University (no. IRT_15R13); National Natural Scientific Foundation
of China (nos. 81430055 and 81372452); International Cooperation
Project of the Ministry of Science and Technology of China (no.
2015DFA31320); Project for Innovative Research Team in Guangxi
Natural Science Foundation (2015GXNSFFA139001); Project of Science
and Technology of Guangxi (nos. 14125008-2-12 and
1599005-2-10).
References
|
1
|
Euhus DM, Gupta RK and Morton DL:
Induction of antibodies to a tumor-associated antigen by
immunization with a whole melanoma cell vaccine. Cancer Immunol
Immunother. 29:247–254. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chiang CL, Coukos G and Kandalaft LE:
Whole tumor antigen vaccines: Where are we? Vaccines (Basel).
3:344–372. 2015. View Article : Google Scholar
|
|
3
|
Nawrocki S and Mackiewicz A: genetically
modified tumour vaccines - where we are today. Cancer Treat Rev.
25:29–46. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ward S, Casey D, Labarthe MC, Whelan M,
Dalgleish A, Pandha H and Todryk S: Immunotherapeutic potential of
whole tumour cells. Cancer Immunol Immunother. 51:351–357. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mach N and Dranoff G: Cytokine-secreting
tumor cell vaccines. Curr Opin Immunol. 12:571–575. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Copier J and Dalgleish A: Overview of
tumor cell-based vaccines. Int Rev Immunol. 25:297–319. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chiang CL, Benencia F and Coukos G: Whole
tumor antigen vaccines. Semin Immunol. 22:132–143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Drake CG, Lipson EJ and Brahmer JR:
Breathing new life into immunotherapy: Review of melanoma, lung and
kidney cancer. Nat Rev Clin Oncol. 11:24–37. 2014. View Article : Google Scholar :
|
|
9
|
Hsueh EC, Essner R, Foshag LJ, Ollila DW,
gammon G, O'Day SJ, Boasberg PD, Stern SL, Ye X and Morton DL:
Prolonged survival after complete resection of disseminated
melanoma and active immunotherapy with a therapeutic cancer
vaccine. J Clin Oncol. 20:4549–4554. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Copier J and Dalgleish A: Whole-cell
vaccines: A failure or a success waiting to happen? Curr Opin Mol
Ther. 12:14–20. 2010.PubMed/NCBI
|
|
11
|
Kraman M, Bambrough PJ, Arnold JN, Roberts
EW, Magiera L, Jones JO, gopinathan A, Tuveson DA and Fearon DT:
Suppression of antitumor immunity by stromal cells expressing
fibroblast activation protein-alpha. Science. 330:827–830. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Speiser DE, Baumgaertner P, Barbey C,
Rubio-Godoy V, Moulin A, Corthesy P, Devevre E, Dietrich PY,
Rimoldi D, Liénard D, et al: A novel approach to characterize
clonality and differentiation of human melanoma-specific T cell
responses: Spontaneous priming and efficient boosting by
vaccination. J Immunol. 177:1338–1348. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Galili U: Discovery of the natural
anti-Gal antibody and its past and future relevance to medicine.
Xenotransplantation. 20:138–147. 2013.PubMed/NCBI
|
|
14
|
Park HM, Kim YW, Kim KJ, Kim YJ, Yang YH,
Jin JM, Kim YH, Kim BG, Shim H and Kim YG: Comparative N-linked
glycan analysis of wild-type and α1,3-galactosyltransferase gene
knock-out pig fibroblasts using mass spectrometry approaches. Mol
Cells. 38:65–74. 2015.
|
|
15
|
Galili U: Significance of the evolutionary
α1,3-galactosyltransferase (GGTA1) gene inactivation in preventing
extinction of apes and old world monkeys. J Mol Evol. 80:1–9. 2015.
View Article : Google Scholar
|
|
16
|
Qiu Y, Yun MM, Dong X, Xu M, Zhao R, Han
X, Zhou E, Yun F, Su W, Liu C, et al: Combination of
cytokine-induced killer and dendritic cells pulsed with antigenic
α-1,3-galactosyl epitope-enhanced lymphoma cell membrane for
effective B-cell lymphoma immunotherapy. Cytotherapy. 18:91–98.
2016. View Article : Google Scholar
|
|
17
|
Sato M, Kagoshima A, Saitoh I, Inada E,
Miyoshi K, Ohtsuka M, Nakamura S, Sakurai T and Watanabe S:
Generation of alpha-1,3-galactosyltransferase-deficient porcine
embryonic fibroblasts by CRISPR/Cas9-mediated knock-in of a small
mutated sequence and a targeted toxin-based selection system.
Reprod Domest Anim. 50:872–880. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shimatsu Y, Yamada K, Horii W, Hirakata A,
Sakamoto Y, Waki S, Sano J, Saitoh T, Sahara H, Shimizu A, et al:
Production of cloned NIBS (Nippon Institute for Biological Science)
and α-1, 3-galactosyltransferase knockout MGH miniature pigs by
somatic cell nuclear transfer using the NIBS breed as surrogates.
Xenotransplantation. 20:157–164. 2013.PubMed/NCBI
|
|
19
|
Park CS, Oh SS, Kim YE, Choi SY, Lim HG,
Ahn H and Kim YJ: Anti-alpha-Gal antibody response following
xenogeneic heart valve implantation in adults. J Heart valve Dis.
22:222–229. 2013.PubMed/NCBI
|
|
20
|
Wilczek P, Lesiak A, Niemiec-Cyganek A,
Kubin B, Slomski R, Nozynski J, Wilczek G, Mzyk A and Gramatyka M:
Biomechanical properties of hybrid heart valve prosthesis utilizing
the pigs that do not express the galactose-α-1,3-galactose (α-Gal)
antigen derived tissue and tissue engineering technique. J Mater
Sci Mater Med. 26:53292015. View Article : Google Scholar
|
|
21
|
Galili U: Anti-Gal: An abundant human
natural antibody of multiple pathogeneses and clinical benefits.
Immunology. 140:1–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saethre M, Schneider MK, Lambris JD,
Magotti P, Haraldsen G, Seebach JD and Mollnes TE: Cytokine
secretion depends on Galalpha (1,3)Gal expression in a pig-to-human
whole blood model. J Immunol. 180:6346–6353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wilhite T, Ezzelarab C, Hara H, Long C,
Ayares D, Cooper DK and Ezzelarab M: The effect of Gal expression
on pig cells on the human T-cell xenoresponse. Xenotransplantation.
19:56–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zibara K, Awada Z, Dib L, El-Saghir J,
Al-Ghadban S, Ibrik A, El-Zein N and El-Sabban M: Anti-angiogenesis
therapy and gap junction inhibition reduce MDA-MB-231 breast cancer
cell invasion and metastasis in vitro and in vivo. Sci Rep.
5:125982015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin SS, Parker W, Everett ML and Platt JL:
Differential recognition by proteins of alpha-galactosyl residues
on endothelial cell surfaces. Glycobiology. 8:433–443. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miguel A, Herrero MJ, Sendra L, Botella R,
Algás R, Sánchez M and Aliño SF: Comparative antitumor effect among
GM-CSF, IL-12 and GM-CSF+IL-12 genetically modified tumor cell
vaccines. Cancer Gene Ther. 20:576–581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Golumbek PT, Azhari R, Jaffee EM, Levitsky
HI, Lazenby A, Leong K and Pardoll DM: Controlled release,
biodegradable cytokine depots: A new approach in cancer vaccine
design. Cancer Res. 53:5841–5844. 1993.PubMed/NCBI
|
|
28
|
Lu X, He J, Li X and Zhao Y: The
relationship between malignant and tumor-associated cells provides
a new strategy for targeted diagnosis and therapy. OncoImmunology.
2:e262952013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abdel-Motal UM, Guay HM, Wigglesworth K,
Welsh RM and Galili U: Immunogenicity of influenza virus vaccine is
increased by anti-gal-mediated targeting to antigen-presenting
cells. J Virol. 81:9131–9141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mangold A, Szerafin T, Hoetzenecker K,
Hacker S, Lichtenauer M, Niederpold T, Nickl S, Dworschak M, Blumer
R, Auer J, et al: Alpha-Gal specific Igg immune response after
implantation of bioprostheses. Thorac Cardiovasc Surg. 57:191–195.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Galili U, Repik PM, Anaraki F,
Mozdzanowska K, Washko G and gerhard W: Enhancement of antigen
presentation of influenza virus hemagglutinin by the natural human
anti-Gal antibody. Vaccine. 14:321–328. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abdel-Motal U, Wang S, Lu S, Wigglesworth
K and Galili U: Increased immunogenicity of human immunodeficiency
virus gp120 engineered to express Galalpha1-3Galbeta1-4glcNAc-R
epitopes. J Virol. 80:6943–6951. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Henion TR, Gerhard W, Anaraki F and Galili
U: Synthesis of alpha-gal epitopes on influenza virus vaccines, by
recombinant alpha 1,3galactosyltransferase, enables the formation
of immune complexes with the natural anti-Gal antibody. Vaccine.
15:1174–1182. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zinkernagel RM, Ehl S, Aichele P, Oehen S,
Kündig T and Hengartner H: Antigen localisation regulates immune
responses in a dose- and time-dependent fashion: A geographical
view of immune reactivity. Immunol Rev. 156:199–209. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thomas DA and Massagué J: TGF-beta
directly targets cytotoxic T cell functions during tumor evasion of
immune surveillance. Cancer Cell. 8:369–380. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamaguchi Y, Tsumura H, Miwa M and Inaba
K: Contrasting effects of TGF-beta 1 and TNF-alpha on the
development of dendritic cells from progenitors in mouse bone
marrow. Stem Cells. 15:144–153. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Steinman RM, Hawiger D, Liu K, Bonifaz L,
Bonnyay D, Mahnke K, Iyoda T, Ravetch J, Dhodapkar M, Inaba K, et
al: Dendritic cell function in vivo during the steady state: A role
in peripheral tolerance. Ann NY Acad Sci. 987:15–25. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rook AH, Kehrl JH, Wakefield LM, Roberts
AB, Sporn MB, Burlington DB, Lane HC and Fauci AS: Effects of
transforming growth factor beta on the functions of natural killer
cells: Depressed cytolytic activity and blunting of interferon
responsiveness. J Immunol. 136:3916–3920. 1986.PubMed/NCBI
|
|
39
|
Ikeda H, Old LJ and Schreiber RD: The
roles of IFN gamma in protection against tumor development and
cancer immunoediting. Cytokine Growth Factor Rev. 13:95–109. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
De Giovanni C, Nicoletti G, Landuzzi L,
Astolfi A, Croci S, Comes A, Ferrini S, Meazza R, Iezzi M, Di Carlo
E, et al: Immunoprevention of HER-2/neu transgenic mammary
carcinoma through an interleukin 12-engineered allogeneic cell
vaccine. Cancer Res. 64:4001–4009. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun Y, Jurgovsky K, Möller P, Alijagic S,
Dorbic T, Georgieva J, Wittig B and Schadendorf D: Vaccination with
IL-12 gene-modified autologous melanoma cells: Preclinical results
and a first clinical phase I study. Gene Ther. 5:481–490. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fuji N, Fujiwara H, Ueda Y, Taniguchi F,
Yoshimura T, Oka T and Yamagishi H: Augmentation of local antitumor
immunity in the liver by tumor vaccine modified to secrete murine
interleukin 12. Gene Ther. 6:1120–1127. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lai C, Ye B, Hou X, Duan S, Wei X, Chen C,
Zeng X, Liang W, Zhou S, Hu N, et al: Anti-tumor effect of folic
acid-conjugated chitosan nanoparticles containing IL-33 gene in
hepatocellular carcinoma. Cell Conmmun. 1:30–40. 2014.
|