Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignant tumors (1).
According to data since 2010, HCC ranks fifth among the most
commonly diagnosed malignant tumors in men and eighth in women
(1). The number of patients
diagnosed with HCC has continued to increase (2). The disease can be radically cured by
surgical removal; the first choice for HCC treatment in China and
many other countries (3). Through
ultrasound screening for patients with liver cirrhosis or
hepatitis, numerous patients suitable for hepatectomy have been
identified (4). Moreover, with the
development of surgical technology and preoperative management,
hepatectomy for HCC has become a safe surgery with rare
complications (4). Over the past 30
years, the survival rate of HCC patients who have undergone
hepatectomy has been markedly improved (5). Nevertheless, HCC mainly follows the
occurrence of viral hepatitis and liver cirrhosis in China and
Southeast Asia (6). The long-term
effects are unsatisfactory. Postoperative occurrence is the primary
cause of treatment failure (7).
Neoplasm staging is of great significance (5). It is conducive to the development of
treatment strategies and the prognostic assessment of patients
(8). However, it provides
standardized platforms for the evaluation of new treatment methods
and the comparison of curative effects. As for HCC, there are many
treatments to this disease, including surgical re-resection, TACE,
PEIT, RFA, microwave therapy, radiotherapy, cryotherapy and liver
transplant (9). Wherein, surgical
re-resection is still the most effective treatment (9). The appropriate treatment should be
selected according to the clinicopathological features of the
recurrent carcinoma, characteristics of the patients, reserve
functions of the liver and the general conditions of the patients
(7).
Recurrence and metastasis are important factors
influencing the curative effects. Since the time of intrahepatic
recurrence affects the survival rate of patients after recurrence,
there is an urgent need for indicators able to accurately predict
the postoperative prognosis of HCC patients (10). Currently, there are numerous studies
concerning the prognostic factors of HCC after surgical
re-resection both at home and abroad (11). These factors include
clinicopathological factors of the primary and recurrent tumors,
the origin of HCC, time of recurrence, general conditions of the
patients and surgical methods (11). However, due to the different liver
diseases and non-uniform standard for radical resection, there are
differences in the markers influencing the postoperative prognosis
of HCC patients (12).
The mature microRNA (miRNA) is a type of non-coding
and single-stranded RNA molecule with 19–25 nucleotides (10). The precursor of miRNA is referred to
as pri-miRNA. It is double-stranded RNA consisting of 70–100
nucleotides. After cutting and unwinding, it develops into mature
miRNA (13). miRNA sequences are
highly conserved among different species, which indicates that
these molecules play an important role in the development,
proliferation, differentiation and apoptosis in organisms (13). However, a number of studies suggest
that there are obvious differences in the miRNA profile in
different diseases and on gene expression (13,14).
This indicates that certain miRNAs can be regarded as biomarkers
and used as diagnostic and prognostic indicators of malignant
tumors (15).
A large amount of research results have shown that
the overexpression or mutation of epidermal growth factor receptor
(EGFR) exists in many tumor cells and tumor tissues (16). EGFR refers to the transmembrane
receptor with RTK activity. It is coded by c-erbB1 genes. The
ligands of EGFR include EGF, TGF, AREG, HB-EGF, EPR and β-cytokine
(17). It regulates the biological
activity of cells by mediating various signaling pathways (18). The overexpression or mutation of
EGFR is often associated with the poor prognosis, rapid metastasis,
short-term recurrence and short survival time of epithelium tumors
such as breast, gastrointestinal, ovarian and cervical cancer
(19).
PI3K is a kinase that is involved in multiple
cellular signaling pathways. As regards Akt, it is an important
factor in the downstream of PI3K (20). It is able to regulate cell
proliferation and prevent cell apoptosis. PI3K/Akt is one of the
principal downstream signaling pathways of the ERBB family of
receptor tyrosine kinases (21).
The cytoplasmic domain of ERBB3 activates PI3K through the
phosphorylation of tyrosine. The activation of a catalytic subunit
of PI3K leads to the excessive activation of PI3K in a variety of
tumor tissues (22).
Akt is a serine/threonine-specific protein kinase.
Since it is highly homologous with PKA and PKC, it is also referred
to as protein kinase B (PKB) (23).
It plays an important role in the regulation of cell growth,
proliferation and survival and glucose metabolism. AKT is able to
inhibit Smac/DIABLO, activate and upregulate apoptosis-inducing
factors and inhibit p53 with Mdm2 (23). In addition, it enables the
inhibitory factors of CDK (p21CIP1/WAF1 and
p27KIP1) to move out of the cell nucleus and degrade in
the cytoplasm through phosphorylation, thereby promoting cell
proliferation.
mTOR is an important site in the downstream of the
PI3K signaling pathway. It plays a critical role in tumor
development, invasion, metastasis and angiogenesis. In 90–100% of
HCC cases, the AKT/mTOR signaling pathway is activated, which is
significantly correlated with the recurrence of tumors and reduced
survival rate of patients (21). In
this study, we evaluated the possible associations between
microRNA-133b (miRNA-133b) and the prognosis of patients with HCC
in light of clinicopathological characteristics after curative
hepatectomy, and investigated the effect of miRNA-133b on the
EGFR/PI3K/Akt/mTOR signaling pathway.
Materials and methods
Patients and follow-up
We analyzed the prospective collected data of 112
HCC patients after curative hepatectomy between February 2010 and
May 2011 at the Chinese General PLA Hospital. The present study was
approved by the Institutional Ethics Committee of the Chinese
General PLA Hospital. Written informed consent was obtained from
all patients. Basic clincal characteristics of all patient with HCC
were collected and are documented in Table I. During the first 2 years after
surgery, patients were followed-up every 2 months, and from 3 years
after surgery, patients were followed-up every 3–4 months.
 | Table I.Clinical characteristics of the
patients with HCC. |
Table I.
Clinical characteristics of the
patients with HCC.
| Variables | All patients
(n=112) | P-value |
|---|
| Age (years) |
| 0.731 |
|
≤55 | 63 |
|
|
>55 | 49 |
|
| Sex, n |
| 0.942 |
|
Female | 58 |
|
|
Male | 54 |
|
| Tumor size (cm),
n |
| 0.178 |
|
≤3.0 | 49 |
|
|
>3.0 | 63 |
|
| Edmondson grade,
n |
| 0.063 |
| I | 12 |
|
| II | 67 |
|
| II | 33 |
|
| Serum AFP levels
(ng/ml) | 75.2±216.8 |
|
| Albumin (g/l) | 38.2±4.5 |
|
| Bilirubin
(µmol/l) | 19.4±11.2 |
|
Reverse transcriptase-quantitative
polymerase chain reaction (RT-qPCR)
The miRNA-133b expression levels in HCC tissues and
matched adjacent normal tissues were detected using qRT-PCR. Total
RNA was extracted from HCC tissue samples and adjacent normal
tissues using TRIzol (Invitrogen, Grand Island, NY, USA) according
to the manufacturer's instructions. Specific cDNA was synthesized
from total RNA (5–10 ng) using TaqMan MicroRNA assays protocol
(Applied Biosystems, Foster City, CA, USA). qRT-PCR (7900HT Fast
Real-Time PCR System) was performed using GeneAmp® Fast
PCR Master Mix (both from Applied Biosystems, Thermo Fisher
Scientific, Inc., Waltham, MA, USA) to analyze the expression level
of miRNA-133b. The qPCR conditions were as follows: 95°C for 5 min;
40 cycles of 94°C for 30 sec; 60°C for 45 sec; 72 for 45 sec. The
following primers were used: miR-133b forward,
5′-GAACCAAGCCGCCCGAGA-3′ and reverse, 5′-CCGCCCTGCTGTGCTGGT-3′;
RNU6B was used as internal control: U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′.
Relative quantification of miRNA-133b expression was evaluated
using the formula, 2−ΔΔCt.
Cell culture
Human HCC cell lines HepG2, SMMC7721, Bel7404 and
HCCM3 were purchased from the Shanghai Cell Bank of the Chinese
Academy of Sciences (Shanghai, China) and cultured in Dulbecco's
modified Eagle's medium (DMEM), supplemented with 10% fetal bovine
serum (FBS) (both from Invitrogen; Thermo Fisher Scientific, Inc.)
100 U/ml penicillin and 100 µg/ml streptomycin in a humidified
atmosphere of 5% CO2 at 37°C.
miRNA transfection
The HepG2 cells (1×105 cells/well) were
seeded into 6-well plates and cultured overnight. Then, the cells
were transfected with 100 nM of negative control mRNA or the
miR-133b mimics (both from Shanghai GenePharma Co., Ltd., Shanghai,
China) using Lipofectamine 2000® (Invitrogen; Thermo
Fisher Scientific, Inc.). At 48 h post-transfection, the
transfected HepG2 cells were prepared for further analysis. EGFR
inhibitor (GW2974 2 µM Sigma Chemical Co. St. Louis, MO, USA) was
added into cell after transfection at 4 h.
Cell viability assay
The transfected HepG2 cells (1×104
cells/well) were seeded into 96-well plates and cultured overnight.
Cell viability was cultured using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Invitrogen; Thermo Fisher Scientific, Inc.) assay for 4 h in a
humidified atmosphere of 5% CO2 at 37°C. The medium was
replaced, and then dimethyl sulfoxide (DMSO) (150 µl) was added
into every well and shaken for 20 min at 37°C. Absorbance was
measured using the EL800 Universal Microplate Reader (BioTek
Instruments, Inc., Winooski, VT, USA) at 570 nm.
Measurements of lactate dehydrogenase
(LDH) activity
The transfected HepG2 cells (1×104
cells/well) were seeded into 96-well plates and cultured overnight.
Cell viability was cultured using lactate dehydrogenase for 1 h at
37°C in darkness. Absorbance was measured using the EL800 Universal
Microplate Reader at 490 nm.
Measurements of the apoptosis
rate
The transfected HepG2 cells (1×105
cells/well) were seeded into 6-well plates and cultured overnight.
The HepG2 cells were added together with 195 µl Annexin V-FITC
binding buffer and 5 µl Annexin V-FITC, and incubated at room
temperature in the dark for 10 min. Then, the cells were stained
with 10 µl propidium iodide (PI) at room temperature in the dark
for 10 min. The apoptosis ratio was recognized with the flow
cytometer FACSVerse (Becton-Dickinson, Heidelberg, Germany).
Measurements of caspase-3/-8
activity
The transfected HepG2 cells (1×105
cells/well) were seeded in 6-well plates and cultured overnight.
The HepG2 cells were incubated with Ac-DEVD-pNA (caspase-3
activity) and Ac-IETD-pNA (caspase-8 activity) at room
temperature in the dark for 2 h. Absorbance was measured using the
EL800 Universal Microplate Reader at 405 nm.
Western blot analysis
The transfected HepG2 cells (1×105
cells/well) were seeded in 6-well plates and cultured overnight.
Cells were washed with ice-cold phosphate-buffered saline (PBS) and
obtained by resuspending the cells in RIPA buffer kit (Beyotime
Institute of Biotechnology, Shanghai, China). The protein
concentration was determined using the Bradford protein assay kit
(Beyotime Institute of Biotechnology). Protein (50 µg) was
subsequently separated using 10–12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and electrotransferred
onto nitrocellulose membranes (EMD; Millipore, Billerica, MA, USA).
The membranes were blocked using 5% skim milk powder and incubated
overnight with primary antibodies: anti-Bax, anti-Bcl-2, anti-PI3K,
anti-p-Akt, anti-p-mTOR and GAPDH at 4°C overnight. The membranes
were then washed with Tris-buffered saline with Tween-20 (TBST) and
incubated with HRP-conjugated goat anti-rabbit immunoglobulin G for
1 h at room temperature. Protein bands were visualized with the
Chemi-Lumi One L western blotting substrate.
Statistical analysis
All data are presented as the mean ± standard error.
The Kaplan-Meier method was used to estimate survival rates, and
the log-rank test was used to assess survival differences between
groups. Data from each group were statistically analyzed using the
Student's t-test. Differences were considered statistically
significant at a p-value of <0.05.
Results
Expression of miRNA-133b in patients
with HCC after curative hepatectomy
We firstly assayed the expression of miRNA-133b in
patients with HCC after curative hepatectomy. As shown in Fig. 1, the expression of miRNA-133b in the
HCC tissues was effectively lower than that in the adjacent normal
tissues. We then assessed that the correlation between miRNA-133b
expression and clinicopathological features of HCC and found that
the tumor size of the HCC patients with low miRNA-133b expression
was larger than that of the HCC patients with high miRNA-133b
expression (Table II). The
Edmondson grade and serum AFP levels of the HCC patients with low
miRNA-133b expression were also higher than these parameters in the
HCC patients with high miRNA-133b expression (Table II).
 | Table II.Correlation between microRNA-133b
expression and clinicopathological features of HCC. |
Table II.
Correlation between microRNA-133b
expression and clinicopathological features of HCC.
| Variables | All patients
(n=112) | Low miRNA-133b | High
miRNA-133b | P-value |
|---|
| Age (years) |
|
|
| 0.892 |
|
≤55 | 63 | 35 | 28 |
|
|
>55 | 49 | 23 | 26 |
|
| Sex |
|
|
| 0.933 |
|
Female | 58 | 31 | 27 |
|
|
Male | 54 | 28 | 26 |
|
| Tumor size
(cm) |
|
|
| 0.009 |
|
≤3.0 | 49 | 35 | 14 |
|
|
>3.0 | 63 | 48 | 15 |
|
| Edmondson
grade |
|
|
| 0.011 |
| I | 12 | 7 | 5 |
|
| II | 67 | 48 | 19 |
|
|
III | 33 | 25 | 8 |
|
| Serum AFP levels
(ng/ml) | 75.2±216.8 | 108.2±301.2 | 53.7±167.2 | 0.001 |
| Albumin
(g/l) | 38.2±4.5 | 39.9±6.1 | 37.3±5.9 | 0.371 |
|
Bilirubin (µmol/l) | 19.4±11.2 | 18.3±10.2 | 20.6±10.8 | 0.782 |
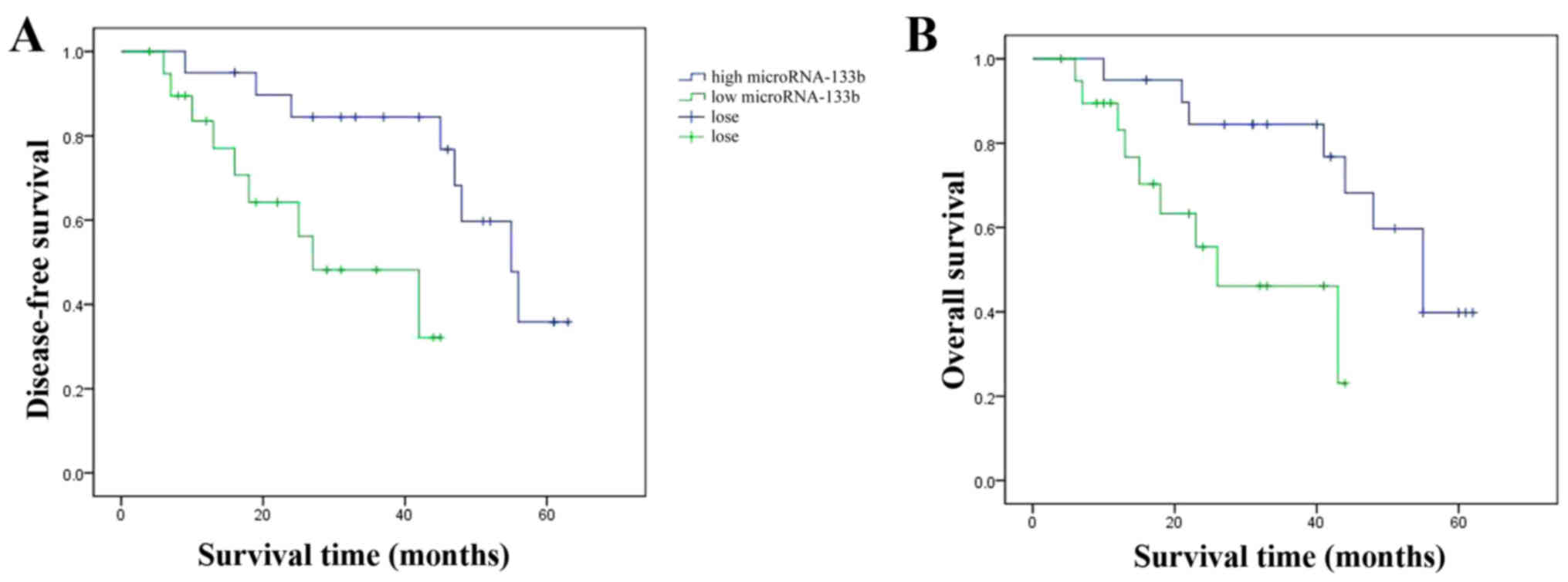
Effects of miRNA-133b expression on
overall and disease-free survival of HCC patients
We aimed to ascertain the affects of miRNA-133b
expression on the overall and disease-free survival of HCC
patients. As shown in Fig. 2, the
overall and disease-free survival of HCC patients with high
miRNA-133b expression was observably extended, compared with the
HCC patients with low miRNA-133b expression.
Expression of miRNA-133b in HCC cell
lines
We detected the expression of miRNA-133b in HCC cell
lines using RT-qPCR. As shown in Fig.
3, the expression of miRNA-133b in the HepG2 cells was lowest
among the HCC cell lines (HepG2, SMMC7721, Bel7404 and HCCM3).
Thus, we selected the HepG2 cells for use in further
experiments.
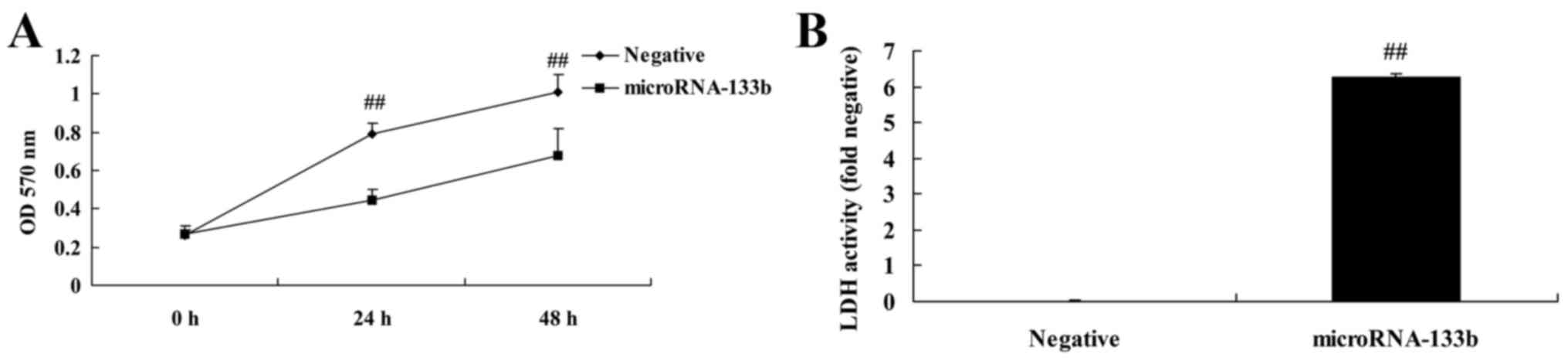
Overexpression of miRNA-133b inhibits
the proliferation of and increases LDH activity in HepG2 cells
We investigated the effects of miRNA-133b on cell
proliferation and LDH activity in HepG2 cells. As shown in Fig. 4, the overexpression of miRNA-133b
significantly inhibited the proliferation of and increased LDH
activity in the HepG2 cells, compared with these parameters in the
negative group.
Overexpression of miRNA-133b induces
the apoptosis of HepG2 cells
We confirmed the effects of miRNA-133b on the
apoptosis of HepG2 cells. As shown in Fig. 5, the overexpression of miRNA-133b
significantly induced the apoptosis of the HepG2 cells, compared
with negative group.
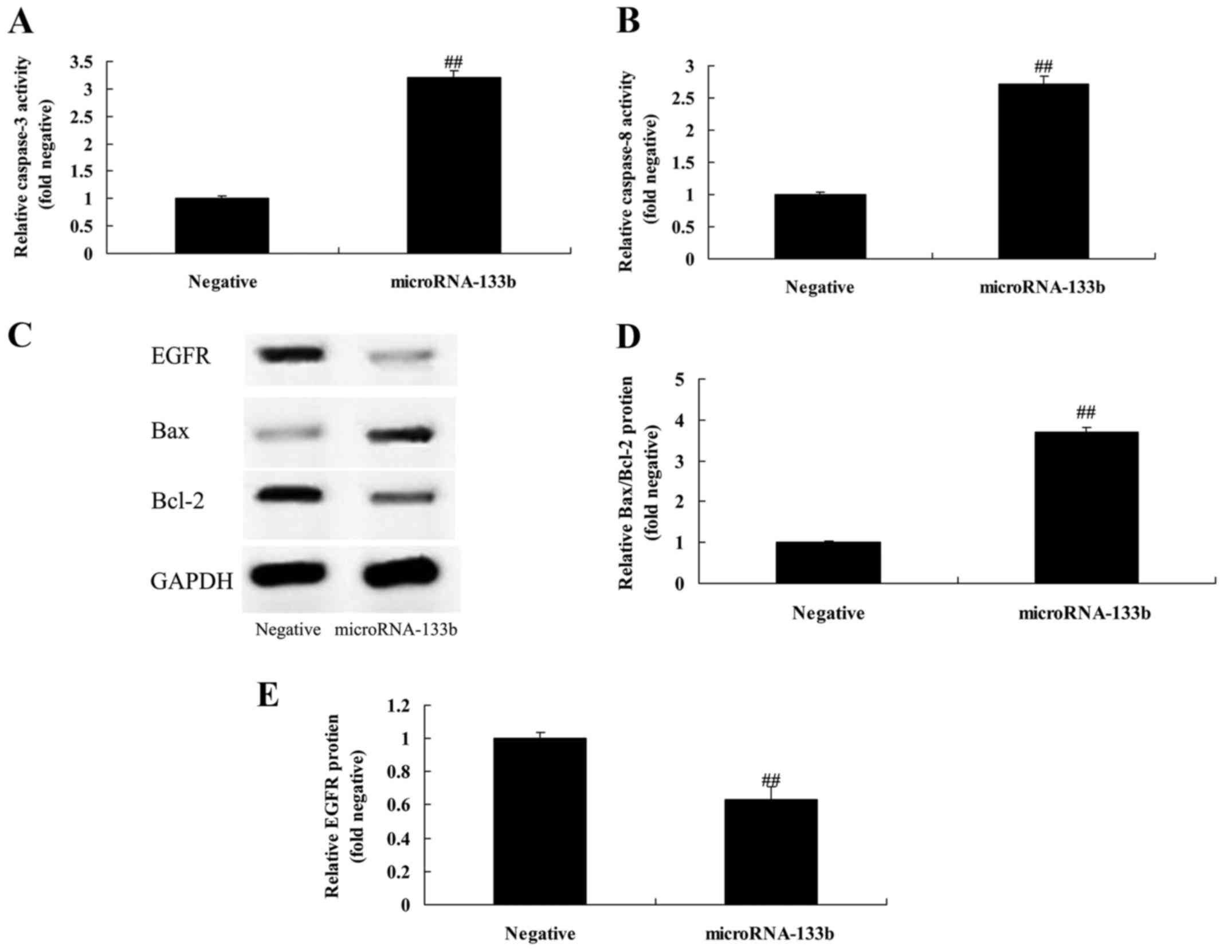
Overexpression of miRNA-133b promotes
caspase-3/-8 activities and increases the Bax/Bcl-2 protein
expression ratio in HepG2 cells
To explore the mechanisms underlying the induction
of apoptosis in the HepG2 cells by miR-133b, we determined
caspase-3/-8 activities and Bax/Bcl-2 protein expression in the
HepG2 cells. As shown in Fig. 6A-D,
the overexpression of miRNA-133b significantly promoted
caspase-3/-8 activities and increased the Bax/Bcl-2 protein
expression ratio in the HepG2 cells, compared with the negative
group.
Overexpression of miRNA-133b
suppresses EGFR protein expression in HepG2 cells
To verify whether EGFR is a direct target of
miRNA-133b, we measured EGFR protein expression by western blot
analysis. As shown in Fig. 6C and
E, the overexpression of miRNA-133b significantly suppressed
EGFR protein expression in the HepG2 cells, compared with the
negative group.
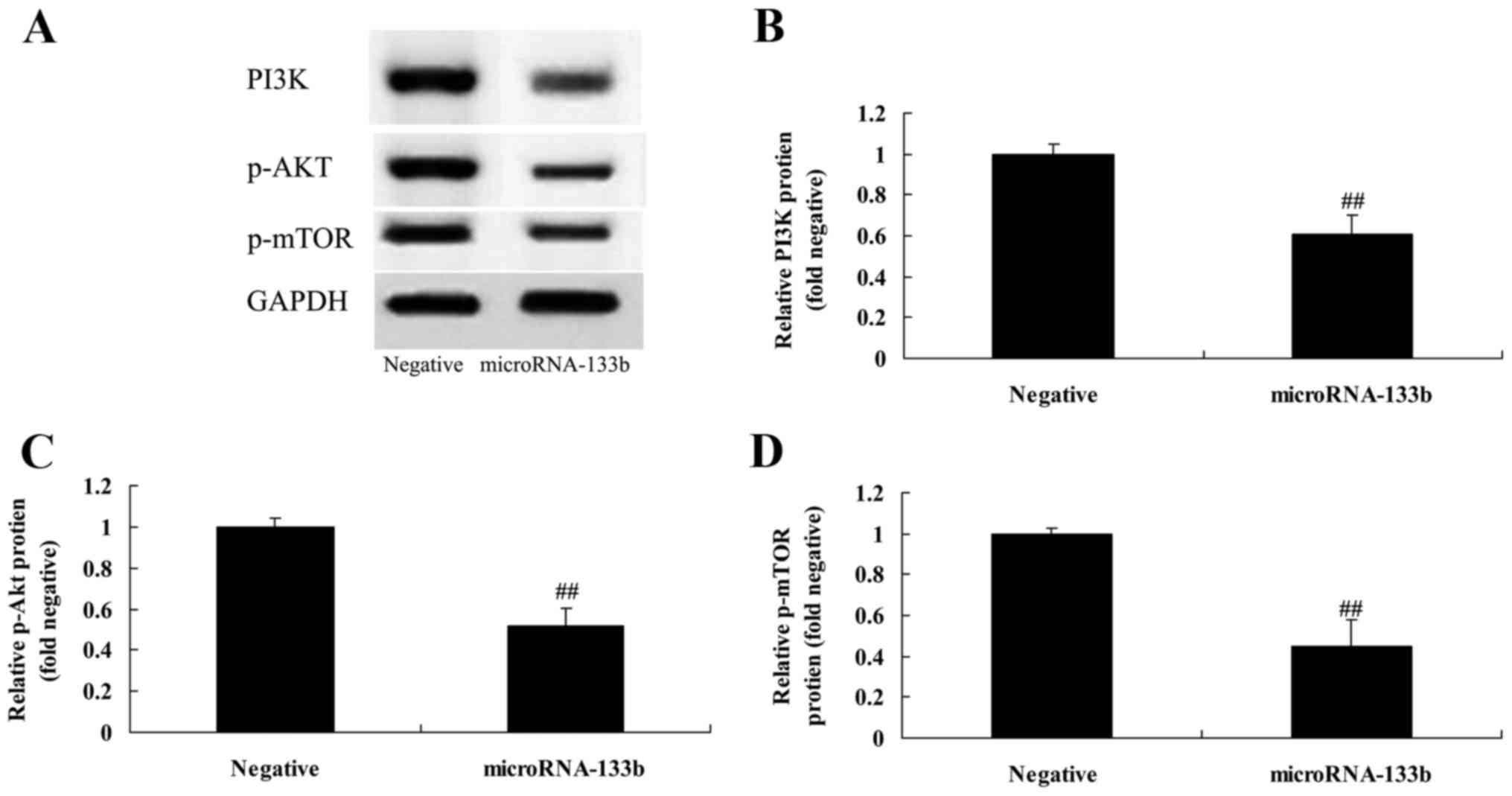
Overexpression of miRNA-133b
suppresses PI3K, p-Akt and p-mTOR protein expression in HepG2
cells
Moreover, we verified that the PI3K/Akt/mTOR
signaling pathway is a direct target of miRNA-133b. We assessed
PI3K, Akt and mTOR protein expression by western blot analysis. As
shown in Fig. 7, the overexpression
of miRNA-133b significantly suppressed PI3K, p-Akt and p-mTOR
protein expression in the HepG2 cells, compared with the negative
group.
EGFR inhibitor enhances the
suppressive effects on EGFR protein expression in HepG2 cells
induced by the overexpression of miRNA-133b
To further investigate the role of EGFR in the
effects of miRNA-133b on the poor of patients with HCC after
curative hepatectomy, we downregulated EGFR expression using a EGFR
inhibitor (GW2974, 2 µM). As shown in Fig. 8A and B, GW2974 significantly
downregulated EGFR expression in the miRNA-133b-overexpressing
HepG2 cells when compared with the miRNA-133b overexpression only
group.
EGFR inhibitor enhances the inhibitory
effects on cell proliferation induced by the overexpression of
miRNA-133b and increases LDH activity even further in HepG2
cells
Furthermore, we investigated the effects of the
downregulation of EGFR on the effects of miRNA-133b on the poor of
patients with HCC after curative hepatectomy. As shown in Fig. 9, the downregulation of EGFR
significantly inhibited cell proliferation and increased LDH
activity in the miRNA-133b-overexpressing HepG2 cells compared with
the miRNA-133b overexpression only group.
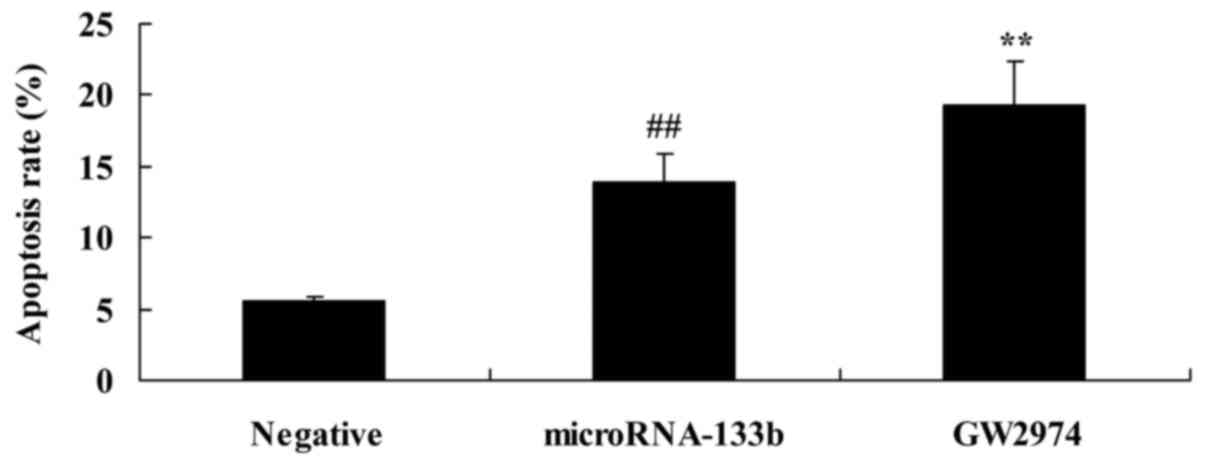
EGFR inhibitor enhances the promoting
effects on the apoptosis of HepG2 cells induced by the
overexpression of miRNA-133b
To further confirm whether the downregulation of
EGFR mediates the promoting effects of miRNA-133b on the apoptosis
of HepG2 cells, the apoptosis rate of HepG2 cells was measured by
flow cytometry. As shown in Fig.
10, the downregulation of EGFR significantly induced the
apoptosis of the miRNA-133b-overexpressing HepG2 cells, compared
with the miRNA-133b overexpression only group.
EGFR inhibitor enhances the promoting
effects on caspase-3/-8 activities and on the Bax/Bcl-2 protein
expression ratio in HepG2 cells induced by the overexpression of
miRNA-133b
In HCC cells, when EGFR expression was
downregulated, the effects on the apoptosis of HepG2 cells
overexpressing miRNA-133b were assessed. As shown in Figs. 8A and C, and 11, the downregulation of EGFR
significantly promoted caspase-3/-8 activities and increased the
Bax/Bcl-2 protein expression ratio in the miRNA-133b-overexpressing
HepG2 cells compared with the miRNA-133b overexpression only
group.
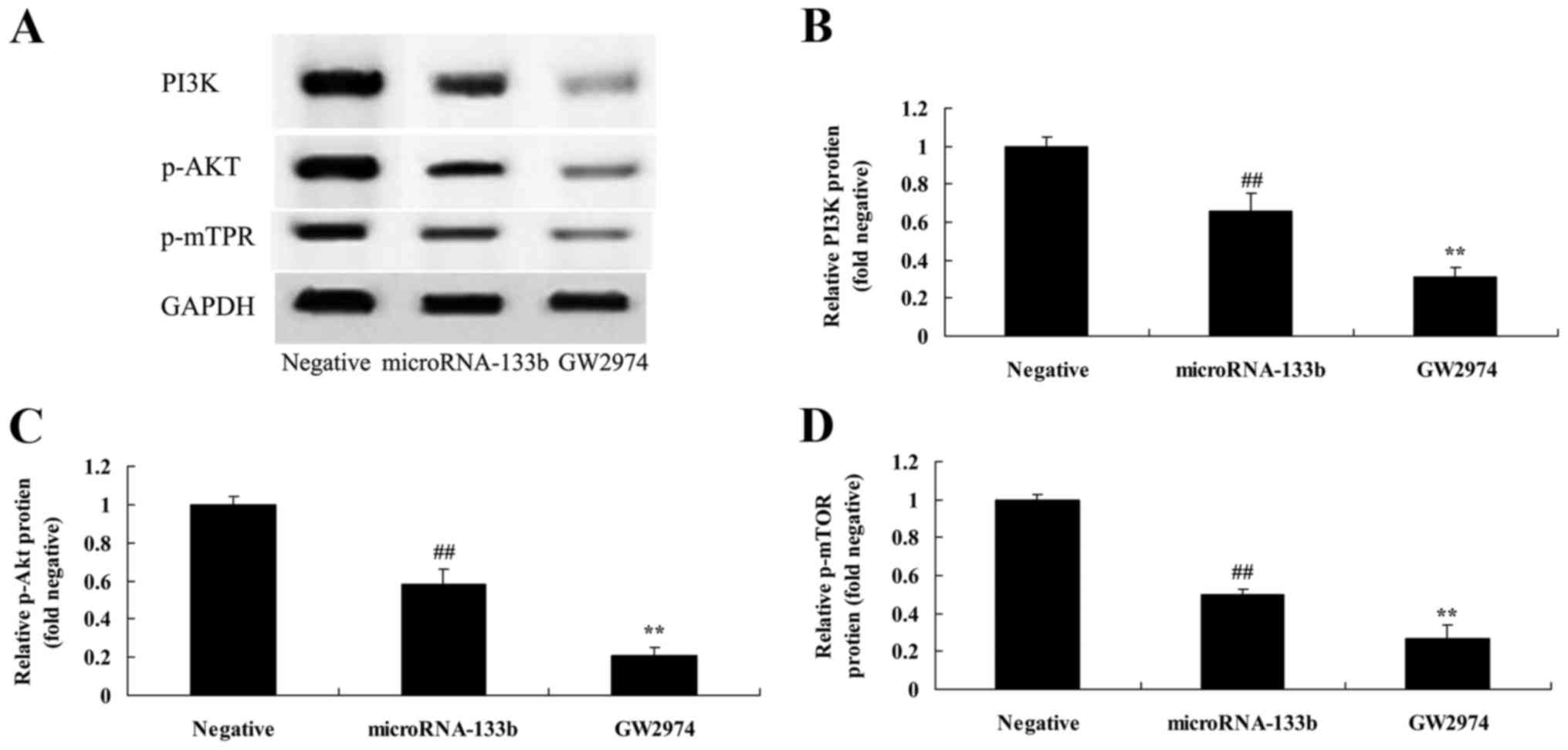
EGFR inhibitor enhances the
suppressive effects on PI3K, p-Akt and p-mTOR protein expression in
HepG2 cells induced by the overexpression of miRNA-133b
To examine whether EGFR regulates miRNA-133b in
regards to HepG2 cell growth, we suppressed EGFR expression and
then analyzed the effects on the PI3K/Akt/mTOR signaling pathway.
As shown in Fig. 12, the
downregulation of EGFR significantly downregulated the
PI3K/Akt/mTOR signaling pathway in miRNA-133b-overexpressing HepG2
cells compared with the miRNA-133b overexpression only group.
Discussion
Hepatocellular carcinoma is one the most common
malignant tumors. Its incidence is increasing. Due to the
technological development of hepatic surgery and the establishment
of new treatments, the disease-free survival of patients has been
extended (24). However, the
recurrence rate remains high. Various foreign documents report that
the recurrence rate of HCC can reach 70–80% within 5 years after
partial hepatectomy. According to domestic documents, the
recurrence rate can be up to 57–81% within 3 years after
hepatectomy. The results of the present study showed that the
overexpression of miRNA-133b inhibited cell proliferation,
increased LDH activity, induced apoptosis and promoted caspase-3/-8
activities and Bax/Bcl-2 protein expression in HepG2 cells.
The activation or mutation of EGFR may cause the
cascade of signaling pathways downstream and finally induce
uncontrollable proliferation of tumor cells (19). The overexpression of EGFR is likely
to be a marker of independent prognosis related to the
proliferation of tumor cells, reduction of radiosensitivity and
high recurrence rate of tumors (25). EGFR gene amplification induces
overexpression of EGFR (25). EGFR
is able to cause excessive activation of its kinase through
spontaneous dimerization (18). In
the present study, the overexpression of miRNA-133b significantly
suppressed EGFR protein expression in HepG2 cells.
The excessive activation of PI3K plays a critical
role in the occurrence and development of HCC (26). There are a number of different
mechanisms mediating the upregulation of PI3K expression. LMP1 is
able to activate PI3K directly, thereby causing the phosphorylation
of Akt (26). With the activation
of NF-κB, the TRAF binding domain in LMP1 becomes the active site
(20). The activation of the
phosphorylation of Akt can further induce the phosphorylation of
downstream molecules (27). In
addition, it may make them participate in cellular metabolism,
proliferation, survival and growth. Upstream molecules of Atk are
blocked by PDK1, which effectively inhibit the growth of tumor
cells. Most HCC cases have sustained activation (26). The phosphorylation and
overexpression of Akt can be detected in HCC tissues and HCC cell
lines.
Activated mTOR can regulate key factors of protein
translation such as p70S6K and 4EBP1 (28). The latter relieves the depression of
eIF4E and finally induces the translation of a series of proteins
that promote cell growth. EIF4E gene amplification and
overexpression are closely linked with the clinical progression of
HCC (29). EMT is obviously
inhibited in HCC cells treated with PI3K and mTOR inhibitors
(30). Thus, EMT of tumor cells is
possibly connected with the PI3K/mTOR pathways (30). In the present study, we found that
the overexpression of miRNA-133b significantly suppressed PI3K,
p-Akt and p-mTOR protein expression in HepG2 cells.
The EGFR/PI3K/Akt/mTOR signaling pathway plays an
important role in the occurrence and development of HCC (31). Key factors of this signaling pathway
have become a research focus. Related drugs are undergoing
pre-clinical trials or clinical trials (32). However, due to the complexity of
this signaling pathway and interaction among different signaling
pathways, the molecular-targeted therapy for HCC merely serves as
an alternative therapy for conventional radiotherapy and
chemotherapy or adjuvant treatment based on conventional
radiotherapy and chemotherapy (33,34).
Our results showed that the suppression of EGFR inhibited cell
proliferation, increased LDH activity, induced apoptosis and
promoted caspase-3/-8 activities and increased Bax/Bcl-2 protein
expression ratio, downregulated PI3K, phosphorylated p-Akt and
phosphorylated-p-mTOR protein expression in the transfected HCC
cells overexpressing miRNA-133b.
In conclusion, our results suggest that the
overexpression of miRNA-133b increases the survival of patients
with HCC after curative hepatectomy, and thus plays protective a
role in HCC progression. miRNA-133b may thus serve as a novel
prognostic biomarker in patients with HCC, namely that patients
with a low expression of this miRNA are predicted to have a poorer
survival. Our findings also indicated that the promoting effects of
miRNA-133b on the survival of patients with HCC, as well as its
suppressive effects on the survival of HCC HepG2 cells are mediated
through the EGFR/PI3K/Akt/mTOR signaling pathway (Fig. 13). These findings provide a
potential therapeutic target for HCC treatment.
Acknowledgements
The present study was supported by the First-Class
General Financial Grant from the China Postdoctoral Science
Foundation (grant no. 2014M562551).
References
|
1
|
Geissler EK, Schnitzbauer AA, Zülke C,
Lamby PE, Proneth A, Duvoux C, Burra P, Jauch KW, Rentsch M, Ganten
TM, et al: Sirolimus use in liver transplant recipients with
hepatocellular carcinoma: A randomized, multicenter, open-label
phase 3 trial. Transplantation. 100:116–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Erhardt A, Kolligs F, Dollinger M, Schott
E, Wege H, Bitzer M, Gog C, Lammert F, Schuchmann M, Walter C, et
al: TACE plus sorafenib for the treatment of hepatocellular
carcinoma: Results of the multicenter, phase II SOCRATES trial.
Cancer Chemother Pharmacol. 74:947–954. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang L, Ge NL, Chen Y, Xie XY, Yin X, Gan
YH, Zhang BH, Zhang JB, Chen RX, Wang YH, et al: Long-term outcomes
and prognostic analysis of radiofrequency ablation for small
hepatocellular carcinoma: 10-year follow-up in Chinese patients.
Med Oncol. 32:772015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirokawa F, Hayashi M, Miyamoto Y, Asakuma
M, Shimizu T, Komeda K, Inoue Y and Uchiyama K: Predictors of poor
prognosis by recurrence patterns after curative hepatectomy for
hepatocellular carcinoma in Child-Pugh classification A.
Hepatogastroenterology. 62:164–168. 2015.PubMed/NCBI
|
|
5
|
Hu BS, Zhao G, Yu HF, Chen K, Dong JH and
Tan JW: High expression of AP-4 predicts poor prognosis for
hepatocellular carcinoma after curative hepatectomy. Tumour Biol.
34:271–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu F, Zhang Y, Peng Z, Gao H, Xu L and
Chen M: High expression of high mobility group box 1 (hmgb1)
predicts poor prognosis for hepatocellular carcinoma after curative
hepatectomy. J Transl Med. 10:1352012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang C, Xiang H, Si H, Guo D and Sun M:
High expression of myofibrillogenesis regulator-1 predicts poor
prognosis for patients with hepatocellular carcinoma after curative
hepatectomy. Int J Clin Exp Pathol. 8:14818–14823. 2015.PubMed/NCBI
|
|
8
|
Hirokawa F, Hayashi M, Asakuma M, Shimizu
T, Inoue Y and Uchiyama K: Risk factors and patterns of early
recurrence after curative hepatectomy for hepatocellular carcinoma.
Surg Oncol. 25:24–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang SL, Liu LP, Sun YF, Yang XR, Fan J,
Ren JW, Chen GG and Lai PB: Distinguished prognosis after
hepatectomy of HBV-related hepatocellular carcinoma with or without
cirrhosis: A long-term follow-up analysis. J Gastroenterol.
51:722–732. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wada H, Yamamoto H, Kim C, Uemura M, Akita
H, Tomimaru Y, Hama N, Kawamoto K, Kobayashi S, Eguchi H, et al:
Association between ephrin-A1 mRNA expression and poor prognosis
after hepatectomy to treat hepatocellular carcinoma. Int J Oncol.
45:1051–1058. 2014.PubMed/NCBI
|
|
11
|
Kobayashi A, Takahashi S, Ishii H, Konishi
M, Nakagohri T, Gotohda N, Satake M, Furuse J and Kinoshita T:
Factors predicting survival in advanced T-staged hepatocellular
carcinoma patients treated with reduction hepatectomy followed by
transcatheter arterial chemoembolization. Eur J Surg Oncol.
33:1019–1024. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ahn S, Hyeon J and Park CK: Metadherin is
a prognostic predictor of hepatocellular carcinoma after curative
hepatectomy. Gut Liver. 7:206–212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mizuguchi Y, Takizawa T, Yoshida H and
Uchida E: Dysregulated miRNA in progression of hepatocellular
carcinoma: A systematic review. Hepatol Res. 46:391–406. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang JT, Liu SM, Ma H, Yang Y, Zhang X,
Sun H, Zhang X, Xu J and Wang J: Systematic review and
meta-analysis: Circulating miRNAs for diagnosis of hepatocellular
carcinoma. J Cell Physiol. 231:328–335. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mahgoub A and Steer CJ: MicroRNAs in the
evaluation and potential treatment of liver diseases. J Clin Med.
5:pii: E522016. View Article : Google Scholar
|
|
16
|
Leng C, Zhang ZG, Chen WX, Luo HP, Song J,
Dong W, Zhu XR, Chen XP, Liang HF and Zhang BX: An integrin
beta4-EGFR unit promotes hepatocellular carcinoma lung metastases
by enhancing anchorage independence through activation of FAK-AKT
pathway. Cancer Lett. 376:188–196. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang YP, Huang LY, Sun WM, Zhang ZZ, Fang
JZ, Wei BF, Wu BH and Han ZG: Insulin receptor tyrosine kinase
substrate activates EGFR/ERK signalling pathway and promotes cell
proliferation of hepatocellular carcinoma. Cancer Lett. 337:96–106.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu H, Gao L, Wang C, Li Y, Ma H, Chen L,
Qin J, Liu B, Liu Y and Liang C: Lower serum soluble-EGFR is a
potential biomarker for metastasis of HCC demonstrated by
N-glycoproteomic analysis. Discov Med. 19:333–341. 2015.PubMed/NCBI
|
|
19
|
Li T, Dong ZR, Guo ZY, Wang CH, Zhi XT,
Zhou JW, Li DK, Chen ZT, Chen ZQ and Hu SY: Mannose-mediated
inhibitory effects of PA-MSHA on invasion and metastasis of
hepatocellular carcinoma via EGFR/Akt/IκBβ/NF-κB pathway. Liver
Int. 35:1416–1429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang J, Zhang Y, Guo Y, Yu C, Chen M, Li
Z, Tian S and Sun C: MicroRNA-3127 promotes cell proliferation and
tumorigenicity in hepatocellular carcinoma by disrupting of
PI3K/AKT negative regulation. Oncotarget. 6:6359–6372. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pellegrino R, Calvisi DF, Neumann O,
Kolluru V, Wesely J, Chen X, Wang C, Wuestefeld T, Ladu S, Elgohary
N, et al: EEF1A2 inactivates p53 by way of PI3K/AKT/mTOR-dependent
stabilization of MDM4 in hepatocellular carcinoma. Hepatology.
59:1886–1899. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu M, Guo J, Li W, Xia H, Lu Y, Dong X,
Chen Y, Xie X, Fu S and Li M: HBx induced AFP receptor expressed to
activate PI3K/AKT signal to promote expression of Src in liver
cells and hepatoma cells. BMC Cancer. 15:3622015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang Q, Zhan L, Cao H, Li J, Lyu Y, Guo
X, Zhang J, Ji L, Ren T, An J, et al: Increased mitochondrial
fission promotes autophagy and hepatocellular carcinoma cell
survival through the ROS-modulated coordinated regulation of the
NFKB and TP53 pathways. Autophagy. 12:999–1014. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brandi G, De Rosa F, Agostini V, di
Girolamo S, Andreone P, Bolondi L, Serra C, Sama C, Golfieri R,
Gramenzi A, et al: Italian Liver Cancer (ITA.LI.CA) Group:
Metronomic capecitabine in advanced hepatocellular carcinoma
patients: A phase II study. Oncologist. 18:1256–1257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lanaya H, Natarajan A, Komposch K, Li L,
Amberg N, Chen L, Wculek SK, Hammer M, Zenz R, Peck-Radosavljevic
M, et al: EGFR has a tumour-promoting role in liver macrophages
during hepatocellular carcinoma formation. Nat Cell Biol.
16:972–981. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu J, Jia L, Ma H, Li Y, Ma Z and Zhao Y:
Axl gene knockdown inhibits the metastasis properties of
hepatocellular carcinoma via PI3K/Akt-PAK1 signal pathway. Tumour
Biol. 35:3809–3817. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang X, Zeng L, Huang J, Zhou H and Liu
Y: Arctigenin, a natural lignan compound, induces apoptotic death
of hepatocellular carcinoma cells via suppression of PI3-K/Akt
signaling. J Biochem Mol Toxicol. 29:458–464. 2015. View Article : Google Scholar
|
|
28
|
Wang H, Zhang C, Xu L, Zang K, Ning Z,
Jiang F, Chi H, Zhu X and Meng Z: Bufalin suppresses hepatocellular
carcinoma invasion and metastasis by targeting HIF-1α via the
PI3K/AKT/mTOR pathway. Oncotarget. 7:20193–20208. 2016.PubMed/NCBI
|
|
29
|
Nemazanyy I, Espeillac C, Pende M and
Panasyuk G: Role of PI3K, mTOR and Akt2 signalling in hepatic
tumorigenesis via the control of PKM2 expression. Biochem Soc
Trans. 41:917–922. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Guo X, Xiong L, Yu L, Li Z, Guo
Q, Li Z, Li B and Lin N: Comprehensive analysis of
microRNA-regulated protein interaction network reveals the tumor
suppressive role of microRNA-149 in human hepatocellular carcinoma
via targeting AKT-mTOR pathway. Mol Cancer. 13:2532014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Horn D, Hess J, Freier K, Hoffmann J and
Freudlsperger C: Targeting EGFR-PI3K-AKT-mTOR signaling enhances
radiosensitivity in head and neck squamous cell carcinoma. Expert
Opin Ther Targets. 19:795–805. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Makinoshima H, Takita M, Saruwatari K,
Umemura S, Obata Y, Ishii G, Matsumoto S, Sugiyama E, Ochiai A, Abe
R, et al: Signaling through the phosphatidylinositol 3-kinase
(PI3K)/mammalian target of rapamycin (mTOR) axis is responsible for
aerobic glycolysis mediated by glucose transporter in epidermal
growth factor receptor (EGFR)-mutated lung adenocarcinoma. J Biol
Chem. 290:17495–17504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ooft ML, Braunius WW, Heus P, Stegeman I,
van Diest PJ, Grolman W, Zuur CI and Mayems SM: Prognostic
significance of the EGFR pathway in nasopharyngeal carcinoma: A
systematic review and meta-analysis. Biomarkers Med. 9:997–1010.
2015. View Article : Google Scholar
|
|
34
|
Brouxhon SM, Kyrkanides S, Teng X, Athar
M, Ghazizadeh S, Simon M, O'Banion MK and Ma L: Soluble E-cadherin:
A critical oncogene modulating receptor tyrosine kinases, MAPK and
PI3K/Akt/mTOR signaling. Oncogene. 33:225–235. 2014. View Article : Google Scholar : PubMed/NCBI
|