Introduction
Ovarian cancer is responsible for more than half of
the deaths related to gynecological malignancies (1,2). Due
to the lack of sensitive tests for the detection of early-stage
disease, which is frequently asymptomatic, 75% of malignant ovarian
tumors are discovered at a late stage, with a 5-year survival rate
of ~30% (3,4). Although, the majority of patients
primarily respond to chemotherapy, the relapse rate is more than
85%. The utilization of serum-soluble tumor antigens, such as
CA-125, a biomarker used for the detection of ovarian cancer has
been limited due to the insufficient specificity and sensitivity of
these antigens, particularly for incipient tumors (5–7).
Increased levels of CA-125, the most widely utilized serum
biomarker for ovarian cancer, occur in merely 50% of stage I
patients and are also present in healthy women (5,6).
Therefore, it is essential to find novel biomarkers for the early
diagnosis, prediction of prognosis, monitoring of therapy and to
develop new treatment methods, such as immunotherapy, for the
management of ovarian cancer (5,6,8).
Proteomic technology has emerged in the last decade
as a powerful device used to reveal the molecular mechanisms of the
tumor microenvironment and malignant cells in patients with cancer
(9). Proteomics has provided
effective insight into the disruption of signaling pathways within
tumor cells, allowing for the detection of new targets for drug
reaction, possible diagnostic markers and prognostic symbols of
outcome and response to therapy (10,11).
The proteome exhibits all the probable gene products of a cell.
Proteins may exist in different forms that vary within a particular
cell or in diverse cells, due to modifications derived from
translational, post-translational, regulatory and degradable
processes that influence protein localization, structure and
function. Proteomic methodology characterizes all proteins in a
biological system, including complex features, such as isoforms,
modifications, functional structures and interactions. Particularly
in cancer biomarker discovery, two-dimensional gel electrophoresis,
mass spectrometry (MS) and protein microarrays, in combination with
advanced bioinformatics, have become useful tools to identify
proteins.
In the present study, we employed a proteomic-based
approach to identify tumor antigens that produce a humoral immune
feedback in patients with ovarian cancer. Proteins from the ovarian
cancer cell line SKOV3 were separated by 2-DE, transferred onto
nitrocellulose (NC) membranes and subsequently sera from ovarian
cancer patients and normal individuals were screened by western
blotting for antibodies which react against separated proteins.
Proteins inducing an immune reaction were isolated, and then
identified by MS. Using this technique various tumor antigens were
discovered to react more frequently with the sera of ovarian cancer
patients compared to normal individuals. These identified
biomarkers may be potential candidates for screening and early
diagnosis of ovarian cancer.
Materials and methods
Sera sample collection
In the present study, 120 sera from patients with
ovarian cancer and 85 sera from normal controls were obtained from
the sera bank in the Cancer Autoimmunity Research Laboratory at the
University of Texas, El Paso (UTEP). The sera were first handled by
our clinical partners. These ovarian cancer sera were obtained at
the initial time of cancer diagnosis, prior to patients being cured
with chemotherapy or radiotherapy. Normal control sera were amassed
during annual health examinations from adults without apparent
evidence of malignancy. Due to regulations regarding studies on
human subjects, the identification number and name of the patients
were not revealed to investigators, and some clinical information
concerning the sera was not available. The present study was
approved by the Institutional Review Board of UTEP and
collaborating institutions. All procedures performed in the present
study were in accordance with the ethical standards of the
Institutional Review Board of UTEP.
Cell culture and cell extracts
The ovarian cancer cell line SKOV3 was purchased
from the American Type Culture Collection (ATCC; Manassas, VA,
USA), and cultured in McCoy's 5A medium (Invitrogen, Carlsbad, CA,
USA) supplemented with 10% fetal bovine serum (FBS), 100 U/ml
penicillin and 100 U/ml streptomycin. The cells were cultured in
75-cm2 Falcon tissue culture flasks and allowed to reach
90% confluence. Thereafter, the cells were rinsed once with McCoy's
5A medium without FBS and unloaded from the flask by incubating the
cells with a solution including trypsin-EDTA (Gibco, Carlsbad, CA,
USA), and subsequently harvested in a 15-ml centrifuge tube for
further study.
Two-dimensional gel electrophoresis
(2-DE) analysis
SKOV3 cells were directly lysed in rehydration
sample buffer [8 M urea, 50 nM dithiothreitol (DTT), 4%
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS),
0.2% Bio-Lyte 3/10 ampholyte, and 0.001% bromophenol blue) as
obtained by Bio-Rad Laboratories (Hercules, CA, USA) and were
vigorously vortexed for 90 min at room temperature (RT). Insoluble
substances were removed by centrifugation at 13,200×rpm for 30 min
at 4°C. The supernatant was collected and the protein concentration
was assessed using the Bradford assay (Bio-Rad Laboratories). For
the first dimensional gel electrophoresis analysis, a total of 150
µg of protein was mixed with rehydration buffer containing a trace
bromophenol blue prepared in proteomic-grade water and applied on a
pH 3.0–10.0, 7-cm isoelectric focusing (IEF) strip (Bio-Rad
Laboratories). IEF was performed at a current of 50 mA/gel, 250 V
for 30 min, followed by 4,000 V for 1.5 h, and an additional 4,000
V for 5 h. Strips were immediately stored at −80°C for 2-DE
analysis. For this analysis, 12% SDS-polyacrylamide gels (SDS-PAGE)
were used. Proteins were subsequently transferred onto NC membranes
for western blot analysis or stained with 0.1% Coomassie blue R-250
prepared in 10% acetic acid. The spots were visualized using
PDQuest 2-DE analysis software as described in the manufacturer's
manual (Bio-Rad Laboratories), as well as in our previous study
(12).
In-gel digestion
Excised gel pieces were destained with 40 mM
NH4HCO3 in 50% acetonitrile (ACN). Reduction
was performed with 5 mM Tris(2-carboxyethyl)phosphine hydrochloride
(TCEP) for 1 h at room temperature followed by alkylation with 50
mM of iodoacetamide (both from Sigma-Aldrich, St. Louis, MO, USA)
at room temperature in the dark for 1 h. The dehydrated gel pieces
were then digested with trypsin in 10 mM
NH4HCO3 for 18 h and peptide digests were
extracted using extraction buffer (1:2 v/v 5% formic acid/ACN).
NanoLC-ESI-MS/MS analysis
The digests were analyzed on an Eksigent
NanoLC™-1D-Plus (AB Sciex, Framingham, MA, USA) coupled to a Thermo
Fisher LTQ XL™ Linear Ion Trap Mass Spectrometer (LTQ XL-MS) as
follows: the digests were loaded onto an online dual trap set-up
(Eksigent Chrom XP NanoLC Trap-Column C18-CL-3 µm 120 Å, 350 µm×0.5
mm) at a flow rate of 1.5 µl/min using channel 1A solution (98%
water, 2% ACN, 0.5% formic acid). Separation was achieved on an
Eksigent Chrom XP NanoLC C18-reverse phase column (3C18-CL-3 µm 120
Å, 0.075×150 mm) using 2 channel mobile phases (solvent 2A, 5%
ACN/0.1% FA; solvent 2B, 80% ACN/0.1% FA, on a linear gradient of
5–45% 2B solvent over 60 min at a flow rate of 300 nl/min). The MS
system was set to perform one full scan (400–1,700 m/z range)
followed by MS/MS scans of the 10 most abundant parent-ions (ESI
voltage, 3 kV; isolation width, 3.0 m/z; 35 normalized collision
energy). The dynamic exclusion was set to collect each parent-ion
twice and then excluded for 120 sec.
Data analysis
The resulting MS/MS spectra (350–5,000 Da,
monoisotopic) were searched against a UniProt protein database
downloaded on April 04, 2013 comprised of Homo sapiens, Bos
Taurus and porcine trypsin using a SEQUEST®
algorithm in Proteome Discoverer 1.4 software (Thermo Scientific,
San Jose, CA, USA). The parameters for database search were: i) 2.0
and 1.0 Da for peptide and fragment mass tolerance respectively;
ii) full digest using trypsin after K/R with up to two missed
cleavages allowed; and iii) methionine (M) oxidation as a fixed
modification, and cysteine (C) carbamidomethylation, and
deamidation of asparagine (N), and glutamine (Q) as variable
modifications. At least two peptides were used for assignment of
proteins and search results were filtered for a false discovery
rate (FDR) of 1% employing a decoy search strategy utilizing a
reverse database.
Enzyme-linked immunosorbent assay
(ELISA)
The HSP70 protein was purchased from Abcam
(Cambridge, MA, USA). This protein was diluted in
phosphate-buffered saline (PBS) with a final concentration of 1
µg/ml for coating polystyrene 96-well microtiter plates (Thermo
Scientific, Waltham, MA, USA). Plates were then blocked with
gelatin post-coating solution at room temperature for 2 h. The
antigen-coated wells were subsequently incubated with human sera
diluted at 1:100 with serum diluent for 2 h at room temperature.
The goat anti-human IgG-HRP and the substrate
2,2′-azino-bis-3-ethylbenzo-thiazoline-6-sulfonic acid (ABTS) (both
from Invitrogen, Grand Island, NY, USA) were applied as detection
reagents. The average optical density (OD) value was assessed at a
wavelength of 405 nm for data analysis. The cut-off value
designating a positive reaction was the mean OD of 45 normal human
sera (NHS) added to 3 standard deviations (SDs).
Indirect immunofluorescence (IIF)
assay
Hep-2 cell slides (MBL International Corporation,
Woburn, MA, USA) were applied to identify the autoantibodies in
cancer sera. Polyclonal anti-HSP70 antibody (Sigma-Aldrich) (1:50
dilution) and human sera (1:80 dilution) were incubated at room
temperature for 1 h. FITC-conjugated goat anti-human IgG with a
1:200 dilution, and anti-mouse IgG Fab2 Alexa Fluor with a 1:100
dilution were employed as the secondary antibodies, respectively.
IIF images were obtained with a laser scanning confocal microscope
(LSM 700; Zeiss, New York, NY, USA), using a 20X objective and
processed by ZEN 2009 software (Zeiss, Dublin, CA, USA).
Immunohistochemistry (IHC) with tissue
array slides
Ovarian cancer tissue array slides with normal
controls are commercially available (US Biomax, Inc., Rockville,
MD, USA), and were applied to detect the expression of the HSP70
protein. The slides were deparaffinized with xylene and dehydrated
with ethanol of different strengths. Antigen retrieval was
performed using microwave-heating methods and Trilogy™ pretreatment
solution for 20 min at 100̊C and then the slides were cooled down
at room temperature for 50 min. Avidin and biotin block solutions
were employed to block nonspecific binding of antibodies for 10
min. The slides were incubated with polyclonal anti-HSP70 antibody
(1:50 dilution) overnight at 4°C. An HRP detection system and a DAB
substrate kit were applied as detecting reagents. After
counterstaining with hematoxylin, the slides were dehydrated and
mounted. Eventually, the sections were observed using a microscope
(DM1000; Leica, Heidelberg, Germany).
Statistical analysis
Statistical analysis was performed using SPSS 21.0
software. Data were analyzed using a χ2 test and
expressed as the mean ± 3SD of OD values with normal controls as
determined by ELISA. The results were considered to represent a
statistically significant difference when P-values were
<0.01.
Results
Identification of immunoreactive
proteins in ovarian cancer by LC-MS/MS
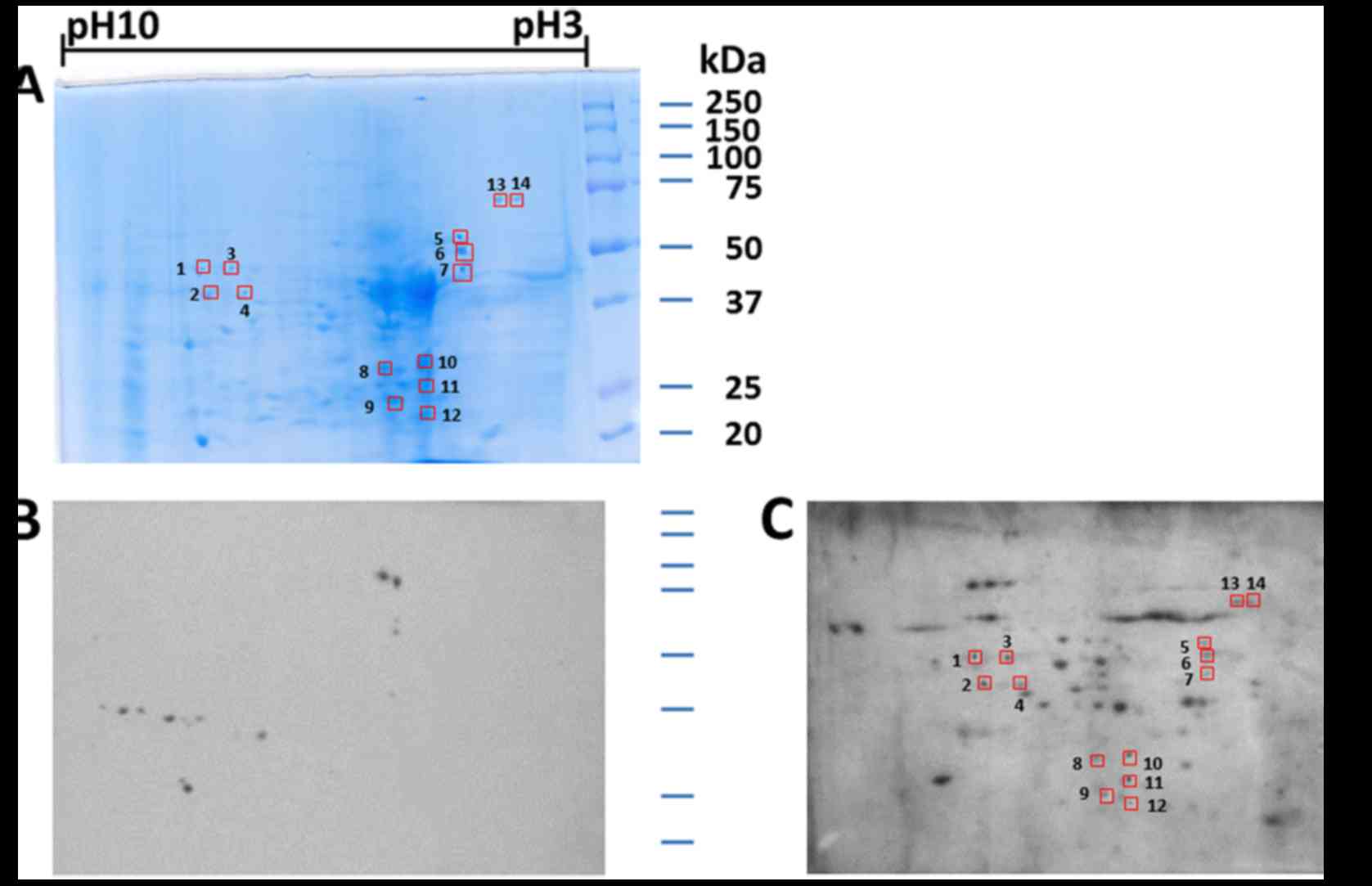
As indicated in Fig.
1, a proteome-based approach was employed to identify valuable
TAAs as biomarkers in ovarian cancer. Concisely, proteins extracted
from SKOV3 cells were separated by 2-DE and later transferred onto
NC membranes. The pool of sera from 10 patients with ovarian cancer
was immunoscreened by western blot analysis. The pool of 10 normal
human sera was applied as the control. The results obtained
revealed a number of protein spots which were visible on the NC
membranes. By comparing and matching protein spots on both
membranes with the equivalent protein spots on the 2-DE gels
(Fig. 2A), 14 immunoreactive spots
were detected with the sera from patients with ovarian cancer
(Fig. 2C), but not with the sera
from the normal controls (Fig. 2B).
In the subsequent study, 14 immunoreactive protein spots were
excised from the SDS-PAGE gels, digested with trypsin and further
analyzed by LC-MS/MS. The resulting MS/MS spectra were identified
using the UniProt protein database, which is a comprehensive
database for human protein sequences. As described in Table I, 13 of the 14 protein spots were
identified by LC-MS/MS. Despite repeated efforts, we could not
identify one spot (spot# 8).
 | Table I.Summary of the identified protein
spots by mass spectrometry. |
Table I.
Summary of the identified protein
spots by mass spectrometry.
| Spot no. | Accession no. | Identified
proteins | Molecular mass
(kDa) | Protein
functions |
|---|
| 1 | P06733 | α-enolase | 47.1 | Glycolytic enzyme
expressed in most human tissues |
| 2 | P06733 | α-enolase | 47.1 | Glycolytic enzyme
expressed in most human tissues |
| 3 | P06733 | α-enolase | 47.1 | Glycolytic enzyme
expressed in most human tissues |
| 4 | P06733 | α-enolase | 47.1 | Glycolytic enzyme
expressed in most human tissues |
| 5 | Q53G71 | Calreticulin
variant | 46.9 | Calcium-binding
chaperone that promotes folding, oligomeric assembly and quality
control in the endoplasmic reticulum |
| 6 | Q53G71 | Calreticulin
variant | 46.9 | Calcium-binding
chaperone that promotes folding, oligomeric assembly and quality
control in the endoplasmic reticulum |
| 7 | Q53G71 | Calreticulin
variant | 46.9 | Calcium-binding
chaperone that promotes folding, oligomeric assembly and quality
control in the endoplasmic reticulum |
| 8 | H7C469 | Uncharacterized
protein | 40.4 |
| 9 | P04083 | Annexin A1 | 38.7 |
Calcium/phospholipid-binding protein which
promotes membrane fusion and is involved in exocytosis This protein
regulates phospholipase A2 activity |
| 10 | Q5VU66 | Tropomyosin 3 | 32 | Binds to actin
filaments in muscle and non-muscle cells Plays a central role, in
association with the troponin complex, in the calcium dependent
regulation of vertebrate striated muscle contraction |
| 11 | Q5HY57 | Emerin | 24.9 | Stabilizes and
promotes the formation of a nuclear actin cortical network.
Inhibits β-catenin activity by preventing its accumulation in the
nucleus |
| 12 | O43399 | Tumor protein
D54 | 22.2 | A marker for breast
cancer and acute lymphoblastic leukemia |
| 13 | P08107 | Heat shock 70 kDa
protein | 70 | A chaperone, that
binds to nascent polypeptides to facilitate correct folding |
| 14 | P08107 | Heat shock 70 kDa
protein | 70 | A chaperone, that
binds to nascent polypeptides to facilitate correct folding |
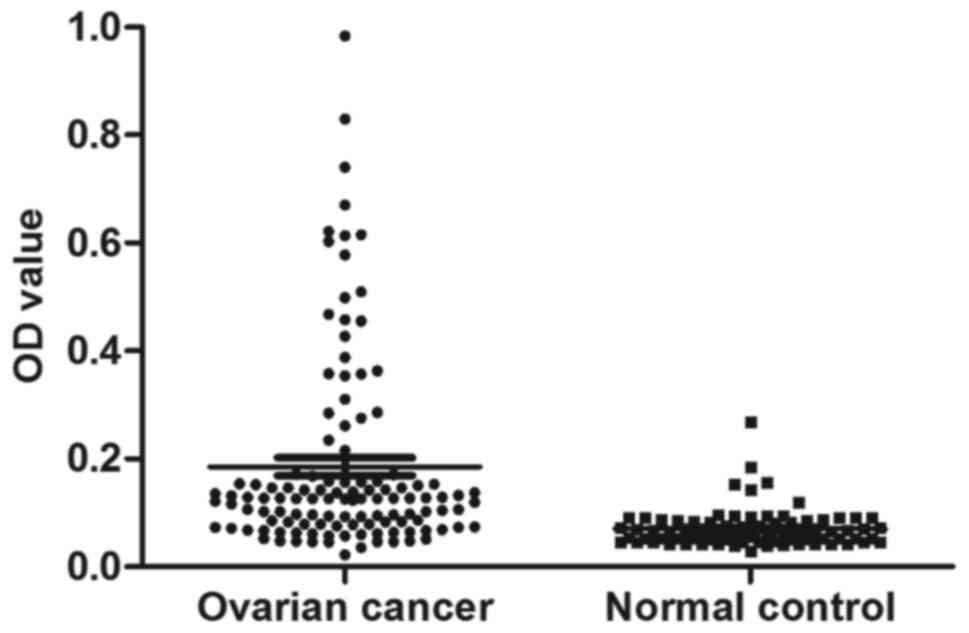
Prevalence of autoantibody against
HSP70 in ovarian cancer
The serum level of anti-HSP70 autoantibody was
evaluated by ELISA as described in Materials and methods. All in
all, 120 sera from patients with ovarian cancer and 85 sera from
normal human individuals were available in the present study. As
demonstrated in Table II, the
prevalence of the anti-HSP70 antibody was 21.7% (26/120) in ovarian
cancer, which was significantly higher than that in the NHS group
(2.35%, 2/85) (P<0.01). Titers of autoantibody against HSP70 in
human sera are displayed in Fig. 3.
The average titer of the anti-HSP70 antibody in ovarian cancer sera
was higher than that in the NHS group (P<0.01).
 | Table II.Frequency of the autoantibody against
HSP70 in human sera by ELISA. |
Table II.
Frequency of the autoantibody against
HSP70 in human sera by ELISA.
| Type of serum | No. tested | Autoantibody to
HSP70 n (%) |
|---|
| Ovarian cancer | 120 | 26
(21.7)a |
| NHS | 85 | 2
(2.35) |
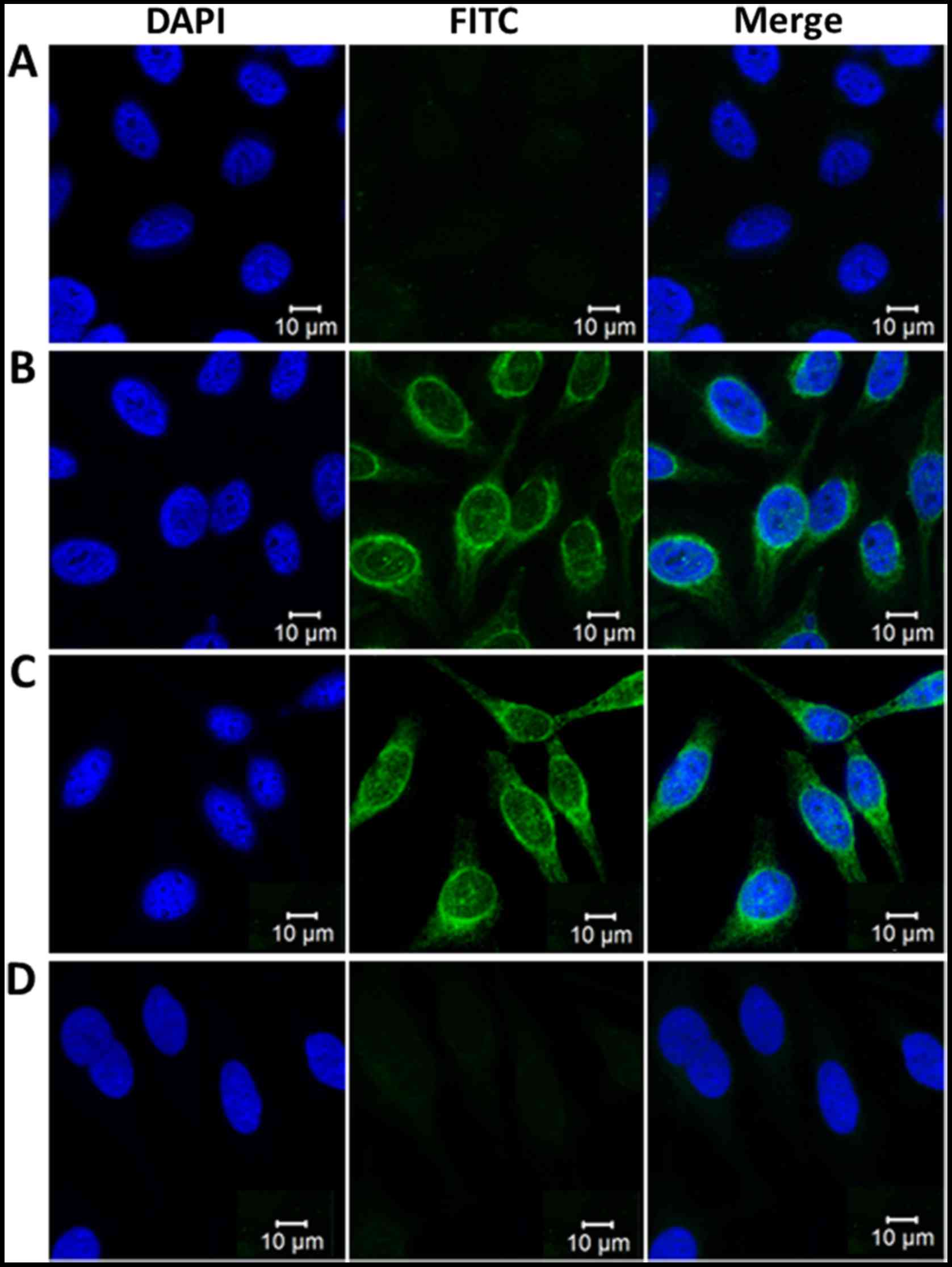
IIF staining pattern of HSP70 in Hep-2
cells
To further ascertain the reactivity of the
anti-HSP70 antibody in ovarian cancer sera and the intracellular
localization of HSP70, Hep-2 cell slides were employed in an
indirect IIF assay to detect positive anti-HSP70-ovarian cancer
sera using ELISA. As described in Fig.
4, a representative ovarian cancer serum sample positive for
the anti-HSP70 antibody as determined by ELISA had an intense
cytoplasmic staining pattern, which was analogous to the staining
pattern obtained by the polyclonal anti-HSP70 antibody which is
largely located in the cytoplasm. The fluorescent cytoplasmic
staining was significantly attenuated when the same ovarian cancer
serum sample was pre-absorbed with recombinant HSP70 protein.
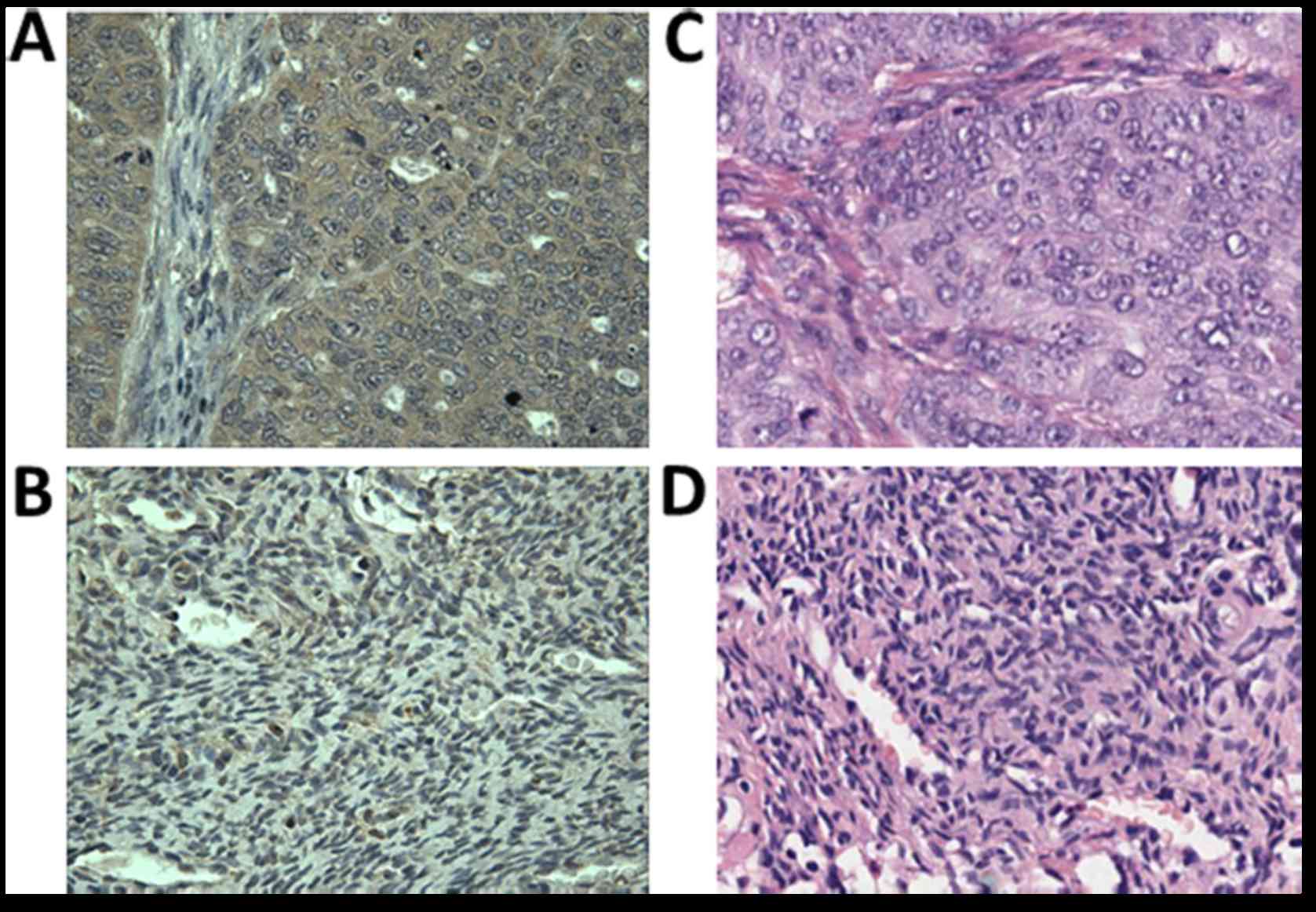
Expression of HSP70 in ovarian cancer
and adjacent normal breast tissues by IHC
To further substantiate the representative protein
as a biomarker in ovarian cancer, it is important to recognize its
expression in ovarian cancer tissue specimens by IHC. In the
present study, the expression of HSP70 in ovarian cancer and
adjacent normal ovarian tissues were detected by IHC with tissue
array slides. Tissue array slides involving 52 breast cancer tissue
specimens and 20 adjacent normal breast tissue specimens were
purchased for the present study. The polyclonal anti-HSP70 antibody
was applied to immunostain the tissue specimens. As displayed in
Table III, 49 of the 52 ovarian
cancer tissues were positively stained (94.2%), while 3 of the 20
adjacent normal breast tissues were positively stained (15%). We
concluded that HSP70 was significantly overexpressed in ovarian
cancer tissues when compared with the normal control (P<0.01).
The expression of HSP70 in ovarian cancer and in the normal
controls is demonstrated in Fig.
5.
 | Table III.Expression of HSP70 in ovarian cancer
tissues and normal controls. |
Table III.
Expression of HSP70 in ovarian cancer
tissues and normal controls.
| Type of
tissues | No. tested | HSP70 positive n
(%) |
|---|
| Ovarian cancer | 52 | 49
(94.2)a |
| Normal
controls | 20 |
3(15) |
Discussion
Proteomics became feasible with the advancement in
two-dimensional polyacrylamide gel electrophoresis (2-DE) (for
protein separation with high reproducibility and resolution), and
in mass spectrometry (MS) (for protein identification). Although,
various techniques have been developed for protein expression
analysis (13–15), the conjunction of 2-DE and MS is
presently the most extensively used (16–19).
In 2001, Brichory et al proposed a proteomic-based method to
identify tumor antigens and related autoantibodies in cancers
(20). To date, this approach has
reported several novel autoantibodies in various malignancies.
Autoantibodies against triosephosphate isomerase, superoxide
dismutase, Annexin I and II and PGP 9.5 have been revealed as
valuable markers in lung cancer (20,21).
Autoantibodies against heat-shock protein 70 (HSP70), eukaryotic
translation elongation factor 2 (eEF2), peroxiredoxin,
heterogeneous nuclear ribonucleoprotein A2 (hnRNP A2),
apoptosis-inducing factor (AIE), triosephosphate isomerase (TIM)
and prostatic binding protein are more prevalent in patients with
hepatocellular carcinoma (HCC) (22,23).
Similarly, autoantibodies against HSP70 and peroxiredoxin VI have
been reported as potential diagnostic biomarkers in esophageal
squamous cell carcinoma (24,25).
Our previous studies employed an immunoproteomic approach to widely
screen sera from patients with certain types of cancer including
esophageal squamous cell carcinoma (ESCC) and HCC, and sera from
patients with precancerous diseases such as liver fibrosis to
characterize and identify the potential tumor-associated antigens
(TAAs) (12,26,27).
In the present study, a total of 14 protein spots were visualized
on 2-DE gels and 7 proteins were finally identified by
LC-MS/MS.
The cellular and molecular roles of the identified
proteins were recorded in the study. A majority of these proteins
were reported to be associated with cell proliferation, cell
migration, cell invasion and signaling transduction. In order to
explore the types of cancer related to these identified proteins,
literature searches were performed using PubMed (http://www.ncbi.nlm.nih.gov/pubmed/). α-enolase,
a glycolytic enzyme, could be a novel pancreatic ductal
adenocarcinoma (PDA)-related antigen (28). Calreticulin has been related to the
proliferation and migration of tumor cells and is associated with a
poor prognosis for breast, gastric and esophageal cancer patients
(29–31). Annexin A1, is an intracellular
protein that is aberrantly expressed in various types of cancer,
involving breast (32,33), gastric (34–36)
and esophageal cancer (37,38). Dysregulation of Annexin A1 is
associated with the occurrence of metastasis, invasion and drug
resistance of cancers (39). It has
been documented that tropomyosin 3 could play a vital role in
hepatocarcinogenesis and serve as an important serological
biomarker in the early detection of ovarian cancer (40,41).
Emerin, as a member of the nuclear lamina-related family, may play
a certain role in papillary thyroid carcinoma (42). The tumor protein D54 gene was first
identified in human breast cancer (43), and then it was established that it
is overexpressed in prostate and ovarian carcinomas (44–47).
Several studies have reported that HSP70 is frequently
overexpressed in tumor cells, where it has anti-apoptotic functions
and acts as a survival protein (48). In the present study, we mainly
concentrated on the representative protein HSP70 to evaluate
whether this protein can serve as a potential TAA biomarker in the
immunodiagnosis of ovarian cancer. As demonstrated above, other
identified proteins, including α-enolase, calreticulin, Annexin A1,
tropomyosin 3, emerin and tumor protein D54 associated with cancer,
may be further explored to detect whether they are useful TAAs in
ovarian cancer.
To date, various studies have been carried out to
detect the autoantibody against HSP70 in various types of cancer
such as liver, esophageal, head and neck cancer, as well as
nasopharyngeal cancer (12,25,48,49).
The present study showed that the frequency of the autoantibody
against HSP70 in sera from patients with ovarian cancer was 21.7%
and confirmed that the levels of serum HSP70 autoantibody were
significantly higher in ovarian cancer patients than in healthy
individuals. The results support the possibility that the
autoantibody against HSP70 may be useful as a novel diagnostic
marker for detecting ovarian cancer.
Heat shock proteins are known to be overexpressed in
a variety of cancers, with HSP70 being the most studied among the
family (50). The human HSP70
family consists of at least 8 highly homologous members that differ
from each other in their intracellular expression and localization
patterns (51). Among them, the
major stress-inducible HSP70 has an important role in cell survival
under stressful conditions. HSP70 is usually overexpressed in
different cancer cells and is presumed to contribute to the
development of tumors (52,53). The expression level of HSP70 is
found to be a diagnostic yardstick in several types of cancers,
since HSP70 overexpression may be correlated with cancerous cell
proliferation (54), increased
clinical stage (55,56) and shorter overall survival (57). HSP70 could be a useful tumor marker
to identify patients with HCC and early-stage prostate cancer
(58,59). A high expression level of HSP70 has
been correlated with poor prognosis in breast cancer, acute myeloid
leukemia, rectum and endometrial cancer (60–64).
Our results demonstrated that the significant high
expression of HSP70 in ovarian cancer tissues may be one of the
reasons why HSP70 induced strong autoantibody response in ovarian
cancer sera compared to normal individuals. Whether HSP70 can serve
as a diagnostic biomarker in ovarian cancer detection, and the
mechanisms of how it can cause humoral immune responses in ovarian
cancer patients remain to be further explored.
The detection of this humoral response may be
helpful not only as a molecular biomarker for immunodiagnosis, but
also to guide effective immunotherapies in malignancies. Further
investigation is needed to evaluate the sensitivity and specificity
of the autoantibody against HSP70 in substantial validation studies
and to consider the mechanisms underlying the function of immune
responses against HSP70 in ovarian cancer. With regards to the
detection of novel autoantibodies, the proteomic-based approach
that we used in the present study is a powerful device helping to
identify biomarker proteins which may be of clinical use in the
future.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (81172086), the
Project for Tackling Key problems in Science and Technology of
Henan Province (1621024100 44), Zhongyuan Scholars Program of Henan
Province (162101510006), the Major Project of Science and
Technology in Henan Province (161100311400) and the Key Scientific
Research Projects of Henan Province (16A330007).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barua A, Bradaric MJ, Kebede T, Espionosa
S, Edassery SL, Bitterman P, Rotmensch J and Luborsky JL:
Anti-tumor and anti-ovarian autoantibodies in women with ovarian
cancer. Am J Reprod Immunol. 57:243–249. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Naora H, Montz FJ, Chai CY and Roden RB:
Aberrant expression of homeobox gene HOXA7 is associated
with müllerian-like differentiation of epithelial ovarian tumors
and the generation of a specific autologous antibody response. Proc
Natl Acad Sci USA. 98:15209–15214. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karabudak AA, Hafner J, Shetty V, Chen S,
Secord AA, Morse MA and Philip R: Autoantibody biomarkers
identified by proteomics methods distinguish ovarian cancer from
non-ovarian cancer with various CA-125 levels. J Cancer Res Clin
Oncol. 139:1757–1770. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gagnon A, Kim JH, Schorge JO, Ye B, Liu B,
Hasselblatt K, Welch WR, Bandera CA and Mok SC: Use of a
combination of approaches to identify and validate relevant
tumor-associated antigens and their corresponding autoantibodies in
ovarian cancer patients. Clin Cancer Res. 14:764–771. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Santucci L, Bruschi M, Ghiggeri GM and
Candiano G: The latest advancements in proteomic two-dimensional
gel electrophoresis analysis applied to biological samples. Methods
Mol Biol. 1243:103–125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JM and Kohn EC: Proteomics as a
guiding tool for more effective personalized therapy. Ann Oncol.
21:(Suppl 7). vii205–vii210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hays JL, Kim G, Giuroiu I and Kohn EC:
Proteomics and ovarian cancer: Integrating proteomics information
into clinical care. J Proteomics. 73:1864–1872. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Looi KS, Nakayasu ES, Diaz RA, Tan EM,
Almeida IC and Zhang JY: Using proteomic approach to identify
tumor-associated antigens as markers in hepatocellular carcinoma. J
Proteome Res. 7:4004–4012. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Opiteck GJ, Ramirez SM, Jorgenson JW and
Moseley MA III: Comprehensive two-dimensional high-performance
liquid chromatography for the isolation of overexpressed proteins
and proteome mapping. Anal Biochem. 258:349–361. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Davidsson P, Westman A, Puchades M,
Nilsson CL and Blennow K: Characterization of proteins from human
cerebrospinal fluid by a combination of preparative two-dimensional
liquid-phase electrophoresis and matrix-assisted laser
desorption/ionization time-of-flight mass spectrometry. Anal Chem.
71:642–647. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tragas C and Pawliszyn J: On-line coupling
of high performance gel filtration chromatography with imaged
capillary isoelectric focusing using a membrane interface.
Electrophoresis. 21:227–237. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsugita A, Kawakami T, Uchida T, Sakai T,
Kamo M, Matsui T, Watanabe Y, Morimasa T, Hosokawa K and Toda T:
Proteome analysis of mouse brain: Two-dimensional electrophoresis
profiles of tissue proteins during the course of aging.
Electrophoresis. 21:1853–1871. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Langen H, Takács B, Evers S, Berndt P,
Lahm HW, Wipf B, Gray C and Fountoulakis M: Two-dimensional map of
the proteome of Haemophilus influenzae. Electrophoresis.
21:411–429. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Colvis CM, Duglas-Tabor Y, Werth KB,
Vieira NE, Kowalak JA, Janjani A, Yergey AL and Garland DL:
Tracking pathology with proteomics: Identification of in vivo
degradation products of alphaB-crystallin. Electrophoresis.
21:2219–2227. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jung E, Heller M, Sanchez JC and
Hochstrasser DF: Proteomics meets cell biology: The establishment
of subcellular proteomes. Electrophoresis. 21:3369–3377. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brichory FM, Misek DE, Yim AM, Krause MC,
Giordano TJ, Beer DG and Hanash SM: An immune response manifested
by the common occurrence of annexins I and II autoantibodies and
high circulating levels of IL-6 in lung cancer. Proc Natl Acad Sci
USA. 98:9824–9829. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brichory F, Beer D, Le Naour F, Giordano T
and Hanash S: Proteomics-based identification of protein gene
product 9.5 as a tumor antigen that induces a humoral immune
response in lung cancer. Cancer Res. 61:7908–7912. 2001.PubMed/NCBI
|
|
22
|
Takashima M, Kuramitsu Y, Yokoyama Y,
Iizuka N, Harada T, Fujimoto M, Sakaida I, Okita K, Oka M and
Nakamura K: Proteomic analysis of autoantibodies in patients with
hepatocellular carcinoma. Proteomics. 6:3894–3900. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li L, Chen SH, Yu CH, Li YM and Wang SQ:
Identification of hepatocellular-carcinoma-associated antigens and
autoantibodies by serological proteome analysis combined with
protein microarray. J Proteome Res. 7:611–620. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fujita Y, Nakanishi T, Hiramatsu M,
Mabuchi H, Miyamoto Y, Miyamoto A, Shimizu A and Tanigawa N:
Proteomics-based approach identifying autoantibody against
peroxiredoxin VI as a novel serum marker in esophageal squamous
cell carcinoma. Clin Cancer Res. 12:6415–6420. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fujita Y, Nakanishi T, Miyamoto Y,
Hiramatsu M, Mabuchi H, Miyamoto A, Shimizu A, Takubo T and
Tanigawa N: Proteomics- based identification of autoantibody
against heat shock protein 70 as a diagnostic marker in esophageal
squamous cell carcinoma. Cancer Lett. 263:280–290. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang J, Wang K, Zhang J, Liu SS, Dai L
and Zhang JY: Using proteomic approach to identify tumor-associated
proteins as biomarkers in human esophageal squamous cell carcinoma.
J Proteome Res. 10:2863–2872. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peng B, Huang X, Nakayasu ES, Petersen JR,
Qiu S, Almeida IC and Zhang JY: Using immunoproteomics to identify
alpha-enolase as an autoantigen in liver fibrosis. J Proteome Res.
12:1789–1796. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cappello P, Tomaino B, Chiarle R, Ceruti
P, Novarino A, Castagnoli C, Migliorini P, Perconti G, Giallongo A,
Milella M, et al: An integrated humoral and cellular response is
elicited in pancreatic cancer by alpha-enolase, a novel pancreatic
ductal adenocarcinoma-associated antigen. Int J Cancer.
125:639–648. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bini L, Magi B, Marzocchi B, Arcuri F,
Tripodi S, Cintorino M, Sanchez JC, Frutiger S, Hughes G, Pallini
V, et al: Protein expression profiles in human breast ductal
carcinoma and histologically normal tissue. Electrophoresis.
18:2832–2841. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen CN, Chang CC, Su TE, Hsu WM, Jeng YM,
Ho MC, Hsieh FJ, Lee PH, Kuo ML, Lee H, et al: Identification of
calreticulin as a prognosis marker and angiogenic regulator in
human gastric cancer. Ann Surg Oncol. 16:524–533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Du XL, Hu H, Lin DC, Xia SH, Shen XM,
Zhang Y, Luo ML, Feng YB, Cai Y, Xu X, et al: Proteomic profiling
of proteins dysregulted in Chinese esophageal squamous cell
carcinoma. J Mol Med. 85:863–875. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yom CK, Han W, Kim SW, Kim HS, Shin HC,
Chang JN, Koo M, Noh DY and Moon BI: Clinical significance of
annexin A1 expression in breast cancer. J Breast Cancer.
14:262–268. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shen D, Nooraie F, Elshimali Y, Lonsberry
V, He J, Bose S, Chia D, Seligson D, Chang HR and Goodglick L:
Decreased expression of annexin A1 is correlated with breast cancer
development and progression as determined by a tissue microarray
analysis. Hum Pathol. 37:1583–1591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng TY, Wu MS, Lin JT, Lin MT, Shun CT,
Huang HY, Hua KT and Kuo ML: Annexin A1 is associated with gastric
cancer survival and promotes gastric cancer cell invasiveness
through the formyl peptide receptor/extracellular signal-regulated
kinase/integrin beta-1-binding protein 1 pathway. Cancer.
118:5757–5767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jorge YC, Mataruco MM, Araújo LP, Rossi
AF, de Oliveira JG, Valsechi MC, Caetano A, Miyazaki K, Fazzio CS,
Thomé JA, et al: Expression of annexin-A1 and galectin-1
anti-inflammatory proteins and mRNA in chronic gastritis and
gastric cancer. Mediators Inflamm. 2013:1528602013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu G, Wang J, Chen Y, Wang X, Pan J, Li Q
and Xie K: Tissue microarray analysis reveals strong clinical
evidence for a close association between loss of annexin A1
expression and nodal metastasis in gastric cancer. Clin Exp
Metastasis. 25:695–702. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang KL, Wu TT, Resetkova E, Wang H,
Correa AM, Hofstetter WL, Swisher SG, Ajani JA, Rashid A, Hamilton
SR, et al: Expression of annexin A1 in esophageal and
esophagogastric junction adenocarcinomas: Association with poor
outcome. Clin Cancer Res. 12:4598–4604. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hu N, Flaig MJ, Su H, Shou JZ, Roth MJ, Li
WJ, Wang C, Goldstein AM, Li G, Emmert-Buck MR, et al:
Comprehensive characterization of annexin I alterations in
esophageal squamous cell carcinoma. Clin Cancer Res. 10:6013–6022.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guo C, Liu S and Sun MZ: Potential role of
Anxa1 in cancer. Future Oncol. 9:1773–1793. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lam CY, Yip CW, Poon TC, Cheng CK, Ng EW,
Wong NC, Cheung PF, Lai PB, Ng IO, Fan ST, et al: Identification
and characterization of tropomyosin 3 associated with
granulin-epithelin precursor in human hepatocellular carcinoma.
PLoS One. 7:e403242012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tang HY, Beer LA, Tanyi JL, Zhang R, Liu Q
and Speicher DW: Protein isoform-specific validation defines
multiple chloride intracellular channel and tropomyosin isoforms as
serological biomarkers of ovarian cancer. J Proteomics. 89:165–178.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kinsella MD, Hinrichs B, Cohen C and
Siddiqui MT: Highlighting nuclear membrane staining in thyroid
neoplasms with emerin: Review and diagnostic utility. Diagn
Cytopathol. 41:497–504. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Byrne JA, Tomasetto C, Garnier JM, Rouyer
N, Mattei MG, Bellocq JP, Rio MC and Basset P: A screening method
to identify genes commonly overexpressed in carcinomas and the
identification of a novel complementary DNA sequence. Cancer Res.
55:2896–2903. 1995.PubMed/NCBI
|
|
44
|
Balleine RL, Fejzo MS, Sathasivam P,
Basset P, Clarke CL and Byrne JA: The hD52 (TPD52) gene is a
candidate target gene for events resulting in increased 8q21 copy
number in human breast carcinoma. Genes Chromosomes Cancer.
29:48–57. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang R, Xu J, Saramäki O, Visakorpi T,
Sutherland WM, Zhou J, Sen B, Lim SD, Mabjeesh N, Amin M, et al:
PrLZ, a novel prostate-specific and androgen-responsive gene
of the TPD52 family, amplified in chromosome 8q21.1 and
overexpressed in human prostate cancer. Cancer Res. 64:1589–1594.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rubin MA, Varambally S, Beroukhim R,
Tomlins SA, Rhodes DR, Paris PL, Hofer MD, Storz-Schweizer M,
Kuefer R, Fletcher JA, et al: Overexpression, amplification, and
androgen regulation of TPD52 in prostate cancer. Cancer Res.
64:3814–3822. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Byrne JA, Balleine RL, Fejzo Schoenberg M,
Mercieca J, Chiew YE, Livnat Y, St Heaps L, Peters GB, Byth K,
Karlan BY, et al: Tumor protein D52 (TPD52) is overexpressed and a
gene amplification target in ovarian cancer. Int J Cancer.
117:1049–1054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shukla S, Pranay A, D'Cruz AK, Chaturvedi
P, Kane SV and Zingde SM: Immunoproteomics reveals that cancer of
the tongue and the gingivobuccal complex exhibit differential
autoantibody response. Cancer Biomark. 5:127–135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tong YQ, Zhang ZJ, Liu B, Huang J, Liu H,
Liu Y, Guo FJ, Zhou GH, Xie PL, Li YH, et al: Autoantibodies as
potential biomarkers for nasopharyngeal carcinoma. Proteomics.
8:3185–3193. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cohen M, Dromard M and Petignat P: Heat
shock proteins in ovarian cancer: A potential target for therapy.
Gynecol Oncol. 119:164–166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tavaria M, Gabriele T, Kola I and Anderson
RL: A hitchhiker's guide to the human Hsp70 family. Cell Stress
Chaperones. 1:23–28. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jäättelä M: Escaping cell death: Survival
proteins in cancer. Exp Cell Res. 248:30–43. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Aghdassi A, Phillips P, Dudeja V,
Dhaulakhandi D, Sharif R, Dawra R, Lerch MM and Saluja A: Heat
shock protein 70 increases tumorigenicity and inhibits apoptosis in
pancreatic adenocarcinoma. Cancer Res. 67:616–625. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ralhan R and Kaur J: Differential
expression of Mr 70,000 heat shock protein in normal, premalignant,
and malignant human uterine cervix. Clin Cancer Res. 1:1217–1222.
1995.PubMed/NCBI
|
|
55
|
Lazaris AC, Theodoropoulos GE, Aroni K,
Saetta A and Davaris PS: Immunohistochemical expression of C-myc
oncogene, heat shock protein 70 and HLA-DR molecules in malignant
cutaneous melanoma. Virchows Arch. 426:461–467. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kaur J, Srivastava A and Ralhan R:
Expression of 70-kDa heat shock protein in oral lesions: Marker of
biological stress or pathogenicity. Oral Oncol. 34:496–501. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Alexiou D, Karayiannakis AJ, Syrigos KN,
Zbar A, Sekara E, Michail P, Rosenberg T and Diamantis T: Clinical
significance of serum levels of E-selectin, intercellular adhesion
molecule-1, and vascular cell adhesion molecule-1 in gastric cancer
patients. Am J Gastroenterol. 98:478–485. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Abe M, Manola JB, Oh WK, Parslow DL,
George DJ, Austin CL and Kantoff PW: Plasma levels of heat shock
protein 70 in patients with prostate cancer: A potential biomarker
for prostate cancer. Clin Prostate Cancer. 3:49–53. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chuma M, Sakamoto M, Yamazaki K, Ohta T,
Ohki M, Asaka M and Hirohashi S: Expression profiling in multistage
hepatocarcinogenesis: Identification of HSP70 as a molecular marker
of early hepatocellular carcinoma. Hepatology. 37:198–207. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Nanbu K, Konishi I, Mandai M, Kuroda H,
Hamid AA, Komatsu T and Mori T: Prognostic significance of heat
shock proteins HSP70 and HSP90 in endometrial carcinomas. Cancer
Detect Prev. 22:549–555. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ciocca DR, Clark GM, Tandon AK, Fuqua SA,
Welch WJ and McGuire WL: Heat shock protein hsp70 in patients with
axillary lymph node-negative breast cancer: Prognostic
implications. J Natl Cancer Inst. 85:570–574. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Thanner F, Sütterlin MW, Kapp M, Rieger L,
Kristen P, Dietl J, Gassel AM and Müller T: Heat-shock protein 70
as a prognostic marker in node-negative breast cancer. Anticancer
Res. 23:1057–1062. 2003.PubMed/NCBI
|
|
63
|
Thomas X, Campos L, Mounier C, Cornillon
J, Flandrin P, Le QH, Piselli S and Guyotat D: Expression of
heat-shock proteins is associated with major adverse prognostic
factors in acute myeloid leukemia. Leuk Res. 29:1049–1058. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sun XF, Zhang H, Carstensen J, Jansson A
and Nordenskjöld B: Heat shock protein 72/73 in relation to
cytoplasmic p53 expression and prognosis in colorectal
adenocarcinomas. Int J Cancer. 74:600–604. 1997. View Article : Google Scholar : PubMed/NCBI
|