Introduction
Rhus verniciflua Stokes (R.
verniciflua) has long been used as a traditional herbal
medicine, mainly in East Asia, including South Korea, Japan and
China. R. verniciflua Stokes is a medicinal plant used for
the treatment of gastrointestinal diseases as well as for relieving
symptoms possibly caused by cancer since the 15th century in the
East Asian region (1). Previous
research has revealed that the extract of R. verniciflua has
various therapeutic effects, encompassing antioxidant,
anti-proliferative, anti-inflammatory and antitumor activities
(2–7). Additionally, increasing evidence from
experimental studies indicates that the R. verniciflua
extract decreases oxidative stress and prevents tumor progression,
although the molecular mechanisms of these pharmacological effects
remain to be determined. According to a recent study of an ethanol
extract of R. verniciflua, it efficiently inhibited human
lymphoma cell growth, which was evaluated by confirming the
apoptotic changes based on increased nuclear fragmentation, the
suppressed fluorescence intensity of nuclei stained with propidium
iodide and the obvious DNA fragments visualized through the DNA
laddering in human lymphoma cells (8).
Apoptosis is a form of programmed cellular death
characterized by its abnormal morphological features, including
cellular nuclear shrinkage, nuclear fragmentation, cytoplasmic
blebbing, chromatin condensation and caspase activation (9,10). Two
pathways regulate the apoptotic process: the extrinsic pathway
involving the activation of death receptors and the intrinsic or
mitochondrial pathway. The anti-apoptotic Bcl-2 family consists of
apoptotic mediators that play important roles in the process of
programmed cell death. This Bcl-2 family of proteins includes
several pro-apoptotic and anti-apoptotic molecules, such as Bax and
Bcl-2, which regulate the cellular commitment to apoptosis
(11).
The activation of nuclear factor-κB (NF-κB) plays a
pivotal role in regulating the pathological changes during tumor
progression from cellular proliferation to the invasion to other
organs. The suppression of NF-κB transcription factor activation is
involved in inhibiting tumor cell growth by inducing apoptosis.
NF-κB and extracellular signaling-regulated kinase (ERK) have long
been regarded as the main signaling pathways contributing to
several aspects of tumorigenesis, such as cell proliferation and
cell death (12,13). Numerous antitumor drugs are known to
trigger cell death by inducing apoptosis. However, the
pharmacological effects of the R. verniciflua extract in
inhibiting cell growth via the induction of the apoptotic process
in human chronic myelogenous leukemia K562 cells remain
unclear.
In the present study, we examined the mechanism by
which the effects of R. verniciflua activity in human
chronic myelogenous leukemia K562 cells are mediated, including the
induction of apoptosis via the caspase-dependent apoptotic effects,
as well as the suppression of the expression of the NF-κB
transcription factor and the activation of the mitogen-activated
protein kinase (MAPK) pathway.
Materials and methods
Extraction of R. verniciflua
R. verniciflua (14,15)
grown in Yeosu (Korea) was purchased from Kyung Hee Pharmaceuticals
(Wonju, Korea). Firstly, 1 kg of R. verniciflua was roasted
at 180°C for 1 h, and then extracted twice with sterile distilled
water for 3 h. The supernatant was evaporated and freeze-dried and
the extract was obtained as a 43 g powder (yield, 4.3%). A
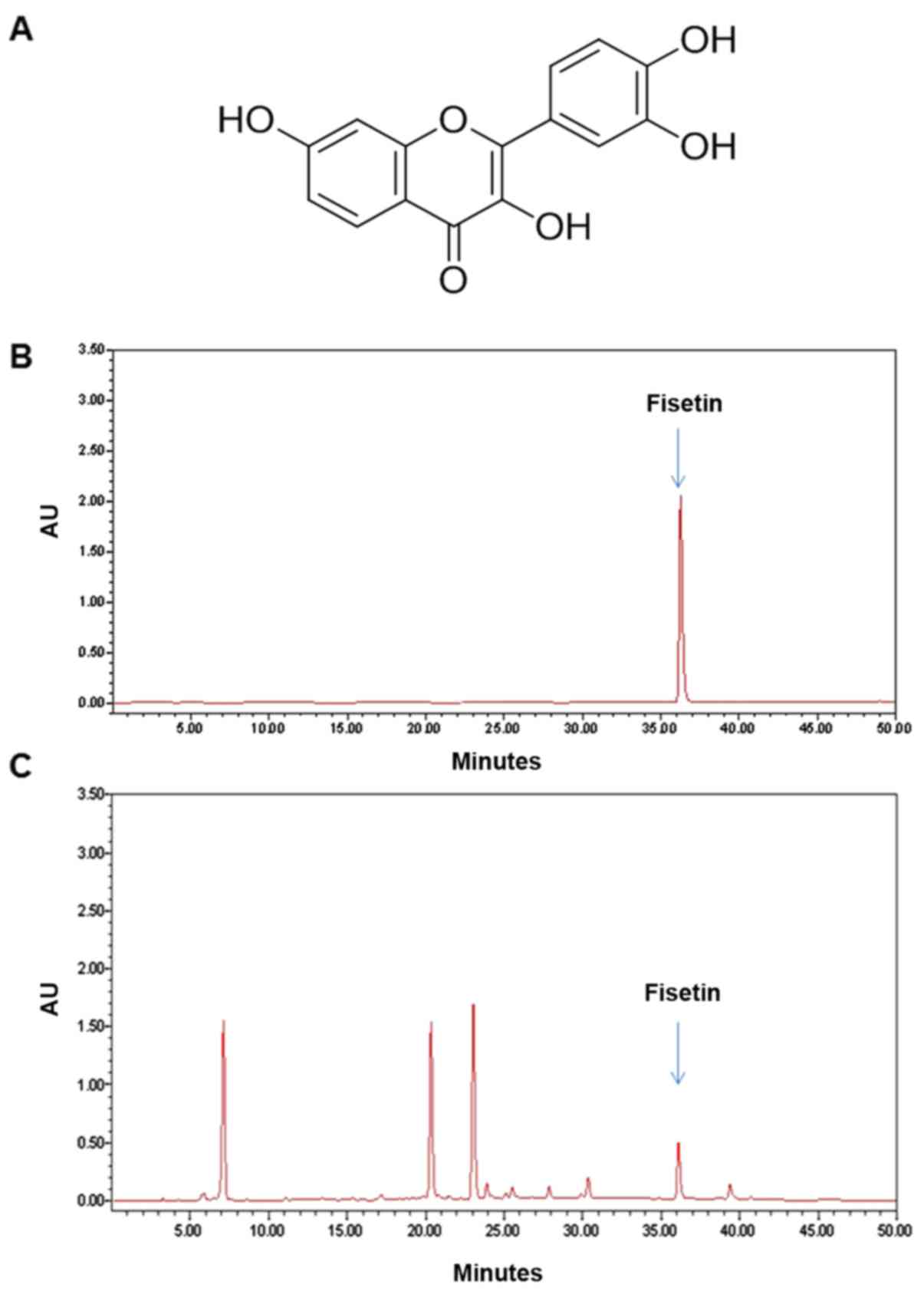
constituent analysis using high-performance liquid chromatography
(HPLC) revealed that fisetin is one of the major components of the
R. verniciflua extract (Fig.
1).
Cell culture
The human chronic myelogenous leukemia K562 cell
line was purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA). The cells were cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Grand Island, NY, USA) containing
10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml
streptomycin. The cells were incubated at 37°C under a humidified
atmosphere of 5% CO2 and 95% air.
Cell viability
CellTiter 96 AQueous One Solution
(Promega, Madison, WI, USA) was used to assess the intensity of the
cellular proliferation. The cells were seeded at a density of
1×104 cells/well in 96-well plates and cultivated in
different concentrations of R. verniciflua extract at 37°C
for 24, 48 and 72 h. A colorimetric assay with PMS/MTS solution
determined the cell viability. The absorbance value was determined
at 490 nm, with reference subtraction at 650 nm.
Flow cytometry
The cells were incubated under treatment with 100,
200 and 300 µg/ml of R. verniciflua extract for 24, 48 and
72 h. After incubation, the cells were harvested and then washed
with phosphate-buffered saline (PBS). Apoptosis was detected by the
FITC Annexin V apoptosis detection kit (BD Biosciences, San Diego,
CA, USA). The cells were incubated in 5 µl FITC-conjugated Annexin
V and 5 µl propidium iodide (PI) with 100 µl binding solution for
15 min at room temperature in the dark. Annexin V-FITC and PI
fluorescence were determined by flow cytometry. Apoptosis was
assessed with a FACSCalibur device using CellQuest software (Becton
Dickinson and Company, Franklin Lakes, NJ, USA).
Apoptosis assay
To detect apoptosis in K562 cells, apoptotic cells
were quantitatively analyzed using a cell death detection ELISAplus
kit (Roche Molecular Biochemicals, Mannheim, Germany). The cells
(1×104) were incubated with 100, 200 and 300 µg/ml R.
verniciflua extract for 24, 48 and 72 h. Subsequently the cells
were lysed with 200 µl lysis buffer. Cell lysates were assayed to
detect DNA fragments using the cell death ELISAplus kit according
to the manufacturer's instructions. DNA fragmentation was estimated
at 405 nm relative to the untreated control level. A caspase
colorimetric assay kit (R&D Systems, Minneapolis, MN, USA) was
used to assess the enzymatic changes of caspase proteases. The K562
cells were treated with 100, 200 and 300 µg/ml of R.
verniciflua extract for 24, 48 and 72 h. The cells were
harvested and the cell pellets obtained were lysed with 50 µl lysis
buffer on ice for 10 min. The concentration of protein in the
supernatant (cytosolic extract) was assessed using a BCA protein
assay kit (Thermo Fisher Scientific, Rockford, IL, USA).
Caspase-3-like protease activity was determined using methods for
identifying the proteolytic cleavage of substrates, including
DEVD-pNA (caspase-3 substrate). These colorimetric substrates were
dissolved with an assay buffer. After incubation with the
solubilized substrates at 37°C for 1 h in the dark, the intensity
of color production in the lysates was assessed with a microplate
reader capable of detecting the absorbance at a wavelength of 405
nm and we compared the caspase-3 activity with the level of the
control. To assess the effect of co-treatment with a caspase
inhibitor on cell viability, K562 cells (1×104 cells)
were pretreated with a pan-caspase inhibitor, Z-VAD-FMK, or a
caspase-3-specific inhibitor, Z-DEVD-FMK (R&D Systems) for 2 h.
Subsequently, we treated the K562 cells with 300 µg/ml R.
verniciflua extract. Following the caspase inhibitor and the
R. verniciflua extract treatments, the PMS and MTS reagents
were added to the cells. A colorimetric assay determined the
absorbance of the colored formazan product at 490 nm with
background subtraction at 650 nm.
RNA extraction and real-time PCR
procedures
Total RNA was extracted and purified from cultured
cells using an RNeasy Mini kit according to the manufacturer's
instructions (Qiagen, Hilden, Germany). First-strand cDNA was then
synthesized from 1 µg of RNA template using a reverse transcriptase
system (Promega). Random hexamers primed the reverse transcription
reaction and the primer sequences and product sizes were as
follows: Bcl-2 (5′-GATTGATGGGATCGTTGCCTTA-3′,
5′-CCTTGGCATGAGATGCAGGA-3′; 200 bp), Bax
(5′-GGATGCGTCCACCAAGAAG-3′, 5′-GCCTTGAGCACCAGTTTGC-3′; 216 bp),
Mcl-1 (5′-CTCATTTCTTTTGGTGCCTTT-3′, 5′-CCAGTCCCGTTTTGTCCTTAC-3′;
117 bp), survivin (5′-GGCCCAGTGTTTCTTCTGCTT-3′,
5′-GCAACCGGACGAATGCTTT-3′; 91 bp), β-actin
(5′-GCGAGAAGATGACCCAGATC-3′, 5′-GGATAGCACAGCCTGGATAG-3′; 77 bp).
Real-time PCR was performed on a StepOnePlus Real-Time PCR system
(Applied Biosystems, Foster, CA, USA) with the Power SYBR-Green PCR
Master Mix (Applied Biosystems). We performed the PCR with 1 µl
cDNA in 20 µl reaction mixtures that consisted of 10 µl Power
SYBR-Green PCR Master Mix, 2 µl primers and 7 µl PCR water. The
amplifying reactions were processed with an initial denaturing step
of the target DNA at 95°C for 10 min, followed by 40 cycles of 95°C
for 15 sec and 60°C for 1 min. The formula 2−(target gene
− β-actin) was used to calculate the crossing
point of target genes with β-actin and the relative expression
amounts were quantified.
Immunoblot analysis
K562 cells were harvested and washed with cold PBS
to remove the medium, and then lysed in lysis buffer containing 1
mM PMSF (Cell Signaling Technology, Boston, MA, USA). The
concentration of protein contained in the incubated cells was
assessed using a BCA protein assay, according to the manufacturer's
instructions. The protein sample (30 µg) was separated by 12%
SDS-PAGE electrophoresis and transferred onto nitrocellulose
membranes. The membranes were rewetted and blocked with 5% blocking
buffer for 1 h at room temperature, and then incubated overnight
with human antibodies against NF-κB p65 (cat. no. 8242),
phosphorylated (p-)NF-κB p65 (cat. no. 3031), p38 MAPK (cat. no.
9228), p-p38 MAPK (cat. no. 9215), MEK (cat. no. 4694), p-MEK (cat.
no. 9154), ERK (cat. no. 4696) and p-ERK1/2 (cat. no. 4376; all
from Cell Signaling Technology) and β-actin (cat. no. A5441;
Sigma-Aldrich Co.) diluted 1:1,000 with Tris-buffered saline
containing 0.05% Tween-20 (TBS-T). After 1 h washing with TBS-T
solution, the membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies (rabbit; cat. no. 7074;
mouse; cat. no. 7076; both from Cell Signaling Technology) diluted
1:2,500 in TBS-T solution for 1 h at room temperature. These
incubated membranes were subsequently washed with TBS-T solution
for 1 h and the proteins were determined using an Amersham ECL
Prime reagent kit (GE Healthcare Life Sciences, Little Chalfont,
UK). The protein expression from cultured cells was detected using
a Davinch-Chemi Chemiluminescence Imaging system (Davinch-K Co.,
Ltd., Seoul, Korea).
Statistical analysis
Values are expressed as the mean ± SD. Student's
t-test was used to evaluate differences between the control group
and the R. verniciflua extract-treated groups. The degree of
inhibition of apoptosis was determined by the differences between
the R. verniciflua extract-treated sample and the samples
treated with a combination of caspase inhibitor and R.
verniciflua extract. The data were analyzed statistically using
GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA).
p<0.05 and p<0.01 were considered to indicate statistically
significant differences.
Results
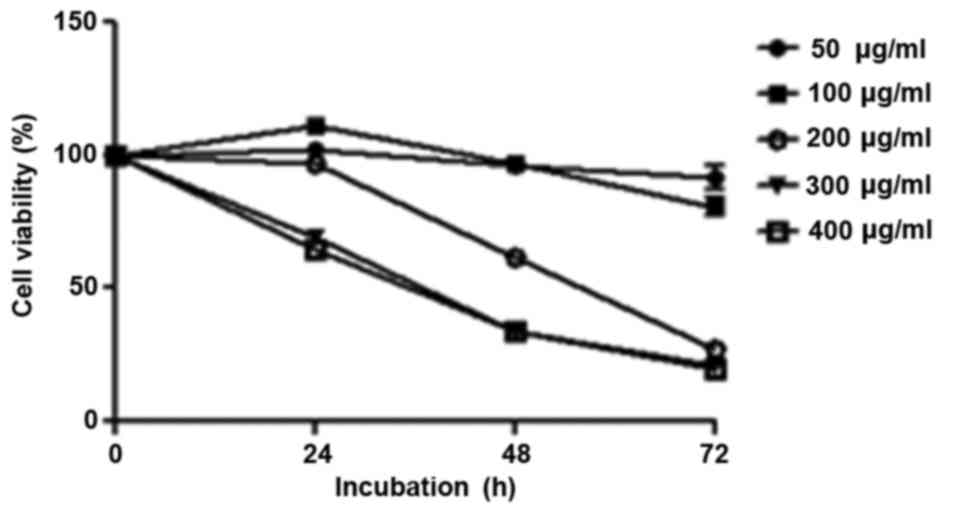
R. verniciflua extract inhibits cell
proliferation
K562 cells were treated with increasing
concentrations of R. verniciflua extract (0–500 µg/ml) for
24, 48 and 72 h. The effects of R. verniciflua extract on
K562 cellular proliferation were evaluated using a PMS/MTS
solution. In the 200 and 300 µg/ml treated groups, the
IC50 values were 211 and 457 µg/ml respectively when
calculated by the cell viabilities of the three time-points. The
morphologies of the cells were not changed with those
concentrations. R. verniciflua extract suppressed cell
proliferation in a dose- and time-dependent manner in K562 cells
(Fig. 2).
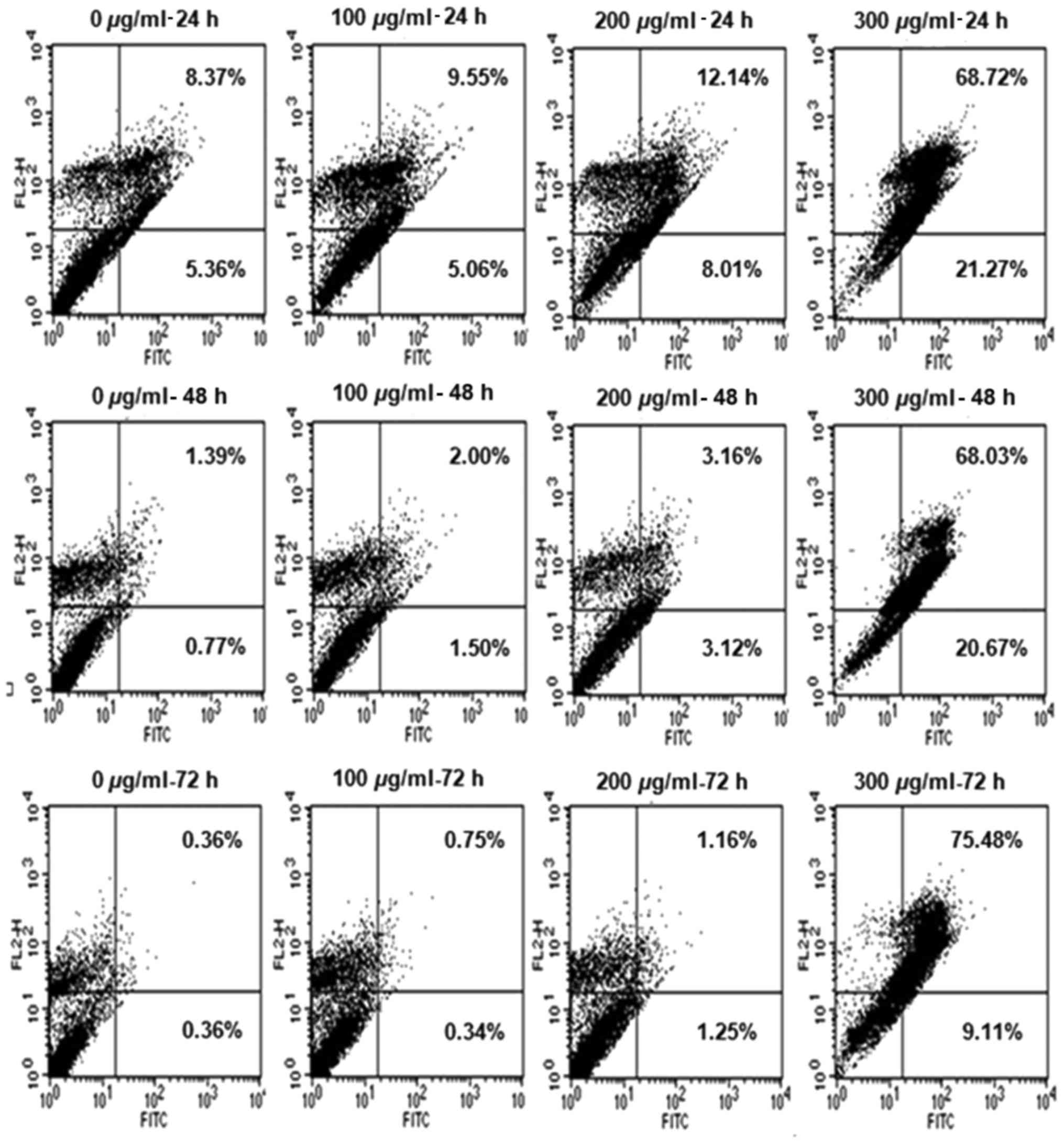
R. verniciflua extract induces cell
cycle progression
The K562 cells were treated with increasing
concentrations of 100, 200 and 300 µg/ml R. verniciflua
extract for 24, 48 and 72 h. To determine whether the R.
verniciflua extract induced cell-cycle arrest, the apoptotic
cell distribution was assessed by flow cytometry. The R.
verniciflua extract increased the number of apoptotic cells in
the early and late stages in a dose- and time-dependent manner when
compared with the controls (Fig.
3).
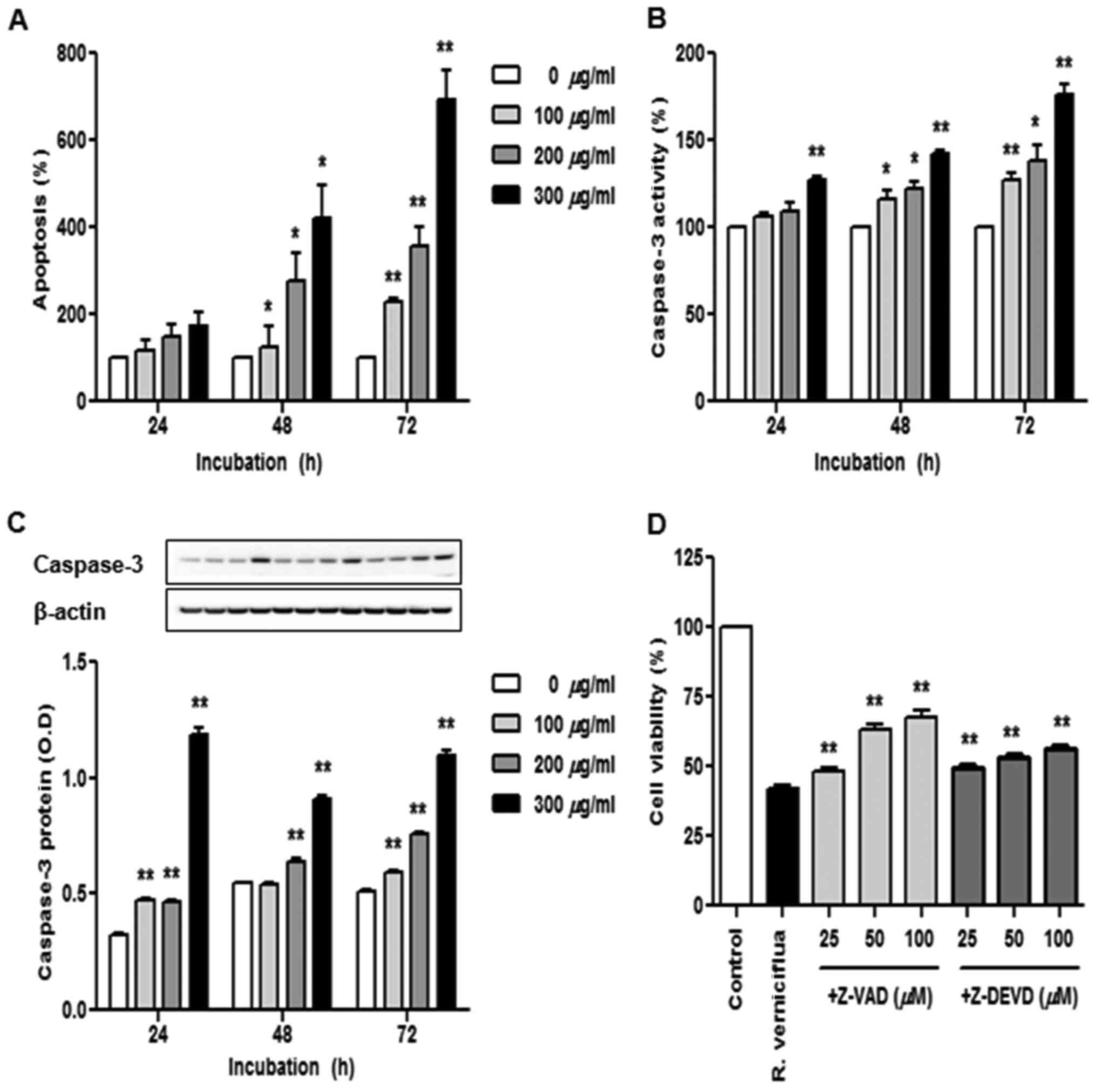
R. verniciflua extract induces cell
apoptosis and caspase activation
The K562 cells were treated with 100, 200 and 300
µg/ml R. verniciflua extract for 24, 48 and 72 h and we used
a cell death detection ELISA assay to identify apoptotic cells
(Fig. 4A). The numbers of apoptotic
cells were significantly increased in a dose-and time-dependent
manner under the treatment of R. verniciflua extract.
Caspase-3 activity was assayed using a colorimetric ELISA and the
level of caspase-3 protein was assessed by immunoblotting.
Caspase-3 activity and protein expression were enhanced in a dose-
and time-dependent manner following treatment with R.
verniciflua extract (Fig. 4B and
C). To determine whether the caspase-3 activation was
associated with the R. verniciflua extract-induced
apoptosis, K562 cell proliferation was detected by a PMS/MTS
solution with R. verniciflua extract treatment. Pretreatment
with the pan-caspase inhibitor Z-VAD-FMK and the caspase-3
inhibitor Z-DEVD-FMK on K562 cells increased R. verniciflua
extract-induced cell proliferation (Fig. 4D).
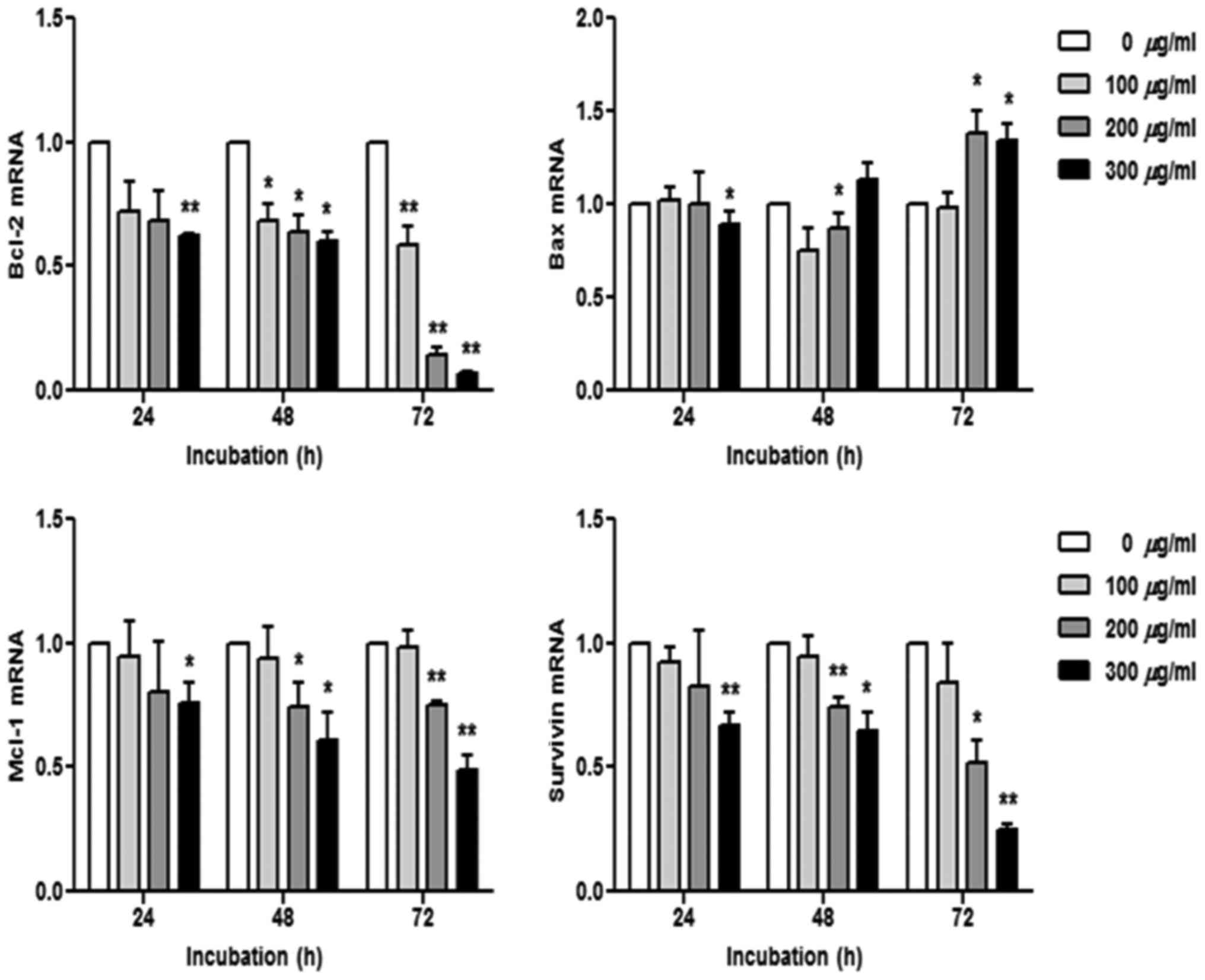
R. verniciflua extract regulates mRNA
transcription
The K562 cells were treated with 100, 200 and 300
µg/ml of R. verniciflua extract for 24, 48 and 72 h.
Subsequently, the mRNA levels of the apoptotic genes Bcl-2, Bax,
Mcl-1 and survivin were assessed using real-time PCR. The mRNA
levels of Bcl-2, Mcl-1 and survivin were decreased in a dose- and
time-dependent manner, whereas the Bax level was increased
(Fig. 5).
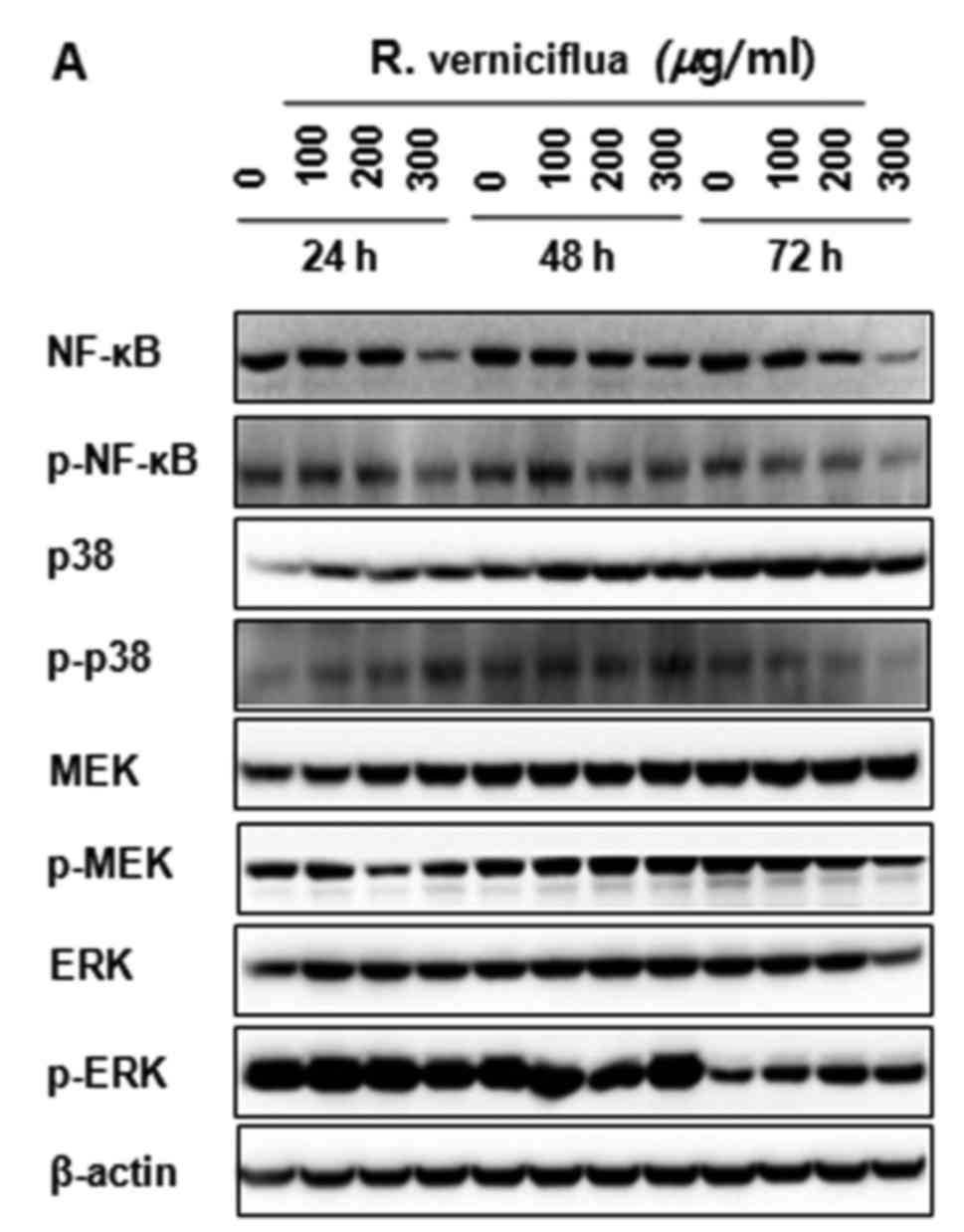
R. verniciflua extract inactivates
NF-κB p65 and the activity of MAPK signaling
The K562 cells were treated with 100, 200 and 300
µg/ml of R. verniciflua extract for 24, 48 and 72 h. The
expression of NF-κB and MAPK proteins were analyzed by
immunoblotting. The levels of the activation and phosphorylation of
important proteins in the K562 cells were inhibited significantly.
The levels of NF-κB p65 and of p38 MAPK, MEK and ERK1/2
phosphorylation were markedly suppressed (Fig. 6A and B).
Discussion
R. verniciflua Stokes has long been used as a
therapeutic medicinal herb in Asian countries. To investigate the
potent pharmacological effect of R. verniciflua extract
against K562 leukemia cells, we identified the effect of R.
verniciflua extract on the viability of the K562 cells. R.
verniciflua extract inhibited K562 cell proliferation, as
previously reported (16).
Experimental results from the present study indicated that R.
verniciflua extract not only blocked K562 cells in early and
late stages of apoptosis but also increased the apoptotic cell
numbers. A recent study reported a quantitative decrease in the
number of A431 cells in the G1 phase and cellular arrest in the
sub-G0/G1 phase after treatment with triptolide (17).
Caspases are central components of the mechanism
responsible for apoptosis (18). To
determine the molecular mechanism of apoptosis in K562 cells, we
revealed that treatment with R. verniciflua extract
increased intracellular caspase-3 activity and enhanced the levels
of caspase-3 and cleaved caspase-3 protein. This result was
confirmed by co-treatment using a pan-caspase inhibitor and
caspase-3-specific inhibitor, which led to an inverse in R.
verniciflua extract-mediated cell proliferation. These results
indicated that R. verniciflua extract-induced apoptosis
pathway was associated with caspase activation in K562 cells. We
then found that the K562 cells exhibited reduced mRNA expression
levels of Bcl-2, Mcl-1 and survivin with R. verniciflua
extract treatment, whereas the Bax level was increased. These
results indicated that R. verniciflua extract induced
apoptosis through the regulation of anti- and pro-apoptotic genes
and the activation of caspase-3. Tubeimoside-1 increased the
apoptotic activities of caspase-3, −8 and −9, whereas a
caspase-3-specific inhibitor significantly inhibited
tubeimoside-1-induced apoptosis in HepG2 cells.
Tubeimoside-1-induced apoptosis also decreased Bcl-2 and increased
Bak, with no change in Bax levels (19).
NF-κB is a key regulator consisting of a variety of
transcription factors related to both pro- and anti-apoptotic
processes. Bcl-2 and Bcl-XL are anti-apoptotic molecules
belonging to the Bcl-2 family and their expression level is
regulated by the activation of the NF-κB (20,21).
NF-κB is a critical transcription factor that plays a pivotal role
in the expression of apoptosis-related proteins (22). In the present study, R.
verniciflua extract inhibited the NF-κB activity. Therefore,
blocking of the NF-κB signaling pathway may be effective at
inducing apoptosis in K562 cells. Triptolide induced apoptosis of
human anaplastic thyroid carcinoma cells by suppressing the NF-κB
expression. It also downregulated Bcl-2 and Bcl-XL,
which were transcriptionally mediated by NF-κB-dependent and
p53-independent mechanisms (23).
Our results indicated that R. verniciflua extract induced
apoptosis by the downstream inhibition of the NF-κB signaling and
the expression of the gene encoding Bcl-2.
MAPK pathway is an important regulator of cell
death, proliferation, differentiation and autophagy (24,25).
We demonstrated that R. verniciflua extract inhibited MAPK
signaling in the K562 cells. Gleditsia sinensis thorn
induced p38 MAPK, ERK1/2 and JNK phosphorylation in human colon
cancer cells (26). Guibi-tang
(GBT) treatment upregulated the expression of p38, ERK and JNK,
which were retained during apoptosis in the A431 cells (27). Ponicin inhibited cell growth by
arresting G1-cell cycle and inducing apoptosis in HT29 cells. The
Akt and MEK signaling pathway were also blocked by ponicin, whereas
the p38 MAPK signaling was activated (28). In the present study, our results
revealed that R. verniciflua extract inhibited K562 cell
proliferation by inducing apoptosis and suppressing the MAPK
signaling pathways.
In conclusion, in the present study, we demonstrated
that R. verniciflua extract induced apoptosis, contributing
to the inhibition of K562 cell proliferation, which is partly
mediated by caspase activation, inhibition of the NF-κB activity
and suppression of MAPK signaling. These results indicated the need
for further research on the in vivo effect of the R.
verniciflua extract.
References
|
1
|
Choi W, Jung H, Kim K and Lee S, Yoon S,
Park J, Kim S, Cheon S, Eo W and Lee S: Rhus verniciflua stokes
against advanced cancer: A perspective from the Korean Integrative
Cancer Center. J Biomed Biotechnol. 2012:8742762012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lim KT, Hu C and Kitts DD: Antioxidant
activity of a Rhus verniciflua Stokes ethanol extract. Food Chem
Toxicol. 39:229–237. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kitts DD and Lim KT: Antitumorigenic and
cytotoxic properties of an ethanol extract derived from Rhus
verniciflua Stokes (RVS). J Toxicol Environ Health A. 64:357–371.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JD, Huh JE, Jeon G, Yang HR, Woo HS,
Choi DY and Park DS: Flavonol-rich RVHxR from Rhus verniciflua
Stokes and its major compound fisetin inhibits inflammation-related
cytokines and angiogenic factor in rheumatoid arthritic
fibroblast-like synovial cells and in vivo models. Int
Immunopharmacol. 9:268–276. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee JC, Lim KT and Jang YS: Identification
of Rhus verniciflua Stokes compounds that exhibit free radical
scavenging and anti-apoptotic properties. Biochim Biophys Acta.
1570:181–191. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JC, Lee KY, Kim J, Na CS, Jung NC,
Chung GH and Jang YS: Extract from Rhus verniciflua Stokes is
capable of inhibiting the growth of human lymphoma cells. Food Chem
Toxicol. 42:1383–1388. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jang HS, Kook SH, Son YO, Kim JG, Jeon YM,
Jang YS, Choi KC, Kim J, Han SK, Lee KY, et al: Flavonoids purified
from Rhus verniciflua Stokes actively inhibit cell growth and
induce apoptosis in human osteosarcoma cells. Biochim Biophys Acta.
1726:309–316. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee JC, Kim J and Jang YS: Ethanol-eluted
extract of Rhus verniciflua stokes inhibits cell growth and induces
apoptosis in human lymphoma cells. J Biochem Mol Biol. 36:337–343.
2003.PubMed/NCBI
|
|
9
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wyllie AH, Kerr JF and Currie AR: Cell
death: The significance of apoptosis. Int Rev Cytol. 68:251–306.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Levine B, Sinha S and Kroemer G: Bcl-2
family members: Dual regulators of apoptosis and autophagy.
Autophagy. 4:600–606. 2008. View Article : Google Scholar :
|
|
12
|
Stanciu M, Wang Y, Kentor R, Burke N,
Watkins S, Kress G, Reynolds I, Klann E, Angiolieri MR, Johnson JW,
et al: Persistent activation of ERK contributes to
glutamate-induced oxidative toxicity in a neuronal cell line and
primary cortical neuron cultures. J Biol Chem. 275:12200–12206.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aggarwal BB: Nuclear factor-kappaB: The
enemy within. Cancer Cell. 6:203–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yun-Yang W, Yu-Min D, Fang-Xing Y, Ying X,
Rong-Zhi C and Kennedy JF: Purification and characterization of
hydrosoluble components from the sap of Chinese lacquer tree Rhus
vernicifera. Int J Biol Macromol. 38:232–240. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee SH, Choi WC and Yoon SW: Impact of
standardized Rhus verniciflua stokes extract as complementary
therapy on metastatic colorectal cancer: A Korean single-center
experience. Integr Cancer Ther. 8:148–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi HS, Seo HS, Kim SR, Choi YK, Jang BH,
Shin YC and Ko SG: Anti-inflammatory and anti-proliferative effects
of Rhus verniciflua Stokes in RAW264.7 cells. Mol Med Rep.
9:311–315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park SW and Kim YI: Triptolide induces
apoptosis of PMA-treated THP-1 cells through activation of
caspases, inhibition of NF-κB and activation of MAPKs. Int J Oncol.
43:1169–1175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J and Yuan J: Caspases in apoptosis and
beyond. Oncogene. 27:6194–6206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yin Y, Chen W, Tang C, Ding H, Jang J,
Weng M, Cai Y and Zou G: NF-κB, JNK and p53 pathways are involved
in tubeimoside-1-induced apoptosis in HepG2 cells with oxidative
stress and G2/M cell cycle arrest. Food Chem Toxicol.
49:3046–3054. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burlacu A: Regulation of apoptosis by
Bcl-2 family proteins. J Cell Mol Med. 7:249–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kuwana T and Newmeyer DD: Bcl-2-family
proteins and the role of mitochondria in apoptosis. Curr Opin Cell
Biol. 15:691–699. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reed JC: Apoptosis-targeted therapies for
cancer. Cancer Cell. 3:17–22. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu W, Hu H, Qiu P and Yan G: Triptolide
induces apoptosis in human anaplastic thyroid carcinoma cells by a
p53-independent but NF-kappaB-related mechanism. Oncol Rep.
22:1397–1401. 2009.PubMed/NCBI
|
|
24
|
Rubinfeld H and Seger R: The ERK cascade:
A prototype of MAPK signaling. Mol Biotechnol. 31:151–174. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cui Q, Tashiro S, Onodera S, Minami M and
Ikejima T: Autophagy preceded apoptosis in oridonin-treated human
breast cancer MCF-7 cells. Biol Pharm Bull. 30:859–864. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee SJ, Park K, Ha SD, Kim WJ and Moon SK:
Gleditsia sinensis thorn extract inhibits human colon cancer cells:
The role of ERK1/2, G2/M-phase cell cycle arrest and p53
expression. Phytother Res. 24:1870–1876. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yim NH, Kim A, Liang C, Cho WK and Ma JY:
Guibitang, a traditional herbal medicine, induces apoptotic death
in A431 cells by regulating the activities of mitogen-activated
protein kinases. BMC Complement Altern Med. 14:3442014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Du J, Chen C, Sun Y, Zheng L and Wang W:
Ponicidin suppresses HT29 cell growth via the induction of G1 cell
cycle arrest and apoptosis. Mol Med Rep. 12:5816–5820. 2015.
View Article : Google Scholar : PubMed/NCBI
|