Introduction
Breast cancer is one of the most frequently
diagnosed cancers, and is the second leading cause of
cancer-related death in women, with an estimated 232,670 new cases
identified and 40,000 deaths in the US alone in 2014 (1). Current therapies such as surgery,
hormone therapy, chemotherapy, radiation therapy or complementary
therapies are not always effective ways for fighting breast cancer,
with some causing toxicities and unwanted effects. However, to the
best of our knowledge, natural products have immune regulatory
functions and less unwanted effects than chemical compounds.
Studies have reported that natural products have regulatory actions
in tumor immunity, such as activation of T lymphocytes,
facilitation of the function of CTL and natural killer (NK) cell
secretion of cytokines (2–4).
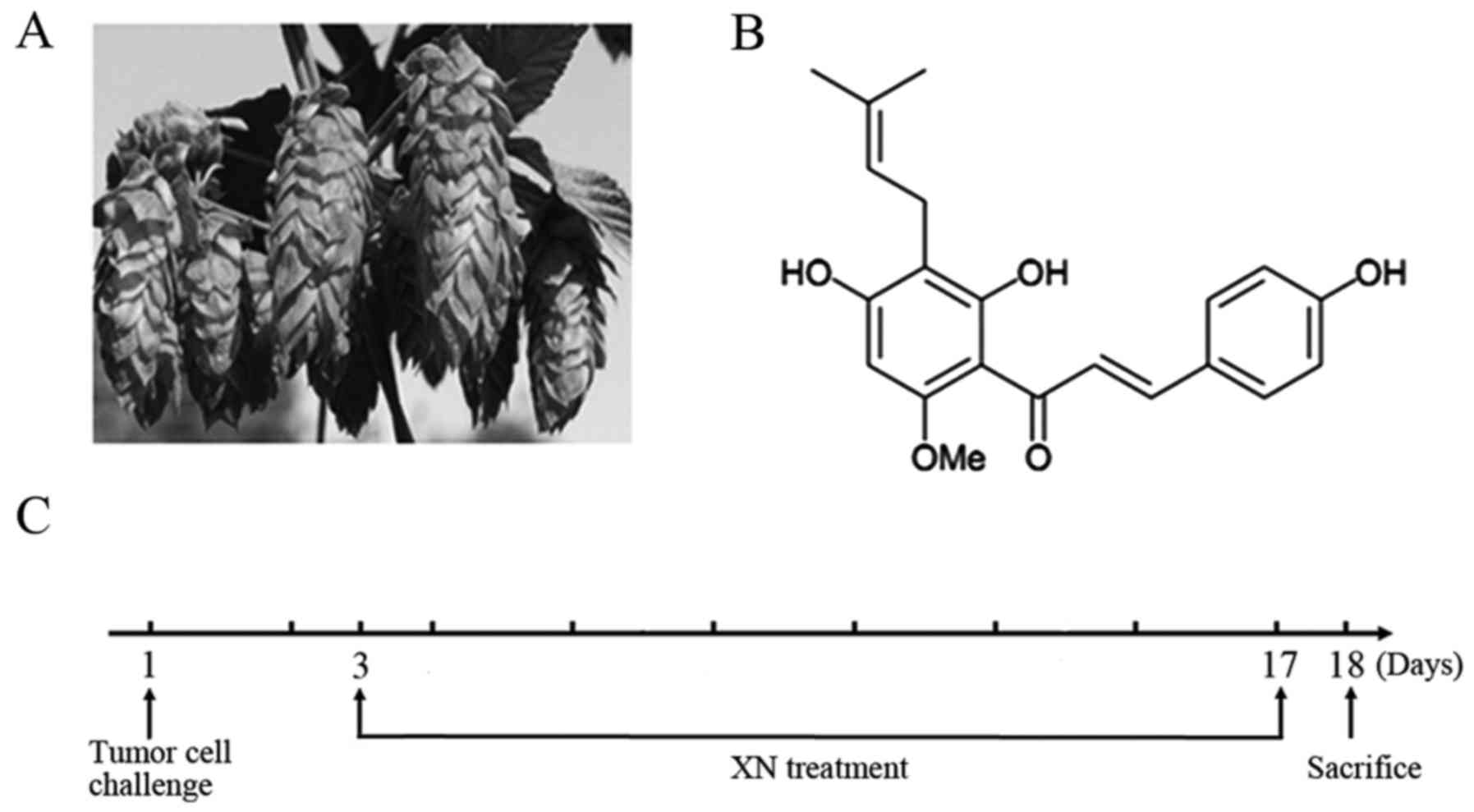
Xanthohumol (XN) is a prenylflavonoid found in hop
plants (Humulus lupulus) (Fig.
1A), and has various biological properties. Previous
epidemiologic studies have reported that XN reduces the risk of
breast, prostate and pancreatic cancer (5–7). It
can also decrease cell growth by inducing cycle arrest and
apoptosis, which is dependent upon downregulation of Notch
signaling pathway in hepatocellular carcinoma and human pancreatic
cancer cells (8,9), as well as inhibition of nuclear
factor-κB (NF-κB) activation (10).
Nevertheless, to the best of our knowledge, there has been no study
on XN and tumor immunity.
Helper T lymphocytes are in a core position with
respect to tumor immunity. According to the type of cytokines
secreted, they can be divided into mainly two subsets: Th1 and Th2,
which differentiate from naive T lymphocytes (Th0) (11). Th1 effector cells produce
characteristic cytokines interleukin (IL)-2, interferon (IFN)-γ and
tumor necrosis factor (TNF)-α, which primarily mediate cellular
immunity, including antitumor immunity. In addition, Th2 effector
cells produce IL-4, IL-5, IL-6, IL-10 and IL-13, which mediate
humoral immunity and promote antibody production (12). The Glimcher research team (Harvard
University) isolated T-bet (T-box protein expressed in T cells), a
Th1-specific transcription factor involved in Th1 differentiation
and transformation, in 2000 (13).
GATA-binding protein-3 (GATA-3) belongs to the GATA family
transcription factors and is a zinc-finger DNA binding protein. It
is a Th2-specific transcription factor and is involved in Th2
differentiation and transformation (14). Furthermore, it is particularly
notorious that T cells express GATA-3 through two pathways, which
are both dependent on STAT6. However, there are different modes of
activation of STAT6: one is auto-activation (15), and the other is activated by IL-4
and T cell receptor (TCR) in co-simulation (16). Recent research has shown that T
cells also express T-bet through two pathways, one is dependent on
STAT4, which is activated by IL-12, and the other is dependent on
STAT1, which is activated by IFN-γ (17). Under normal circumstances, Th1 and
Th2 cytokines maintain a relative balance, which is important for
maintenance of immune equilibrium. However, when dysfunction
occurs, the balance is disturbed, known as ‘the shifting of Th1/Th2
balance’ (18). In addition, this
may cause different types of diseases, such as cancer (19–21),
AIDS (22) and autoimmune diseases
(23). When the balance drifts from
Th1 to Th2, it may cause antitumor immunosuppression (24).
Our previous research suggested that XN inhibits
breast cancer cell survival via modulating the Notch signaling
pathway in vivo and in vitro (25). Meanwhile, preliminary test results
demonstrated that XN enhanced expression of T-bet and weakened
expression of GATA-3. These results led us to believe that XN
exerts various effects on Th1/Th2, and we explored the underlying
effects in the present study.
Materials and methods
Reagents and antibodies
XN (purity >98.6%) was provided by Yumen Tuopu
Science and Technology Development LLC (Yumen, China), in
accordance with the chemical structure of XN as shown in Fig. 1B. Anti-mouse CD3 (FITC), anti-mouse
CD4 (PE), anti-mouse CD8 (PE), anti-mouseCD25 (APC), anti-mouse
IL-4 (APC), anti-mouse IFN-γ (DAPI), anti-mouse perforin (FITC),
anti-mouse granzyme B (FITC), flow cytometry staining buffer,
fixation/permeabilization concentration, fixation/permeabilization
diluent and permeabilization buffer were purchased from
eBioscience, Inc. (San Diego, CA, USA). Mouse IL-2, mouse IL-4,
mouse IL-10 and mouse IFN-γ ELISA kits were purchased from
Elabscience Biotechnology Co., Ltd. (Wuhan, China). Anti-T-bet and
anti-GATA-3 antibodies were purchased from Abcam (Cambridge, UK).
Ki-67 (D3B5) was purchased from Cell Signaling Technology (CST;
Boston, MA, USA). Anti-phospho-STAT4 (Tyr693), anti-STAT4,
anti-STAT6, and anti-phospho-STAT6 (Tyr641) were purchased from
Biosynthesis Biotechnology Co., Ltd. (Beijing, China). Total
protein extraction kit was purchased from BestBio Co., Ltd.
(Shanghai, China). Mouse 1X lymphocyte separation medium was
purchased from Dakew (Shenzhen, China).
Cell line and animals
The 4T1 cell line was purchased from the China
General Microbiological Culture Collection Center (Shanghai,
China). The 6- to 8-week-old BALB/c female mice, weighing 18±2 g,
were purchased from Lanzhou Veterinary Research Institute (Chinese
Academy of Agricultural Sciences, Lanzhou, China). All the animals
were acclimatized for one week. Living environment was maintained
under controlled conditions (24±2°C, 50±10% relative humidity, with
a rhythm of 12 h light/dark cycles).
Treatment of mice
All animal methods in the experiments were in line
with ‘Guidelines for the Humane Treatment of Laboratory Animals’,
which was issued by the Ministry of Science and Technology of the
People's Republic of China in 2006.
Mice were randomly assigned into five groups and
named the healthy, control, 25 mg/kg XN and 50 mg/kg XN groups, and
10 mg/kg cyclophosphamide (Cy) group. Cy has been reported to shift
Th1/Th2 immune response from Th2 to Th1 (26,27);
we used it as a positive treatment. A mouse model of breast cancer
was constructed by subcutaneous injection of 4T1 cells
(1×105-106 cells/ml) into the right posterior
leg. Drugs and solvent were administered by gavage after three days
of injection, and treatment was administered for two weeks. The
healthy and control groups received only solvent. The treatment
schedule is shown in Fig. 1C. Two
weeks later, mice were anesthetized with ether and peripheral blood
was collected by removal of the eyeballs. Finally, tumors and
spleens were removed from the mice.
Calculation of the tumor volume (V) was as follows:
Vtumor = (L × W2)/2 (L, is the longest
diameter; W, is the shortest diameter) (28), and the diameters were measured by
electronic calipers. Tumor inhibitory rate = (tumor weight of
treatment group - tumor weight of control group)/tumor weight of
control group × 100%. Rate of body weight change = (body weight at
the end of experiment - body weight at the beginning of
experiment)/body weight at the beginning of experiment × 100%. The
above experiments were performed at least three times.
Western blot analysis
We homogenized the tumor tissues and obtained total
protein using a total protein extraction kit. Sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12%) was
performed on 30 µg of each sample protein, and then the samples
were transferred to polyvinylidene difluoride membranes (Millipore,
Bedford, MA, USA). Blocking of non-specific protein occurred at
room temperature in Tris-buffered saline-Tween-20 (TBST; TBS
containing 0.01% Tween-20)-5% non-fat milk. Following incubation
with the primary antibody at 4°C overnight, incubation with the
secondary antibody was carried out at room temperature for 4 h.
Membranes were washed with TBST for 5×5 min and peroxidase activity
was visualized with an enhanced chemiluminescence western detection
system (Perkin-Elmer Life Sciences, Boston, MA, USA).
Immunohistochemistry (IHC) and
histopathologic analysis
Tumor tissues (3 µm) were cut and fixed in formalin
buffer after being paraffin-embedded. Paraffin-embedded cancer
tissue sections were deparaffinized and dewatered first. Then,
slides were incubated with 0.3% H2O2 for 10
min and washed with ddH2O for 3×3 min. Antigen retrieval
was undertaken in a pressure cooker for 10 min with 0.01 M citrate
buffer and washed with phosphate-buffered saline (PBS) for 3×5 min.
Bovine serum albumin (BSA) (5%) was applied to the specimen
sections to block non-specific protein for 20 min at room
temperature. The primary antibody was incubated for 2 h at 37°C and
washed with PBS for 3×3 min. After washing, specimen slides were
incubated with the secondary antibody for 20 min at 37°C and washed
with PBS for 3×3 min. Finally, the specimen slides subsequently
underwent 3,3-diaminobenzidine (DBA) staining for 10 min. Slides
were assessed using microscopy; positive cells are shown in brown
or yellow and cell nuclei are shown in blue.
Flow cytometric analysis
The spleen from each sacrificed mouse was
aseptically removed and put into a 35-mm culture dish containing
cold PBS. Total lymphocytes were separated using the Mouse 1X
Lymphocyte Separation Medium. Then, the lymphocytes were
resuspended at a concentration of 1×107 cells/ml and
transferred (100 µl) to a 1.5 ml tube. The antibody was then
labeled. For the Th1 and Th2 subset analysis, Th1 subsets were
labeled as CD3+CD4+IFN-γ+, Th2
subsets were labeled as
CD3+CD4+IL4+ (29,30).
The ratio of Th1/Th2 = percentage of
CD4+IFN-γ+/percentage of
CD4+IL4+. Cell surface antigens CD3 and CD4
were labeled in the dark for 30 min. Then, cells were fixed and
permeabilized in fixation/permeabilization mixture in dark for 1 h.
Lastly, the anti-mouse IFN-γ and IL4, which are intracellular
antigens, were incubated in the dark for 30 min. For the perforin
and granzyme B subset analysis, splenic lymphocytes were fixed and
permeabilized first, and then the anti-mouse perforin and granzyme
B antibodies, which are also intracellular antigens, were incubated
in the dark for 30 min. For the CD8 and CD25 subset analysis, the
CD8 subset was labeled as CD3+CD8+, and the
CD25 subset was labeled as CD3+CD25+. After
antibodies were labeled, lymphocytes were fixed in 1%
paraformaldehyde, and detected by flow cytometer. The above
procedures were performed on ice and repeated at least three
times.
ELISA analysis
Serum was separated from peripheral blood via
centrifugation (12,000 rpm, 10 min, 4°C) and stored at −20°C. Four
cytokines (IL-2, IL-4, IL-10 and IFN-γ), and the key tumor-specific
markers (CA15-3) were detected using mouse cytokines ELISA kit. All
steps were followed in accordance with the manufacture's protocol.
The cytokines and marker were detected within seven days after
serum separation, and above procedures were performed on ice and
repeated at least three times.
Statistical analysis
Values are presented as mean ± standard deviation
(SD) and analyzed by one-way analysis of variance (ANOVA) using
GraphPad Prism 5. The results of flow cytometry were analyzed using
FlowJo 7.6.1. Grayscale quantitative analysis was performed using
Image Pro-Plus 6.0. Probability values <0.05 were considered to
indicate statistical significance.
Results
XN inhibits breast cancer growth in a
mouse model
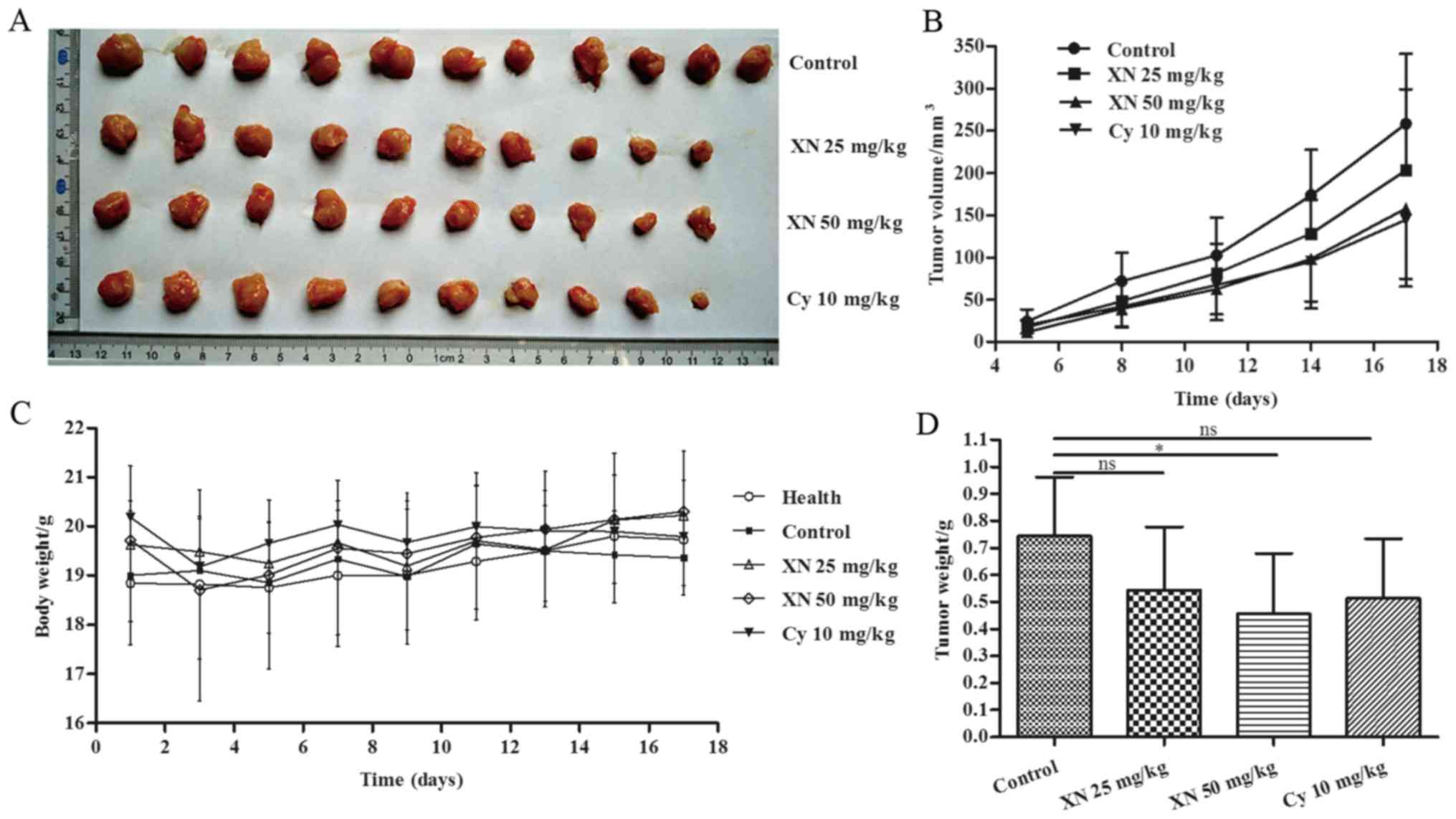
Body weight, tumor volume and tumor weight were
assessed to evaluate the effects of XN on breast cancer. As shown
in Fig. 2A and B, the tumor growth
of the XN-treated groups was evidently slower than that noted in
the control group. Fig. 2D shows
that tumor weight was significantly decreased in the 50 mg/kg
XN-treated group compared with the control group (p=0.036).
Meanwhile, Table I shows that
inhibitory rate of tumor growth in the 25 and 50 mg XN groups and
Cy-treated group was 25.68, 37.72 and 29.85%, respectively. Body
weight did not obviously change in the four groups (Fig. 2C), while 10 mg/kg Cy caused negative
growth rate (−0.93%) (Table I).
 | Table I.Effects of XN on body weight and
tumor growth in the BALB/c-4T1 mice. |
Table I.
Effects of XN on body weight and
tumor growth in the BALB/c-4T1 mice.
| Dose (mg/kg) | No. of mice | Tumor weight
(g) | Inhibitory rate
(%) | Rate of body weight
change (%) |
|---|
| 0 (Healthy) | 11 | – | – | +6.01 |
| 0 (Control) | 11 | 0.732±0.21 | – | +1.20 |
| 25 XN | 10 | 0.544±0.23 | 25.68 | +2.96 |
| 50 XN | 10 | 0.456±0.22 | 37.72 | +1.90 |
| 10 Cy | 10 | 0.514±0.21 | 29.85 |
−0.93 |
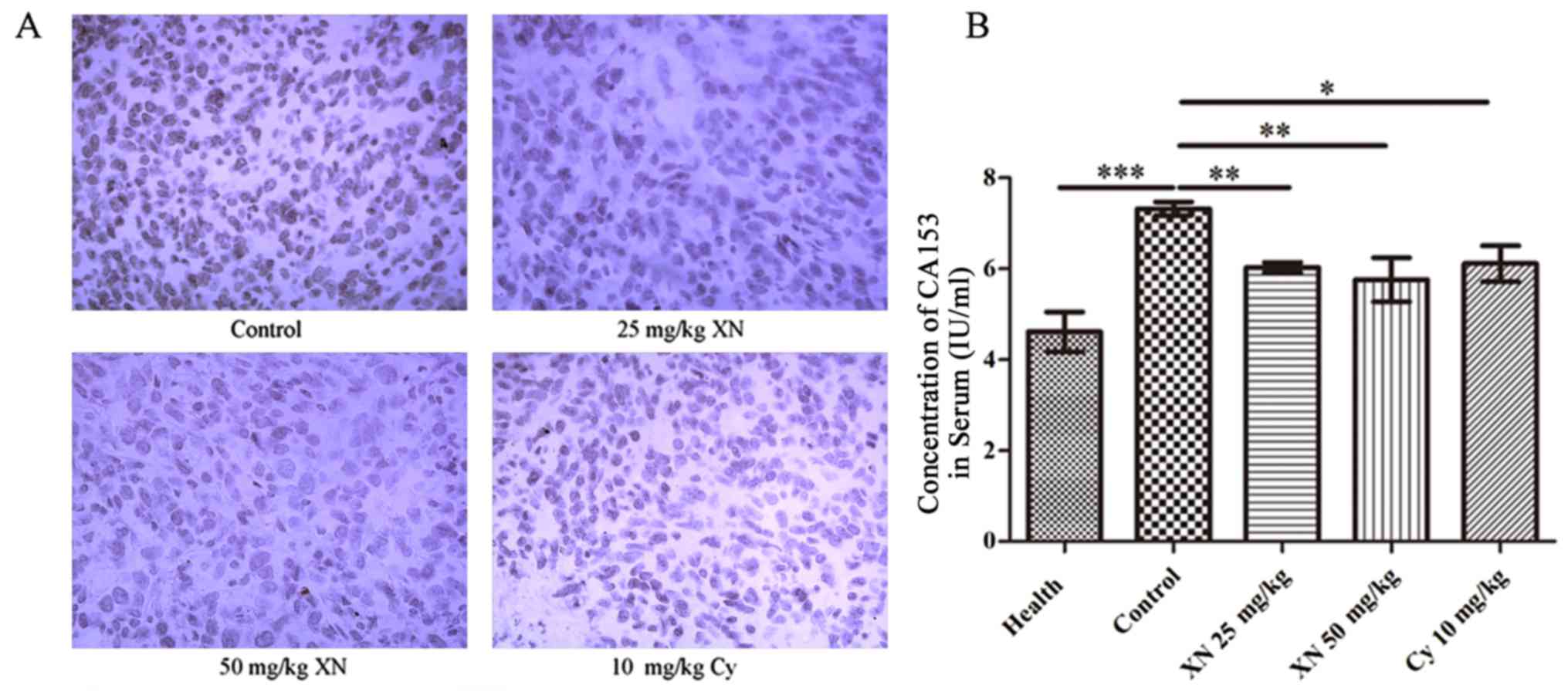
XN inhibits the expression of Ki-67
and CA15-3 in the mouse model
CA15-3 and antigen Ki-67 are the most common markers
for breast cancer (31,32). IHC analysis (Fig. 3A) showed that expression of Ki-67 in
the tumor tissues was significantly inhibited after treatment.
Concomitantly, we detected the concentration of CA15-3 in serum
using mouse CA15-3 ELISA kit. Results demonstrated that the CA15-3
level in the control group was evidently higher than levels in the
other groups (p<0.05) (Fig. 3B).
These results demonstrated that XN restrained the growth of breast
cancer cells in the mouse model.
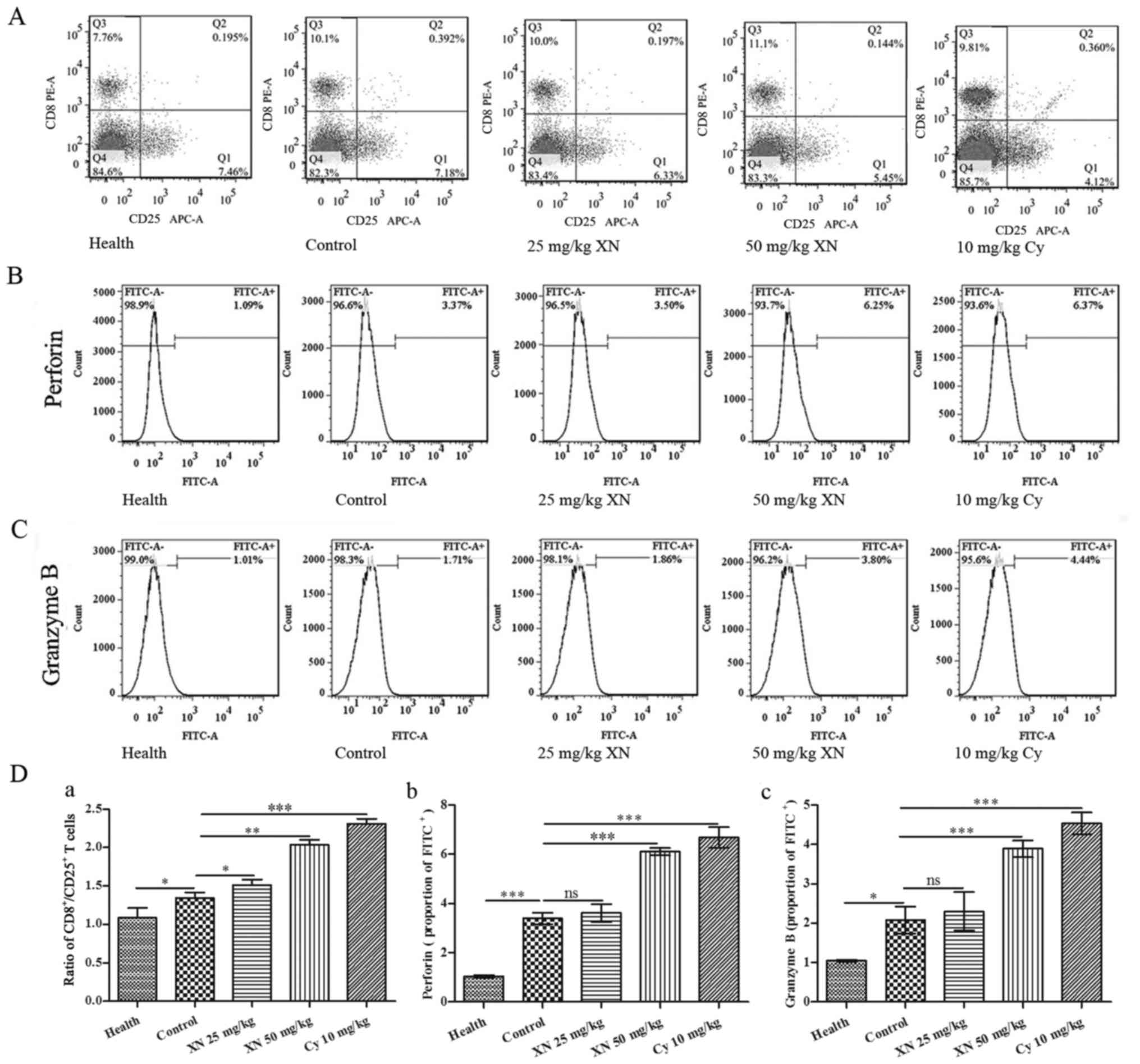
XN activates CTL responses in the
mouse model
CTL responses are very important for cellular
immunity, and it is associated with Th1 polarization (19). The ratio of
CD8+/CD25+ lymphocytes was detected by flow
cytometry. As shown in Fig. 4A and
D-a, XN increased the ratio of CD8+/CD25+
when compared with that noted in the control group. Fig. 4B, C and D-b and -c indicate that
expression of perforin and granular enzyme B were also markedly
upregulated by treatment with XN (compared with control group, all
p<0.05).
XN promotes the Th1/Th2 balance drift
to Th1 polarization in the mouse model
Numerous studies have shown that cancer disrupts the
Th1/Th2 balance, and this is known as ‘balance shifting’ (33–35).
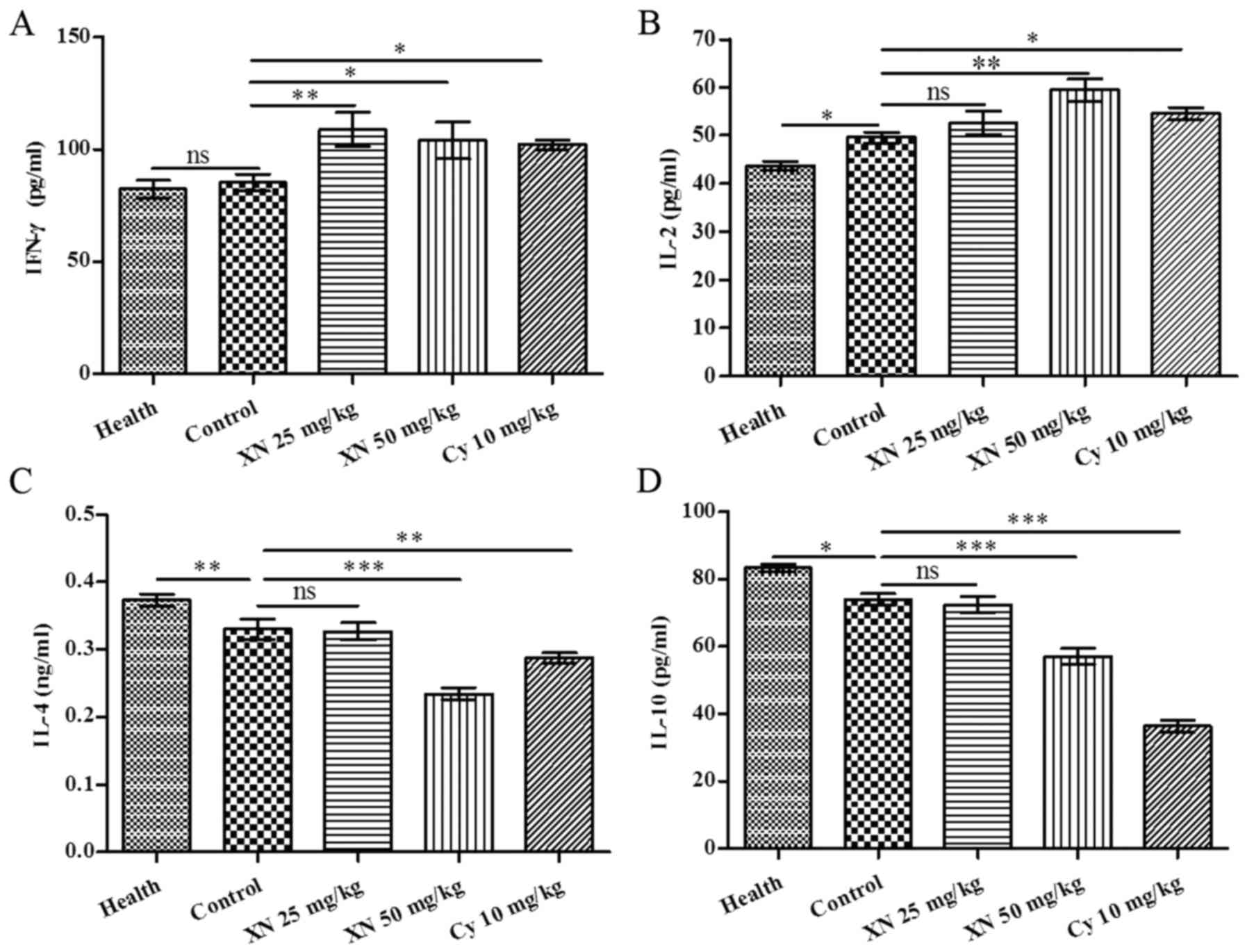
IFN-γ and IL-4 are pivotal cytokines which are produced by Th1 and
Th2 cells, respectively. We investigated Th1 and Th2 cytokines in
serum by ELISA kit. As shown in Fig.
5, expression of Th1 cytokines IL-2 and IFN-γ was markedly
enhanced; in contrast, expression of Th2 cytokines, (including IL-4
and IL-10) was evidently weakened after XN therapy (p<0.05).
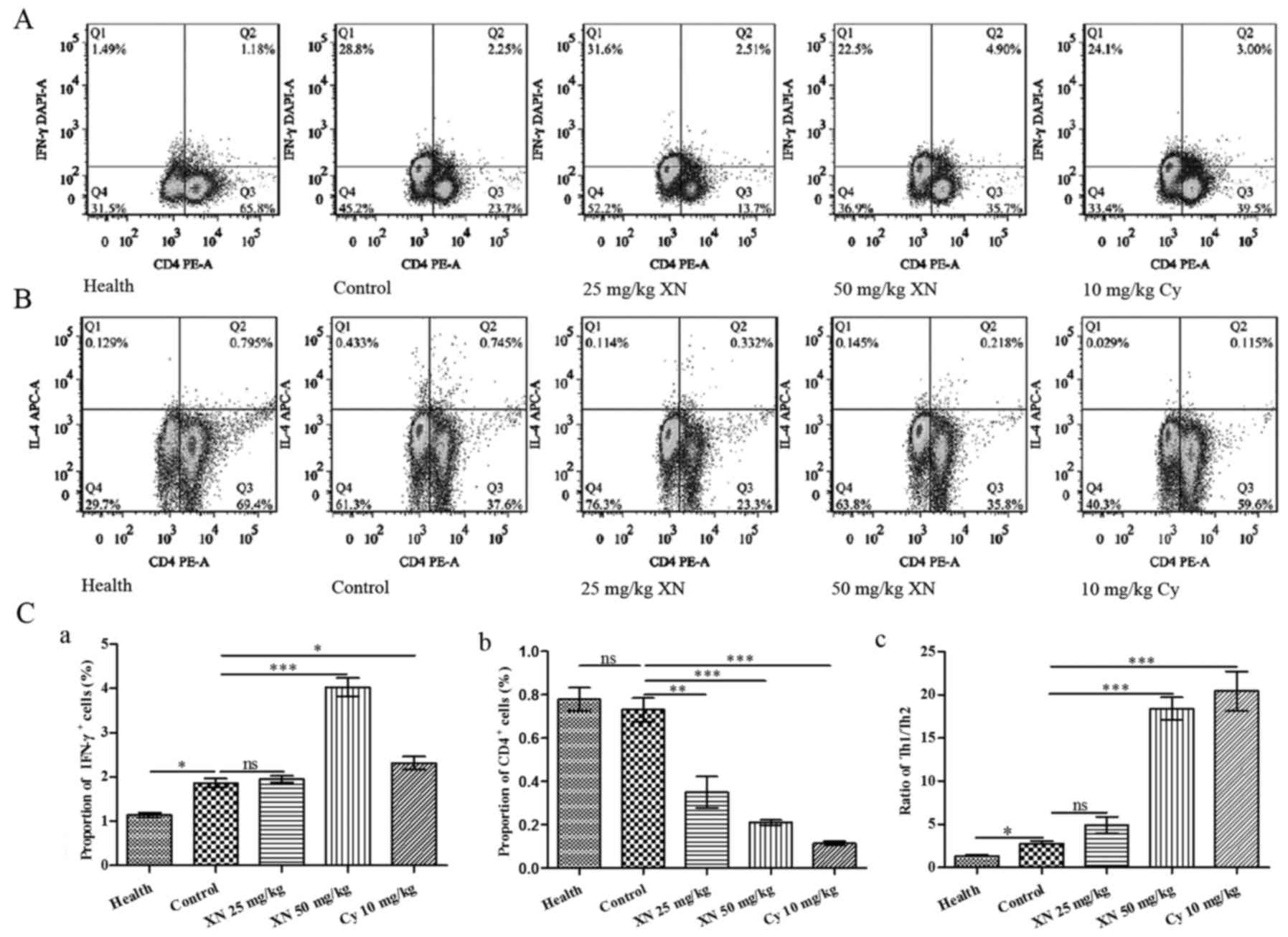
Fig. 6A and B show the flow
cytometric analysis of Th1 (CD4+IFN-γ+) and
Th2 (CD4+IL4+) cells, respectively. Fig. 6C-a and -b indicates that the
percentage of Th1 cells was significantly increased, while the
percentage of Th2 cells was markedly decreased after treatment with
50 mg/kg XN (compared with control group, p<0.05). Fig. 6C-c indicates that the ratio of
Th1/Th2 in the 50 mg/kg XN-treated group was significantly
increased compared with the control group (p<0.05).
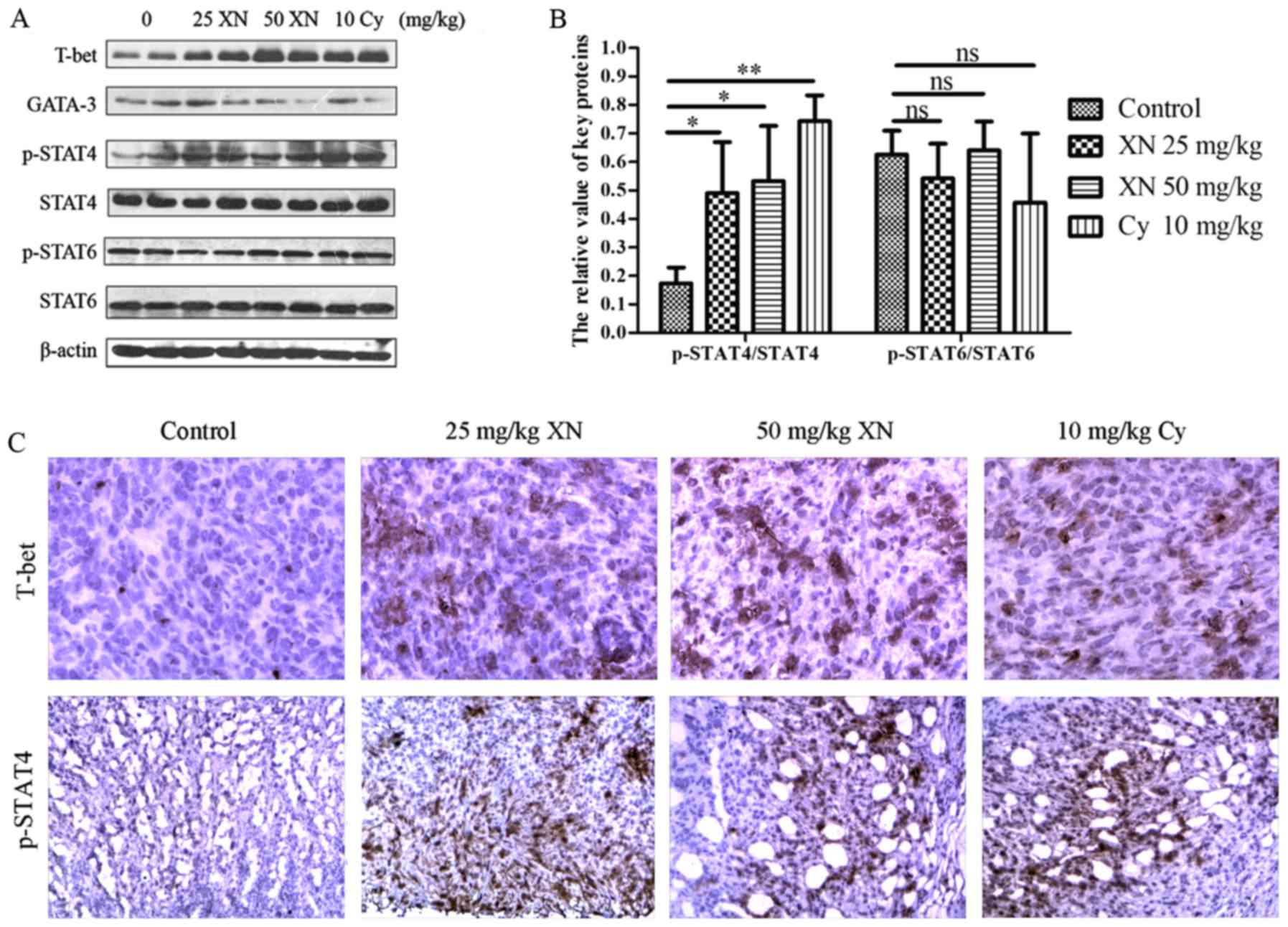
XN activates STAT4, a pivotal factor
in Th1 differentiation, in the mouse model
Based on the above results, XN regulated the Th1/Th2
balance drifting to Th1, indicating that there may be merit in
exploring the mechanisms of the balance drifting. We examined
expression levels of key proteins in Th1 and Th2 development with
western blotting and IHC, respectively. As shown in Fig. 7 the expression of T-bet and p-STAT4
were markedly enhanced, while GATA-3 was significantly weakened
(p<0.05). Meanwhile, p-STAT6 did not obviously change
(p>0.05). Simultaneously, the results of IHC corroborated those
of western blotting. The above results indicated that STAT4,
mediating Th0 differentiate to Th1, may be involved in the
influence of the Th1/Th2 balance by XN.
Discussion
Th1/Th2 is closely related to a variety of tumors
(33,36,37),
and it is also one of the hotspots in immunological therapy of
malignancy. In the present study, we demonstrated that xanthohumol
(XN), a prenylflavonoid found in the hop plant Humulus
lupulus, inhibited tumor growth, enhanced Th1 cytokines and
promote Th1/Th2 balance drifting to Th1 population in a breast
cancer mouse model. Consistent with a previous study, both XN and
combination therapy (ginseng polysaccharides and dendritic cells)
caused an increase in the Th1 population (35). In addition, we observed the
underlying mechanisms and found that XN activated STAT4 when Th1
cytokines were upregulated.
XN exhibits many biological properties, including
anti-obesity (38),
anti-hyperlipidemia (39),
anti-angiogenic (40),
pro-apoptosis and modulation of autophagy (8), anti-invasion (41) and anti-inflammatory activities
(42). A previous study concerning
the safety profile of XN revealed that 1,000 mg/kg XN feeding did
not impair the function of major organs and homoeostasis in the
mouse (43). Choi et al
(44) suggested that XN may
increase IL-2 production in T cells, which means that XN may
promote an immune response mediated by Th1. Cho et al
(45) revealed that XN inhibits
IL-12, which indirectly promotes differentiation of Th1 in the
immune system by activating transcription (STATs) molecules. In
physiological condition, Th1 and Th2 maintain a relative dynamic
balance, but when organisms are in a morbid state, the balance may
be broken (18). These results
raise a potential link between XN and Th1/Th2, and this appears to
be a field worthy of investigation.
The cytotoxic T lymphocyte (CTL) is a type of
crucial effector cell in cellular immunity, and plays an important
role in the process of antitumor immunology. CD8+ CTL
exists as a form of CTL-P, which is a non-activated precursor cell
in vivo. CTL-Ps are activated by related-antigen and in
assistance of Th1 cytokines, and then develop into mature CTLs
(30). Liao et al (19) reported that relative
CD8+/CD25+ cells were evidently increased
following Th2 to Th1 transition in the tumor microenvironment. Liu
et al (46) showed that the
ratio of CD8+/CD25+ T cells was significantly
increased when the CTLs were activated by CoCl2 in the
4T1 cell line. The present study demonstrated that the ratio of
CD8+/CD25+ was observably increased in the T
lymphocyte subset in the spleen in breast cancer-bearing mice;
meanwhile, an increase in expression of perforin and granular
enzyme was also detected. The above results revealed that CTLs were
activated.
The function of Th1 and Th2 cells depends on the
secretion of different cytokines. To investigate the effects of XN
on Th1/Th2 cytokines, we detected serum levels of cytokines
associated with Th1 and Th2 using ELISA kits. Our findings showed
that XN significantly increased expression of Th1 cytokines
(including IL-2 and IFN-γ), and decreased levels of Th2 cytokines
(including IL-4 and IL-10). This is understandable due to the fact
that Th1 and Th2 are mutually inhibitory (47). Moreover, we detected the ratio of
Th1/Th2 cytokines by flow cytometry and found that the ratio was
markedly elevated by XN. Similar studies have been reported in a
variety of tumors. Sparano et al (48) reported that patients with advanced
head and neck squamous cell carcinoma apparently have a decrease in
Th1 cytokines compared with less advanced patients, while an
increase in Th2 cytokines. Liao et al (19) found that combination therapy
promoted cytokine transition from Th2 to Th1 in the tumor
microenvironment. The above results indicate that an imbalance of
Th1/Th2 cytokines is extensively observed in many types of tumors
and most usually in advanced cancers.
To confirm the potential mechanism of XN on Th1/Th2
cytokines further, we determined expression of key factors in the
pathway of Th1 and Th2 differentiation. Th0 cells differentiate to
Th1 and Th2 cells proportionally in the physiological condition
(29). In addition, it has been
proved that activated STAT4 and STAT6 play an irreplaceable role in
the pathway of Th0 differentiation to Th1 and Th2, respectively
(49). However, T-bet and GATA-3
are also pivotal in the two pathways (50,51).
Chen et al (52) reported
that CpG-ODN (cytosine-phosphorothioate-guanine containing
oligodeoxynucleotide), a strong Th1 adjuvant, decreased the
expression of GATA-3 and STAT6 by activating T-bet, STAT1 and STAT4
in a lung cancer mouse model. Guo et al (29) found that mangiferin may be
attributed to the modulation of Th1/Th2 cytokine imbalance via
inhibiting the STAT6 signaling pathway in an asthmatic mouse model.
In the present study, the results showed that XN enhanced
expression of T-bet, while reduced GATA-3. Furthermore, STAT4 was
activated by XN, but there was no significant effect on the level
of p-STAT6. This suggests that STAT4 may play a positive role in
the regulation of XN on Th1/Th2 cytokines.
In summary, the present study illustrated that XN
enhanced the Th1 immunity response, which appears to be more
effective in mediating anticancer function. In addition, a pivotal
molecule in the differentiation of Th1, STAT4, was concurrently
activated, but the underlying mechanisms are still unclear. These
results demonstrated that XN may be a promising candidate for
breast cancer treatment. Nevertheless, further study must be
carried out in different mouse cancer models and molecular docking
is required to confirm the active site of XN.
Acknowledgements
This study was supported by the Science and
Technology Support Projects of Gansu Province (no. 1104FKCA123;
http://www.gsstc.gov.cn). The funders had no role
in study design, data collection and analysis, decision to publish,
or preparation of the manuscript.
References
|
1
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang GL and Lin ZB: The immunomodulatory
effect of lentinan. Yao Xue Xue Bao. 31:86–90. 1996.(In Chinese).
PubMed/NCBI
|
|
3
|
Lei LS and Lin ZB: Effect of Ganoderma
polysaccharides on T cell subpopulations and production of
interleukin 2 in mixed lymphocyte response. Yao Xue Xue Bao.
27:331–335. 1992.PubMed/NCBI
|
|
4
|
Zhang Q and Lin Z: Study on antitumor
activity and mechanism of Ganoderma polysaccharides B. Zhongguo
Zhong Xi Yi Jie He Za Zhi. 19:544–547. 1999.(In Chinese).
PubMed/NCBI
|
|
5
|
Jiang W, Zhao S, Xu L, Lu Y, Lu Z, Chen C,
Ni J, Wan R and Yang L: The inhibitory effects of xanthohumol, a
prenylated chalcone derived from hops, on cell growth and
tumorigenesis in human pancreatic cancer. Biomed Pharmacother.
73:40–47. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Venè R, Benelli R, Minghelli S, Astigiano
S, Tosetti F and Ferrari N: Xanthohumol impairs human prostate
cancer cell growth and invasion and diminishes the incidence and
progression of advanced tumors in TRAMP mice. Mol Med.
18:1292–1302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoshimaru T, Komatsu M, Tashiro E, Imoto
M, Osada H, Miyoshi Y, Honda J, Sasa M and Katagiri T: Xanthohumol
suppresses oestrogen-signalling in breast cancer through the
inhibition of BIG3-PHB2 interactions. Sci Rep. 4:73552014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kunnimalaiyaan S, Sokolowski KM,
Balamurugan M, Gamblin TC and Kunnimalaiyaan M: Xanthohumol
inhibits Notch signaling and induces apoptosis in hepatocellular
carcinoma. PLoS One. 10:e01274642015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kunnimalaiyaan S, Trevino J, Tsai S,
Gamblin TC and Kunnimalaiyaan M: Xanthohumol-mediated suppression
of Notch1 signaling is associated with antitumor activity in human
pancreatic cancer cells. Mol Cancer Ther. 14:1395–1403. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Benelli R, Venè R, Ciarlo M, Carlone S,
Barbieri O and Ferrari N: The AKT/NF-κB inhibitor xanthohumol is a
potent anti-lymphocytic leukemia drug overcoming chemoresistance
and cell infiltration. Biochem Pharmacol. 83:1634–1642. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fowler DH: Rapamycin-resistant effector
T-cell therapy. Immunol Rev. 257:210–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu W, Celeridad M, Sankar S, Webb DR and
Bennett BL: CC-4047 promotes Th1 cell differentiation and
reprograms polarized human Th2 cells by enhancing transcription
factor T-bet. Clin Immunol. 128:392–399. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Szabo SJ, Kim ST, Costa GL, Zhang X,
Fathman CG and Glimcher LH: A novel transcription factor, T-bet,
directs Th1 lineage commitment. Cell. 100:655–669. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu J, Yamane H, Cote-Sierra J, Guo L and
Paul WE: GATA-3 promotes Th2 responses through three different
mechanisms: Induction of Th2 cytokine production, selective growth
of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res.
16:3–10. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ouyang W, Löhning M, Gao Z, Assenmacher M,
Ranganath S, Radbruch A and Murphy KM: Stat6-independent GATA-3
autoactivation directs IL-4-independent Th2 development and
commitment. Immunity. 12:27–37. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kurata H, Lee HJ, O'Garra A and Arai N:
Ectopic expression of activated Stat6 induces the expression of
Th2-specific cytokines and transcription factors in developing Th1
cells. Immunity. 11:677–688. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oh S and Hwang ES: The role of protein
modifications of T-bet in cytokine production and differentiation
of T helper cells. J Immunol Res. 2014:5896722014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kidd P: Th1/Th2 balance: The hypothesis,
its limitations, and implications for health and disease. Altern
Med Rev. 8:223–246. 2003.PubMed/NCBI
|
|
19
|
Liao D, Luo Y, Markowitz D, Xiang R and
Reisfeld RA: Cancer associated fibroblasts promote tumor growth and
metastasis by modulating the tumor immune microenvironment in a 4T1
murine breast cancer model. PLoS One. 4:e79652009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hong M, Jiang Z and Zhou YF: Effects of
thermotherapy on Th1/Th2 cells in esophageal cancer patients
treated with radiotherapy. Asian Pac J Cancer Prev. 15:2359–2362.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoshino S, Yoshimura K, Suzuki N, Iida M,
Yoshida S, Maeda Y, Maeda K, Hazama S and Oka M: Immunoregulatory
effects of PSK on the Th1/Th2 balance and regulatory T-cells in
patients with colorectal cancer. Gan To Kagaku Ryoho. 37:2234–2236.
2010.(In Japanese). PubMed/NCBI
|
|
22
|
Romagnani S, Maggi E and Del Prete G: An
alternative view of the Th1/Th2 switch hypothesis in HIV infection.
AIDS Res Hum Retroviruses. 10:iii–ix. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Crane IJ and Forrester JV: Th1 and Th2
lymphocytes in autoimmune disease. Crit Rev Immunol. 25:75–102.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsuzaki J, Tsuji T, Imazeki I, Ikeda H
and Nishimura T: Immunosteroid as a regulator for Th1/Th2 balance:
Its possible role in autoimmune diseases. Autoimmunity. 38:369–375.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun CZ Zhihong, Feng Liu, et al:
Inhibition of breast cancer cell survival by Xanthohumol via
modulating Notch signaling pathway in vivo and in vitro. Oncology
Letters. 15585229–12;2015.
|
|
26
|
Kast RE: Ribavirin in cancer
immunotherapies: Controlling nitric oxide augments cytotoxic
lymphocyte function. Neoplasia. 5:3–8. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matar P, Rozados VR, Gervasoni SI and
Scharovsky GO: Th2/Th1 switch induced by a single low dose of
cyclophosphamide in a rat metastatic lymphoma model. Cancer Immunol
Immunother. 50:588–596. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Naito S, von Eschenbach AC, Giavazzi R and
Fidler IJ: Growth and metastasis of tumor cells isolated from a
human renal cell carcinoma implanted into different organs of nude
mice. Cancer Res. 46:4109–4115. 1986.PubMed/NCBI
|
|
29
|
Guo HW, Yun CX, Hou GH, Du J, Huang X, Lu
Y, Keller ET, Zhang J and Deng JG: Mangiferin attenuates TH1/TH2
cytokine imbalance in an ovalbumin-induced asthmatic mouse model.
PLoS One. 9:e1003942014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ubukata H, Motohashi G and Tabuchi T,
Nagata H, Konishi S and Tabuchi T: Evaluations of
interferon-γ/interleukin-4 ratio and neutrophil/lymphocyte ratio as
prognostic indicators in gastric cancer patients. J Surg Oncol.
102:742–747. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao J, Zhang Q, Xu J, Guo L and Li X:
Clinical significance of serum miR-21 in breast cancer compared
with CA153 and CEA. Chin J Cancer Res. 25:743–748. 2013.PubMed/NCBI
|
|
32
|
Tan QX, Qin QH, Yang WP, Mo QG and Wei CY:
Prognostic value of Ki67 expression in HR-negative breast cancer
before and after neoadjuvant chemotherapy. Int J Clin Exp Pathol.
7:6862–6870. 2014.PubMed/NCBI
|
|
33
|
Shurin MR, Lu L, Kalinski P, Stewart-Akers
AM and Lotze MT: Th1/Th2 balance in cancer, transplantation and
pregnancy. Springer Semin Immunopathol. 21:339–359. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feili-Hariri M, Falkner DH and Morel PA:
Polarization of naive T cells into Th1 or Th2 by distinct
cytokine-driven murine dendritic cell populations: Implications for
immunotherapy. J Leukoc Biol. 78:656–664. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma J, Liu H and Wang X: Effect of ginseng
polysaccharides and dendritic cells on the balance of Th1/Th2 T
helper cells in patients with non-small cell lung cancer. J Tradit
Chin Med. 34:641–645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Saxena R and Kaur J: Th1/Th2 cytokines and
their genotypes as predictors of hepatitis B virus related
hepatocellular carcinoma. World J Hepatol. 7:1572–1580. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hong CC, Yao S, McCann SE, Dolnick RY,
Wallace PK, Gong Z, Quan L, Lee KP, Evans SS, Repasky EA, et al:
Pretreatment levels of circulating Th1 and Th2 cytokines, and their
ratios, are associated with ER-negative and triple negative breast
cancers. Breast Cancer Res Treat. 139:477–488. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kiyofuji A, Yui K, Takahashi K and Osada
K: Effects of xanthohumol-rich hop extract on the differentiation
of preadipocytes. J Oleo Sci. 63:593–597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Casaschi A, Maiyoh GK, Rubio BK, Li RW,
Adeli K and Theriault AG: The chalcone xanthohumol inhibits
triglyceride and apolipoprotein B secretion in HepG2 cells. J Nutr.
134:1340–1346. 2004.PubMed/NCBI
|
|
40
|
Costa R, Rodrigues I, Guardão L, Lima JQ,
Sousa E, Soares R and Negrão R: Modulation of VEGF signaling in a
mouse model of diabetes by xanthohumol and 8-prenylnaringenin:
Unveiling the angiogenic paradox and metabolism interplay. Mol Nutr
Food Res. 61:doi: 10.1002/mnfr.2016004882017. View Article : Google Scholar
|
|
41
|
Sasazawa Y, Kanagaki S, Tashiro E, Nogawa
T, Muroi M, Kondoh Y, Osada H and Imoto M: Xanthohumol impairs
autophagosome maturation through direct inhibition of
valosin-containing protein. ACS Chem Biol. 7:892–900. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee IS, Lim J, Gal J, Kang JC, Kim HJ,
Kang BY and Choi HJ: Anti-inflammatory activity of xanthohumol
involves heme oxygenase-1 induction via NRF2-ARE signaling in
microglial BV2 cells. Neurochem Int. 58:153–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dorn C, Bataille F, Gaebele E, Heilmann J
and Hellerbrand C: Xanthohumol feeding does not impair organ
function and homoeostasis in mice. Food Chem Toxicol. 48:1890–1897.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Choi JM, Kim HJ, Lee KY, Choi HJ, Lee IS
and Kang BY: Increased IL-2 production in T cells by xanthohumol
through enhanced NF-AT and AP-1 activity. Int Immunopharmacol.
9:103–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cho YC, You SK, Kim HJ, Cho CW, Lee IS and
Kang BY: Xanthohumol inhibits IL-12 production and reduces chronic
allergic contact dermatitis. Int Immunopharmacol. 10:556–561. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu Z, Lv D, Liu S, Gong J, Wang D, Xiong
M, Chen X, Xiang R and Tan X: Alginic acid-coated chitosan
nanoparticles loaded with legumain DNA vaccine: Effect against
breast cancer in mice. PLoS One. 8:e601902013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mosmann TR and Sad S: The expanding
universe of T-cell subsets: Th1, Th2 and more. Immunol Today.
17:138–146. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sparano A, Lathers DM, Achille N,
Petruzzelli GJ and Young MR: Modulation of Th1 and Th2 cytokine
profiles and their association with advanced head and neck squamous
cell carcinoma. Otolaryngol Head Neck Surg. 131:573–576. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pernis AB and Rothman PB: JAK-STAT
signaling in asthma. J Clin Invest. 109:1279–1283. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mowen KA and Glimcher LH: Signaling
pathways in Th2 development. Immunol Rev. 202:203–222. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chang HC, Han L, Goswami R, Nguyen ET,
Pelloso D, Robertson MJ and Kaplan MH: Impaired development of
human Th1 cells in patients with deficient expression of STAT4.
Blood. 113:5887–5890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen J, Wang Y, Mei Z, Zhang S, Yang J, Li
X, Yao Y and Xie C: Radiation-induced lung fibrosis in a
tumor-bearing mouse model is associated with enhanced type-2
immunity. J Radiat Res. 57:133–141. 2016. View Article : Google Scholar : PubMed/NCBI
|