Introduction
Gastric cancer is the fourth most common cancer and
the second leading cause of cancer-related deaths worldwide. Almost
half of the patients present with advanced disease at diagnosis and
the 5-year survival rate is <30% (1). The inflammatory microenvironment has
been revealed to be tightly associated with gastric cancer
development and progression. Multiple types of cells exist in the
gastric cancer microenvironment, including fibroblasts,
macrophages, regulatory T cells, and myeloid-derived suppressor
cells, which have been demonstrated to play important roles in
gastric cancer initiation, growth and metastasis (2). Recent studies have revealed that
neutrophils are a predominant component of the tumor
microenvironment and that these cells are critically involved in
cancer development and progression (3,4).
Neutrophils have been reported to promote gastric cancer growth and
progression by stimulating angiogenesis and suppressing anti-tumor
T-cell responses (5,6).
Increasing evidence has revealed that both human and
mouse neutrophils could be polarized towards a pro-tumor N2
phenotype by tumor-derived factors (7). In a mouse gastrointestinal cancer
liver metastasis model, the polarized neutrophils produced
substantially higher levels of FGF2, a pro-angiogenic molecule, to
support vessel formation and liver metastatic growth (8). In mouse breast cancer, the
IL-17-producing γδ T cells induced neutrophils to expand and
acquire an N2 phenotype, suppressing CD8+ T cell
activation and promoting lung metastasis (9). In human hepatocellular carcinoma
(HCC), neutrophils accumulated at the invading tumor edge and
represented the major source of HGF, which promoted HCC metastasis
via interaction with c-Met in HCC cells (10). In human germinal center B-cell
lymphoma, neutrophils could trigger the activation of the nuclear
factor-κB (NF-κB) pathway in stromal cells, which in turn enhanced
the survival of malignant B cells (11). The multifaceted roles of neutrophils
in the pathogenesis of cancer revealed that a better understanding
of the interplay between neutrophils and tumor cells would provide
a new approach for cancer therapy.
In our previous study, we demonstrated that
neutrophils could act cooperatively with cancer-associated
mesenchymal stem cells (MSC) to promote gastric cancer progression
(12). In the present study, we
further determined the direct effects of neutrophils on gastric
cancer cell proliferation, migration and invasion and explored the
underlying molecular mechanism. We demonstrated that the
interaction with neutrophils promoted gastric cancer cell migration
and invasion through the activation of the ERK pathway and the
induction of epithelial-mesenchymal transition (EMT), indicating a
critical role of neutrophils in gastric cancer metastasis.
Materials and methods
Cell culture
Human gastric cancer cell lines (MGC80-3 and
HGC-27), human promyelocytic leukemia cell line (HL-60), and mouse
gastric cancer cell line (MFC) were purchased from the Institute of
Biochemistry and Cell Biology of the Shanghai Institutes for
Biological Sciences at the Chinese Academy of Sciences (Shanghai,
China). MGC80-3 cells were cultured in high-glucose Dulbecco's
modified Eagle's medium (DMEM), supplemented with 10% fetal bovine
serum (FBS; Gibco, Invitrogen Life Technologies, Carlsbad, CA,
USA). HGC-27, HL-60, and MFC cells were cultured in RPMI-1640
medium containing 10% FBS. HL-60 cells were induced to
differentiate into neutrophil-like cells (HL-60N) by 1.25% dimethyl
sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) for 5 days. All
the cells were cultured in a humidified incubator with 5%
CO2 at 37°C. For the cell co-culture experiment, gastric
cancer cells were seeded into plates in the presence or absence of
HL-60N cells (1:3 ratio).
Conditioned medium (CM)
preparation
MGC80-3, HGC-27 and MFC cells were seeded onto 10-cm
plates at a density of 8×105cells/plate for 24 h in
complete culture medium. The cells were then washed with
phosphate-buffered saline (PBS) twice and cultured in serum-free
medium for another 24 h. The supernatants were collected and the
cell debris was removed by centrifugation at 3,000 × g for 10 min.
The CM (designated as GC-CM) was filtered through a 0.22-mm filter
and stored at −80°C until use. HL-60N cells were seeded in
25-cm2 culture cell flasks at a density of
1×105 cells and treated with GC-CM (1:1 ratio, v/v) for
24 h, then washed with PBS twice and cultured in serum-free medium
for another 24 h. The CM (designated as HL-60 CM) was collected,
filtered and stored at −80°C.
Cell viability assay
For the cell co-culture, 3×103 GC cells
were seeded into each well of 96 well-plates in the presence or
absence of HL-60N cells (1:3 ratio). For the CM treatment, the same
density of gastric cancer cells was seeded into each well of 96
well-plates and treated with or without HL-60N CM (1:1 ratio, v/v).
At different time-points (1, 2 and 3 days) a methyl thiazolyl
tetrazolium (MTT) assay was performed. Twenty microliters of MTT (5
mg/ml) was added into each well and the cells were further
incubated for 4 h. Then, the media were discarded and 150 µl DMSO
was added. The plate was shaken at room temperature for 15 min and
the absorbance was read at 490 nm on an automatic microplate reader
(Bio-Tek Instruments, Winooski, VT, USA).
Cell colony formation assay
Following the co-culture with HL-60N cells or
treatment with HL-60N CM for 48 h, the cells were cultured in
six-well plates at a density of 500 cells/well for 8 days. The
medium was changed every two days. At the end of the experiment,
the cell colonies were fixed with formaldehyde and stained with
crystal violet for 30 min. The number of cell colonies was counted
under a microscope.
Transwell migration assay
After the co-culture with HL-60N cells or treatment
with HL-60N CM for 48 h, the cells were collected and seeded into
the upper chamber (8 µm) at a density of 1×105
cells/well (Corning Inc., Corning, NY, USA). The lower chamber was
filled with 500 µl culture medium supplemented with 10% FBS. Nine
hours later, the cells on the upper surface of the membrane were
removed with a cotton swab. Then, the lower cells were fixed with
formaldehyde and stained with crystal violet for 30 min. The number
of migrated cells was counted under a microscope.
Cell invasion assay
The diluted basement Matrigel (BD Biosciences,
Franklin Lakes, NJ, USA) was added into each chamber and let to
polymerize at 37°C for 30 min. The cells were seeded into the upper
chamber at a density of 2×105 cells/well. The lower
chamber was filled with 500 µl culture medium supplemented with 10%
FBS. The cells were allowed to invade to the lower membrane for 30
h. Subsequently, the cells on the upper surface of the membrane
were removed with a cotton swab. The lower cells were then fixed
with formaldehyde and stained with crystal violet for 30 min. The
number of migrated cells was counted under a microscope.
Quantitative real-time PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) and reverse-transcribed into cDNA
using miScript reverse transcription kit (Bio-Rad, Hercules, CA,
USA). The relative expression of target genes was detected on a
Bio-Rad CFX96 quantitative real-time PCR system with the SYBR Green
method (β-actin served as an internal control). The sequences of
the primers are listed in Table
I.
 | Table I.The sequences of primers for
qRT-PCR. |
Table I.
The sequences of primers for
qRT-PCR.
| Gene | Sequence (5′-3′) |
|---|
| β-actin | F:
5′-CACGAAACTACCTTCAACTCC-3′ |
|
| R:
5′-CATACTCCTGCTTGCTGATC-3′ |
| E-cadherin | F:
5′-CGCATTGCCACATACACTCT-3′ |
|
| R:
5′-TTGGCTGAGGATGGTGTAAG-3′ |
| Slug | F:
5′-CCTGGTTGCTTCAAGGACAC-3′ |
|
| R:
5′-TCCATGCTCTTGCAGCTCTC-3′ |
| Vimentin | F:
5′-GAGCTGCAGGAGCTGAATG-3′ |
|
| R:
5′-AGGTCAAGACGTGCCAGAG-3′ |
| ZEB1 | F:
5′-CAGAAGCCAGTGGTCATGAT-3′ |
|
| R:
5′-GACTGCGTCACATGTCTTTG-3′ |
| Sox2 | F:
5′-ACACCAATCCCATCCACACT-3′ |
|
| R:
5′-GCAAACTTCCTGCAAAGCTC-3′ |
| Oct4 | F:
5′-TTGAGGCTCTGCAGCTTAG-3′ |
|
| R:
5′-GCCGGTTACAGAACCACAC-3′ |
| Nanog | F:
5′-CCTGATTCTTCCACCAGTCC-3′ |
|
| R:
5′-TGCTATTCTTCGGCCAGTTG-3′ |
| IL-1β | F:
5′-TACGAATCTCCGACCACCA-3′ |
|
| R:
5′-GGACCAGACATCACCAAGC-3′ |
| IL-6 | F:
5′-TACATCCTCGACGGCATCTC-3′ |
|
| R:
5′-AGCTCTGGCTTGTTCCTCAC-3′ |
| IL-8 | F:
5′-GCTCTGTGTGAAGGTGCAGTTT-3′ |
|
| R:
5′-TTCTGTGTTGGCGCAGTGT-3′ |
| TNFα | F:
5′-CCTGCCCCAATCCCTTTA-3′ |
|
| R:
5′-TGGTTGCCAGCACTTCACT-3′ |
| IL-1β (mouse) | F:
5′-AGCTTCAGGCAGGCAGTATC-3′ |
|
| R:
5′-TCATCTCGGAGCCTGTAGTG-3′ |
| IL-6 (mouse) | F:
5′-AAGTCCGGAGAGGAGACTTC-3′ |
|
| R:
5′-TGGATGGTCTTGGTCCTTAG-3′ |
| TNFα (mouse) | F:
5′-AACTCCAGGCGGTGCCTATG-3′ |
|
| R:
5′-TCCAGCTGCTCCTCCACTTG-3′ |
Western blot analysis
The cells were washed twice with PBS and lysed with
RIPA buffer containing 1% protease inhibitors. Equal amounts of
proteins were separated on 10% SDS-polyacrylamide gels and
transferred onto polyvinylidene fluoride (PVDF) membranes, followed
by blocking with 5% non-fat milk for 1 h. The membranes were
incubated with primary antibodies overnight at 4°C. The following
primary antibodies were used: anti-T-ERK (4695S; Cell Signaling
Technology, Beverly, MA, USA), anti-p-ERK (4370S; Cell Signaling
Technology), anti-E-cadherin (H-108; Santa Cruz Biotechnology,
Dallas, TX, USA), anti-Slug (9585S; Cell Signaling Technology),
anti-vimentin (5741S; Cell Signaling Technology) and anti-GAPDH
(MB001; Bioworld Technology, St. Louis Park, MN, USA). After
incubation with the secondary antibodies (Bioworld Technology) at
37°C for 1 h, the bands were visualized with an ECL
chemiluminescent detection system.
Enzyme-linked immunosorbent assay
(ELISA)
The HL-60N cells were co-cultured with the HGC-27
cells or treated with the supernatants from the HGC-27 cells for 24
h. The CM of the HL-60N cells was collected. The protein levels of
IL-1β, IL-6, IL-8 and TNFα were assessed using ELISA kits (Bangyi
Biotechnology, Shanghai, China), according to the manufacturer's
protocol. A volume of 100 µl of culture supernatants was added to
each well and the absorbance was assessed at 450 nm. ELISA was
performed in triplicate.
Cell sorting
Mice (615; 6–8 weeks old) were purchased from the
Tianjin Institute of Hematology and Blood Diseases Hospital
(Tianjin, China). The bone marrow was collected and filtered
through a 200-mesh metal screen and centrifugated at 800 × g for 5
min. After the lysis of the red cells, the single cell suspensions
were washed once with PBS containing 2% FBS and incubated with
anti-Ly6G-PE and anti-CD11b-PE Cy7 antibodies for 30 min at 4°C.
The CD11b+Ly6G+ neutrophils were isolated on
a flow cytometric cell sorter (BD FACS Aria II; BD Biosciences).
The present study was approved by the Animal Care and Use Committee
of Jiangsu University.
Statistical analysis
All the results were expressed as the mean ± SD.
Statistical analyses were performed using Student's t-test with
GraphPad Prism version 5.0 software (GraphPad Software, La Jolla,
CA, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Co-culture with HL-60N cells promotes
the migration and invasion of gastric cancer cells
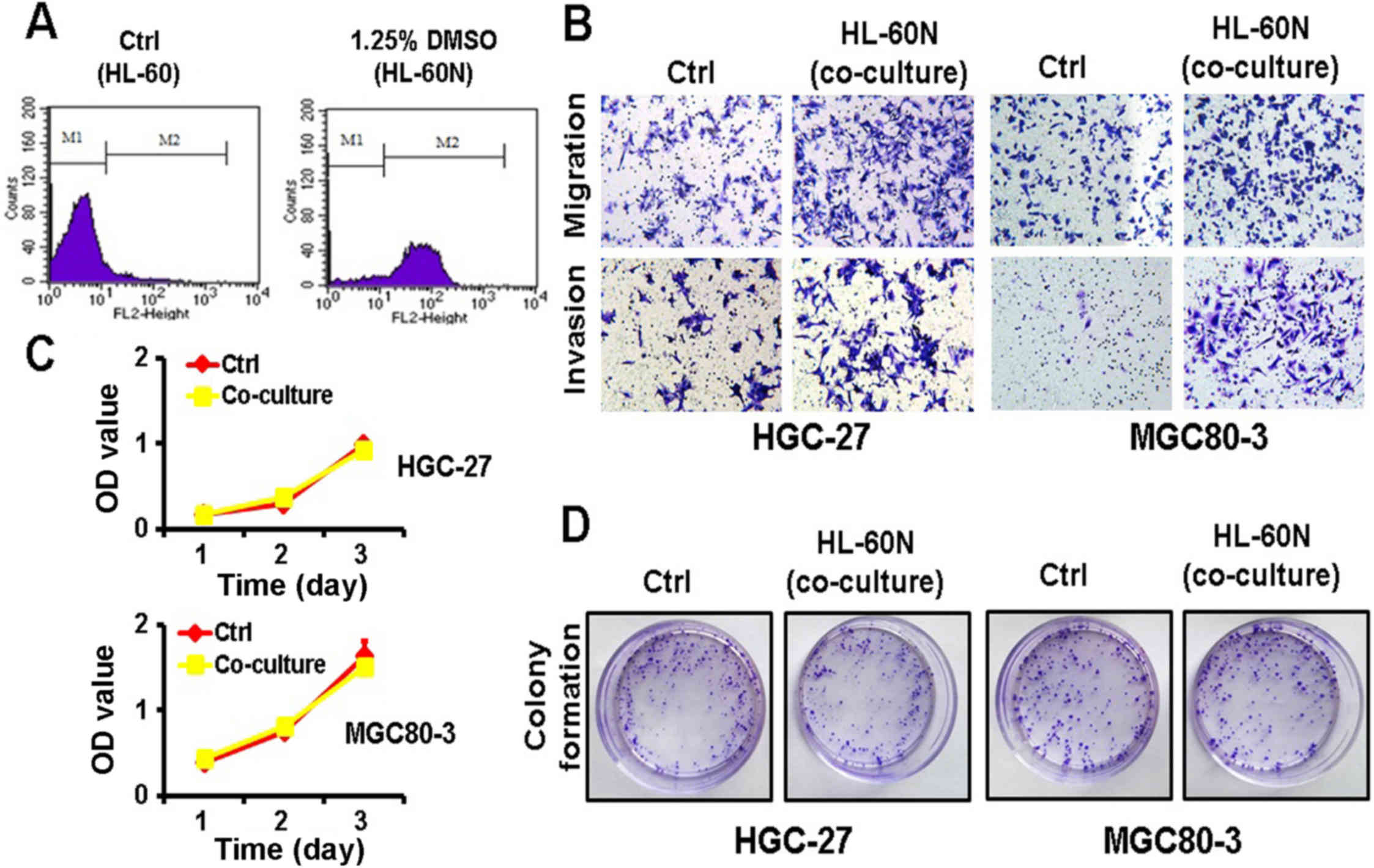
After DMSO induction for 5 days, the expression of
CD11b was notably increased in the HL-60N cells, revealing that the
HL-60 cells were successfully induced into HL-60N cells (Fig. 1A). To examine the potential role of
the HL-60N cells in gastric cancer progression, we co-cultured the
gastric cancer cells with HL-60N cells for 48 h and then examined
their proliferating, migratory and invasive abilities. The results
of the Transwell migration assay and the Matrigel invasion assay
revealed that the co-culture with HL-60N cells markedly increased
the migratory and invasive abilities of both the MGC80-3 and the
HGC-27 cells (Fig. 1B). However,
the results of the MTT and colony formation assays revealed that
the gastric cancer cells co-cultured with the HL-60N cells had no
significant change in their proliferating ability (Fig. 1C and D). Collectively, these results
revealed that the co-culture with HL-60N cells promoted the
migration and invasion of GC cells while it did not affect their
proliferation.
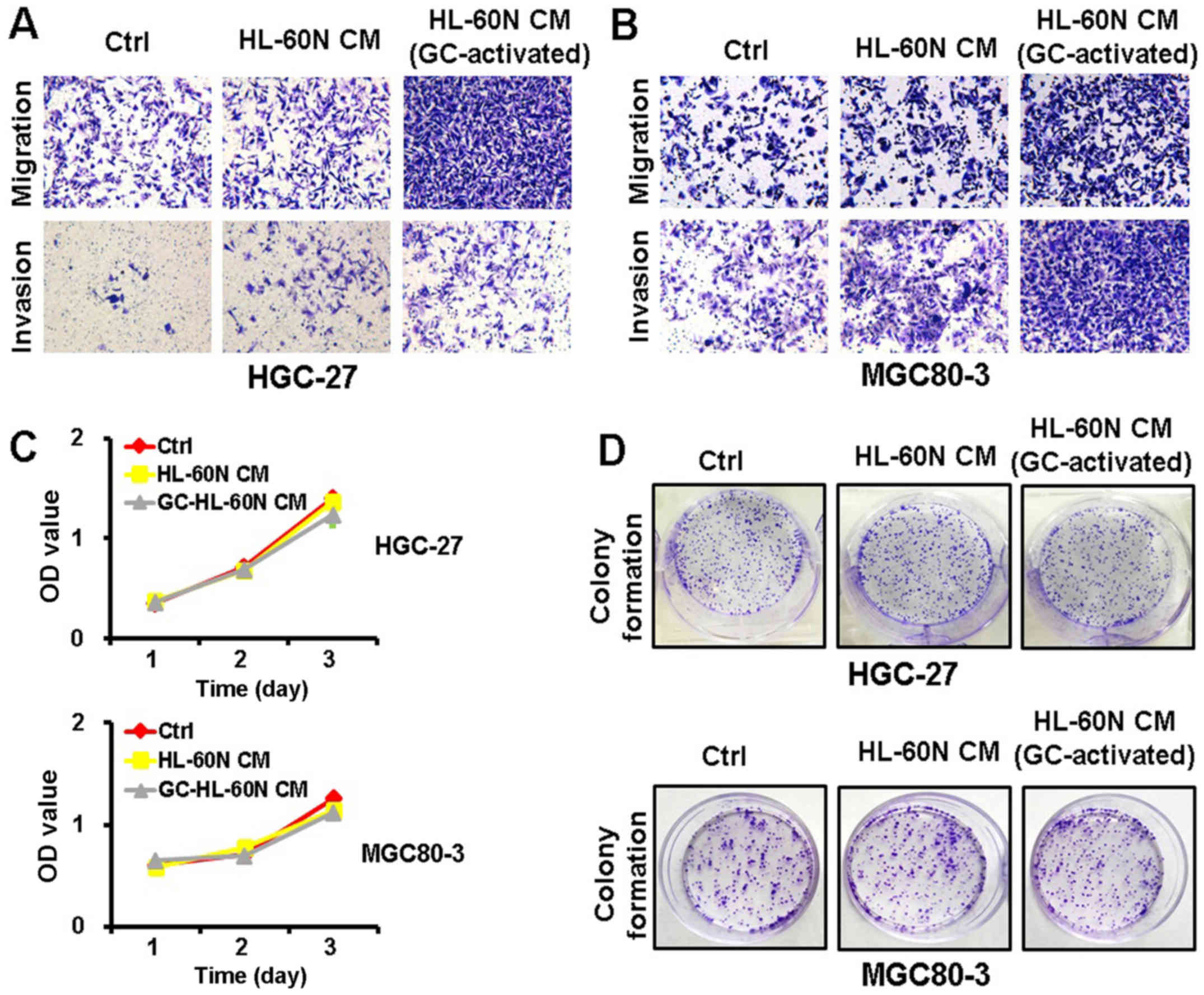
The CM of gastric cancer
(GC)-activated HL-60N cells promotes the migration and invasion of
gastric cancer cells
To examine whether the HL-60N cells promoted gastric
cancer progression through a cell contact-dependent manner, we
collected CM from the HL-60N cells after being co-cultured with
HGC-27 cells for 24 h. We then treated the HGC-27 cells with the CM
for another 24 h. The metastatic potential of the HGC-27 cells was
determined using Transwell invasion assay and Matrigel invasion
assay. As shown in Fig. 2A, the CM
from the HL-60N cells co-cultured with the HGC-27 cells enhanced
the migration and invasion of HGC-27 cells to a greater extent than
that from the HL-60N cells alone. The results of the MTT and colony
formation assay revealed that there was no significant change in
the proliferation of the HGC-27 cells after treatment with the CM
from the activated HL-60N cells (Fig.
2C and D). Similar results were also obtained for the MGC80-3
cells (Fig. 2B and D). These
results revealed that the HL-60N cells, in response to the stimuli
from gastric cancer cells, promoted the migration and invasion of
gastric cancer cells through a paracrine mechanism.
GC-activated HL-60N cells express
increased levels of pro-inflammatory factors
We next determined the expression of
pro-inflammatory factors including the IL-6, IL-8, IL-1β and TNFα
genes in HL-60N cells using qRT-PCR. We found that the co-culture
with gastric cancer cells significantly upregulated the expression
of IL-6, IL-8, IL-1β and TNFα in the HL-60N cells (Fig. 3A). In addition, treatment with the
CM of gastric cancer cells also increased the expression of the
IL-6, IL-8, IL-1β and TNFα genes in HL-60N cells (Fig. 3B). Moreover, the results of ELISA
revealed that either the co-culture with gastric cancer cells or
the treatment with CM of gastric cancer cells improved the
expression levels of IL-6, IL-8, IL-1β and TNFα in HL-60N cells
(Fig. 3C). Collectively, these
results revealed that gastric cancer cells induced HL-60N cells to
acquire a pro-inflammatory phenotype.
 | Figure 3.GC-activated HL-60N cells display
increased expression of pro-inflammatory factors. (A) The HL-60N
cells were co-cultured with HGC-27 cells for 24 h. The expression
of the IL-1β, IL-6, IL-8, and TNF-α genes in the HL-60N cells was
determined using qRT-PCR. (B) The HL-60N cells were treated with
the culture supernatants from HGC-27 cells for 24 h. The expression
of the IL-1β, IL-6, IL-8, and TNF-α genes in the HL-60N cells was
determined using qRT-PCR analysis. (C) The expression of the IL-1β,
IL-6, IL-8 and TNF-α proteins in co-cultured or CM-treated HL-60N
cells was determined using ELISA. *P<0.05; **P<0.01. GC,
gastric cancer; HL-60N, neutrophil-like cells; CM, conditioned
medium; ELISA, enzyme-linked immunosorbent assay. |
Co-culture with HL-60N cells or
treatment with the CM of activated HL-60N cells induces EMT in
gastric cancer cells
We then determined the expression EMT-related genes
in gastric cancer cells. The results of the qRT-PCR analyses
revealed that the co-culture with HL-60N cells or treatment with
the CM of activated HL-60N cells significantly increased the
expression of vimentin, ZEB-1, Slug, Sox2, Nanog and Oct4 in HGC-27
cells (Fig. 4A and B), indicating
that the interaction with neutrophils promoted gastric cancer cell
migration and invasion through the induction of EMT.
Inhibition of the ERK pathway reverses
the promoting effects of the HL-60N cells on gastric cancer cell
migration and invasion
We further determined the activation of the ERK
pathway in HGC-27 cells using western blot analysis. As shown in
Fig. 5A, the activity of p-ERK
started to increase at 15 min after treatment with the CM of
activated HL-60N cells and was maintained at a high level 2 h after
treatment, while the expression of total ERK exhibited no
significant change. To further investigate whether the effects of
the activated HL-60N cells on gastric cancer migration and invasion
were ERK pathway-dependent, we pretreated the gastric cancer cells
with the ERK inhibitor U0126 for 1 h and then incubated the cells
with the CM of activated HL-60N cells. As shown in Fig. 5B, the inhibition of ERK activity
with U0126 could reverse the promoting roles of the activated
HL-60N cells in gastric cancer cell migration and invasion. In
addition, the results of qRT-PCR revealed that the inhibition of
ERK activity with U0126 upregulated the expression of E-cadherin
while it downregulated that of vimentin, ZEB-1, Slug, Sox2, Nanog
and Oct4 in gastric cancer cells (Fig.
5C). Moreover, the results of the western blot analysis
revealed that the inhibition of ERK activity with U0126 upregulated
the expression of E-cadherin while it downregulated that of
vimentin and Slug (Fig. 5D). These
results indicated that the interaction with neutrophils promoted
gastric cancer cell migration and invasion through the activation
of the ERK pathway.
Mouse bone marrow neutrophils
activated by mouse gastric cancer cells promote their migration and
invasion abilities
To confirm the results of the in vitro
experiments, we isolated neutrophils from the bone marrow of 615
mice by cell sorting. Consistent with the observations for the
HL-60N cells, the mouse bone marrow neutrophils also exhibited an
increased expression of IL-6, IL-1β and TNFα when treated with the
CM of MFC cells (Fig. 6A). In
addition, the CM of activated mouse bone marrow neutrophils could
induce the activation of the ERK pathway in mouse gastric cancer
MFC cells (Fig. 6B). Furthermore,
the promoting roles of the activated mouse bone marrow neutrophils
on MFC cell migration and invasion were decreased when the ERK
pathway was inhibited (Fig. 6C).
Collectively, these results revealed that the activated mouse
neutrophils also promoted mouse gastric cancer cell migration and
invasion.
Discussion
In the present study, we demonstrated that human
HL-60N cells (induced from the HL-60 cell line using DMSO) and
primary neutrophils (isolated from mouse bone marrow) could be
activated by gastric cancer to express increased levels of
pro-inflammatory factors, which in turn induced EMT in gastric
cancer cells through the activation of the ERK pathway and promoted
gastric cancer cell migration and invasion, indicating that the
interplay with neutrophils plays a critical role in gastric cancer
metastasis.
Neutrophils have been previously revealed to play
important roles in tumor metastasis (3,4).
Neutrophils could promote tumor metastasis through distinct
mechanisms, which include degrading the extracellular matrix,
aiding the extravasation of tumor cells and increasing the
migratory and invasive abilities of tumor cells. Neutrophils could
release proteinases such as catheptin G and elastase to degrade
thrombospondin-1 (Tsp-1) and facilitate cancer metastasis (13). Neutrophils released increased levels
of oncostatin M (OSM) when stimulated with cancer-derived GM-CSF,
which in turn promoted the motility of breast cancer cells
(14). Glogauer et al
demonstrated that the co-culture with neutrophils increased oral
squamous cell carcinoma cell invasion and induced matrix
degradation, which was associated with the increase of TNFα and
IL-8 in the supernatants from the co-culture system (15). We also found that the co-culture
with gastric cancer cells increased the expression of IL-8 and TNFα
in activated HL-60N cells and mouse bone marrow neutrophils, which
revealed that the induced production of pro-inflammatory factors
(e.g., IL-8 and TNFα) in neutrophils may have profound effects on
the promotion of cancer metastasis.
Neutrophils have been reported to induce EMT in
tumor cells, which significantly increases the migratory and
invasive capacities of tumor cells. Using an oncogenic HRas-induced
zebrafish transformation model, Freisinger and Huttenlocher
revealed that the expression of EMT-related genes, including Slug
and vimentin, was upregulated in the transformed epithelial cells,
which was dependent on the presence of neutrophils (16). Hu et al demonstrated that the
number of intratumoral neutrophils was negatively associated with
E-cadherin expression in human lung cancer tissues (17). Gaida et al revealed that
E-cadherin expression was negatively correlated with neutrophil
infiltration in the tumor tissues of patients with pancreatic
ductal adenocarcinoma (18). Mayer
et al suggested that neutrophils induced EMT in human
ovarian cancer cells to promote their migration (19). EMT has been demonstrated to
critically contribute to gastric cancer development and progression
(20). We revealed that the
co-culture with neutrophils inhibited the expression of E-cadherin
in gastric cancer cells and induced the expression of EMT
transcription factors such as ZEB1 and Slug, which in turn enhanced
the migratory and invasive capacities of gastric cancer cells. The
association between neutrophil infiltration and E-cadherin
expression in human gastric cancer tissues warrants further
investigation.
The signaling pathway that mediates the promotion of
cancer cell migration and invasion by neutrophils varies among
various types of cancer. Neutrophils increase HCC cell migration
through the activation of the Akt pathway (21). In human lung cancer, neutrophils
promoted EMT and enhanced the migration of lung cancer cells via
the activation of the TGF-β/Smad signaling pathway (17). We found that the activated HL-60N
cells and mouse bone marrow neutrophils triggered the activation of
the ERK pathway in gastric cancer cells. The ERK pathway activation
has been previously linked to EMT in gastric cancer (22). Whether other signaling pathways are
also involved in the induction of EMT in gastric cancer cells by
the activated neutrophils needs to be explored in future
studies.
Due to the short life span of human neutrophils, it
is difficult to culture these cells in vitro. In order to
establish a stable and reliable cell model, we selected the HL-60
cell line in the present study. This cell line could be induced to
differentiate into neutrophil-like cells by DMSO and has been
ascertained as a model to study the activity and regulation of
neutrophils in inflammation and cancer (23). In this study, we found that the
HL-60N cells could promote the migration and invasion of gastric
cancer cells as that observed in neutrophils from mouse bone
marrow, revealing the reliability of using this cell line. However,
the limitation of using this cell line is that it may not reflect
the real state of human neutrophils. Further studies are warranted
to assess the effects of human neutrophils on gastric cancer cell
migration and invasion.
In conclusion, our findings revealed that the
interaction with neutrophils promoted gastric cancer cell migration
and invasion through the induction of ΕΜΤ. Our data not only added
new information to the important roles of neutrophils in cancer
metastasis, but also provided a new strategy for the treatment of
gastric cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (nos. 81672416 and 81201660),
the Natural Science Foundation of Jiangsu Province (no.
BK20141303), the Key Research and Development Project of Zhenjiang
(no. SH2015034), the Starting Foundation for Senior Talents of
Jiangsu University (no. 13JDG086), the Qing Lan project of Jiangsu
Province, the ‘333’ project of Jiangsu Province and the Foundation
for Young Academic Leader of Jiangsu University.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma HY, Liu XZ and Liang CM: Inflammatory
microenvironment contributes to epithelial-mesenchymal transition
in gastric cancer. World J Gastroenterol. 22:6619–6628. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang X, Zhang W, Yuan X, Fu M, Qian H and
Xu W: Neutrophils in cancer development and progression: Roles,
mechanisms, and implications (Review). Int J Oncol. 49:857–867.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coffelt SB, Wellenstein MD and de Visser
KE: Neutrophils in cancer: Neutral no more. Nat Rev Cancer.
16:431–446. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li TJ, Jiang YM, Hu YF, Huang L, Yu J,
Zhao LY, Deng HJ, Mou TY, Liu H, Yang Y, et al:
Interleukin-17-producing neutrophils link inflammatory stimuli to
disease progression by promoting angiogenesis in gastric cancer.
Clin Cancer Res. 23:1575–1585. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang TT, Zhao YL, Peng LS, Chen N, Chen W,
Lv YP, Mao FY, Zhang JY, Cheng P, Teng YS, et al: Tumour-activated
neutrophils in gastric cancer foster immune suppression and disease
progression through GM-CSF-PD-L1 pathway. Gut. Mar 8–2017.(Epud
ahead of print). doi: 10.1136/gutjnl-2016-313075. View Article : Google Scholar :
|
|
7
|
Powell DR and Huttenlocher A: Neutrophils
in the tumor microenvironment. Trends Immunol. 37:41–52. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gordon-Weeks AN, Lim SY, Yuzhalin AE,
Jones K, Markelc B, Kim KJ, Buzzelli JN, Fokas E, Cao Y, Smart S,
et al: Neutrophils promote hepatic metastasis growth through
fibroblast growth factor 2-dependent angiogenesis in mice.
Hepatology. 65:1920–1935. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coffelt SB, Kersten K, Doornebal CW,
Weiden J, Vrijland K, Hau CS, Verstegen NJM, Ciampricotti M,
Hawinkels LJAC, Jonkers J and de Visser KE: IL-17-producing γδ T
cells and neutrophils conspire to promote breast cancer metastasis.
Nature. 522:345–348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He M, Peng A, Huang XZ, Shi DC, Wang JC,
Zhao Q, Lin H, Kuang DM, Ke PF and Lao XM: Peritumoral stromal
neutrophils are essential for c-Met-elicited metastasis in human
hepatocellular carcinoma. OncoImmunology. 5:e12198282016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grégoire M, Guilloton F, Pangault C,
Mourcin F, Sok P, Latour M, Amé-Thomas P, Flecher E, Fest T and
Tarte K: Neutrophils trigger a NF-κB dependent polarization of
tumor-supportive stromal cells in germinal center B-cell lymphomas.
Oncotarget. 6:16471–16487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu Q, Zhang X, Zhang L, Li W, Wu H, Yuan
X, Mao F, Wang M, Zhu W, Qian H, et al: The IL-6-STAT3 axis
mediates a reciprocal crosstalk between cancer-derived mesenchymal
stem cells and neutrophils to synergistically prompt gastric cancer
progression. Cell Death Dis. 5:e12952014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
El Rayes T, Catena R, Lee S, Stawowczyk M,
Joshi N, Fischbach C, Powell CA, Dannenberg AJ, Altorki NK, Gao D,
et al: Lung inflammation promotes metastasis through neutrophil
protease-mediated degradation of Tsp-1. Proc Natl Acad Sci USA.
112:pp. 16000–16005. 2015; View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Queen MM, Ryan RE, Holzer RG, Keller-Peck
CR and Jorcyk CL: Breast cancer cells stimulate neutrophils to
produce oncostatin M: Potential implications for tumor progression.
Cancer Res. 65:8896–8904. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Glogauer JE, Sun CX, Bradley G and
Magalhaes MA: Neutrophils increase oral squamous cell carcinoma
invasion through an invadopodia-dependent pathway. Cancer Immunol
Res. 3:1218–1226. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Freisinger CM and Huttenlocher A: Live
imaging and gene expression analysis in zebrafish identifies a link
between neutrophils and epithelial to mesenchymal transition. PLoS
One. 9:e1121832014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu P, Shen M, Zhang P, Zheng C, Pang Z,
Zhu L and Du J: Intratumoral neutrophil granulocytes contribute to
epithelial-mesenchymal transition in lung adenocarcinoma cells.
Tumour Biol. 36:7789–7796. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gaida MM, Steffen TG, Günther F,
Tschaharganeh DF, Felix K, Bergmann F, Schirmacher P and Hänsch GM:
Polymorphonuclear neutrophils promote dyshesion of tumor cells and
elastase-mediated degradation of E-cadherin in pancreatic tumors.
Eur J Immunol. 42:3369–3380. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mayer C, Darb-Esfahani S, Meyer AS, Hübner
K, Rom J, Sohn C, Braicu I, Sehouli J, Hänsch GM and Gaida MM:
Neutrophil granulocytes in ovarian cancer - induction of
epithelial-to-mesenchymal-transition and tumor cell migration. J
Cancer. 7:546–554. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang L, Wu RL and Xu AM:
Epithelial-mesenchymal transition in gastric cancer. Am J Transl
Res. 7:2141–2158. 2015.PubMed/NCBI
|
|
21
|
Wu Y, Zhao Q, Peng C, Sun L, Li XF and
Kuang DM: Neutrophils promote motility of cancer cells via a
hyaluronan-mediated TLR4/PI3K activation loop. J Pathol.
225:438–447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou Q, Wang X, Yu Z, Wu X, Chen X, Li J,
Zhu Z, Liu B and Su L: Transducin (β)-like 1 X-linked receptor 1
promotes gastric cancer progression via the ERK1/2 pathway.
Oncogene. 36:1873–1886. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li XF, Chen DP, Ouyang FZ, Chen MM, Wu Y,
Kuang DM and Zheng L: Increased autophagy sustains the survival and
pro-tumourigenic effects of neutrophils in human hepatocellular
carcinoma. J Hepatol. 62:131–139. 2015. View Article : Google Scholar : PubMed/NCBI
|