Introduction
Leukemia is one of the most common hematological
cancers worldwide (1). Acute
leukemia can be classified into acute lymphoblastic leukemia (ALL)
and acute myeloid leukemia (AML) (2). A high incidence of ALL is found in
children, and AML is the most common acute leukemia in adults
(1,3). Due to the high degree of resistance to
standard chemotherapy, older AML patients have a median survival of
~7 months and the overall 5-year survival is only 5–15% (4,5).
Optimized chemotherapy protocols provide more than 80% cure rates
for childhood ALL (6). ALL patients
often have relapses and the prediction of prognosis remains
difficult (7). Notably, bone marrow
transplantation provides an effective and practical treatment for
some patients, However graft-rejection and relapses are the major
causes of treatment failure in these patients (8).
Gene therapy targeting cancer cells provide a
potential alternative therapeutic strategy for cancer (9). CD40-ligand (CD154) gene therapy
increased leukemia-specific T cells and decreased the number of
leukemia cells (10). The
proliferative activity of the leukemic cells and the clonogenicity
of the primary cells were inhibited by knockdown of polo-like
kinase 1 (11). RNA interference
(RNAi) targeting knockout of M-BCR/ABL mRNA and oncoprotein kill
K562 human leukemic cells (12).
Immature colon carcinoma transcript 1 (ICT1) encodes
an essential mitochondrial protein and an integral component of the
mitoribosome (13,14). ICT1 was revealed to function as a
ribosome-dependent codon-independent peptidyl-tRNA hydrolase
(14). It was originally discovered
by comparison of gene expression pattern between undifferentiated
and differentiated human colon cancer cells (HT29-D4) (13). Lack of ICT1 markedly reduced the
number of HeLa and human hepatoblastoma HepG2 cells, and was able
to induce HeLa cell apoptosis (13). Akabane et al (15) also demonstrated that mutation of the
GGQ-motif of ICT1 led to a loss of cell viability and affected cell
growth. Absence of ICT1 inhibited proliferation of glioblastoma
multiforme cells through the arrest of the cell cycle at the G2/M
phase (16). However, the role of
ICT1 in leukemic cancer is unknown.
Materials and methods
Cell lines and cell culture
U937 leukemia and human embryonic kidney 293
(HEK293) cells were purchased from the Chinese Academy of Sciences
(Shanghai, China). U937 cells were propagated in RPMI-1640 medium
(SH30809.01B; HyClone, Logan, UT, USA) with 10% fetal bovine serum
(FBS; 04-001-1A; Biological Industries, Grand Island, NY, USA).
HEK293T cells were maintained in Dulbecco's modified Eagle's medium
(DMEM; SH30243.01B; HyClone) with 10% FBS.
Construction and transduction of
lentivirus-based vectors
Two independent shRNAs targeting ICT1 were designed
from GenBank (accession no. NM_001545). shRNA (S1) and shRNA (S2)
sequences for ICT1
(5′-GCTGTTAATGCTTGTCTATAACTCGAGTTATAGACAAGCATTAACAGC-3′ and
5′-GCAGAATGTGAACAAAGTGAACTCGAGTTCACTTTGTTCACATTCTGC-3′) were
inserted into pGP vectors (SBI, Mountain View, CA, USA) at the
EcoRI and BamHI sites. ICT1 shRNA scrambled control
was: 5′-TTCTCCGAACGTGTCACGTCTCGAGACGTGACACGTTCGGAGAA-3′. HEK293T
cells were transiently transfected with the mixture of recombinant
pGP vectors, pVSVG-I and pCMVΔR8.92 (Sigma, St. Louis, MO, USA)
using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), in
accordance with the manufacturers instructions. U937 cells were
inoculated at densities of 10,000 cells/well in each of the 12-well
plates and infected with lentiviral particles [shCon, shICT (S1) or
shICT1 (S2)] at a multiplicity of infection (MOI) of 80. At 168 h
post-infection, the infection efficiency was detected by
GFP-positive U937 cells under a fluorescence microscopy.
Quantitative real-time RT-PCR
(qRT-PCR)
After transfection for 7 days, total cellular RNA
was extracted from U937 cells using TRIzol reagent (Gibco, Grand
Island, NY, USA) followed by first-strand cDNA synthesis with the
Invitrogen Superscript first-strand system. qRT-PCR analysis was
performed on Bio-Rad Connet Real-Time PCR platform using SYBR
Premix EX Taq™ (Takara, Dalian, China). The primer sequences were
as follows: ICT1 forward, 5′-CAGCCTGGACAAGCTCTACC-3′ and reverse,
5′-GGAACCTGACTTCTGCCTTG-3′; actin forward, 5′-GTGGACATCCGCAAAGAC-3′
and reverse, 5′-AAAGGGTGTAACGCAACTA-3′; cyclin A2 forward,
5′-GTTCCTCCTTGGAAAGCAAAC-3′ and reverse,
5′-GGGCATCTTCACGCTCTATTT-3′.
The reaction mixture contained 10 µl of 2X SYBR
Premix Ex Taq, 0.5 µl of forward and reverse primers 2.5 µM, 5 µl
of cDNA, and 4.5 µl of ddH2O. The qRT-PCR parameters
were as follows: initial denaturation at 95°C for 1 min, 40 cycles
of amplification at 60°C for 20 min, and final extension at 72°C
for 10 min. ICT1 gene expression levels were assessed using the
2−ΔΔCt method.
Western blotting
For western blotting assay, the U937 cells were
infected with lentiviral particles for 6 days. In short, cells were
washed twice with ice-cold phosphate-buffered saline (PBS) before
being lysed with 1 ml 2X SDS sample buffer [100 mM Tris-Hcl (pH
6.8), 10 mM EDTA, 4% SDS, 10% glycine]. Supernatants were collected
after centrifugation for 20 min at 12,000 × g. A total 30 µg of
protein samples in each lane were electrophoresed by 10% sodium
dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE).
Proteins were then transferred onto polyvinylidene difluoride
(PVDF) membranes (Millipore, Belfor, MA, USA) under the following
conditions: 300 mA, 1.5 h. The membranes were incubated overnight
at 4°C with rabbit anti-ICT1 (cat. no. Ab-147; 1:1,000 dilution;
Abgent, San Diego, CA, USA), rabbit anti-caspase-3 (cat. no. 9661;
1:500 dilution; Cell Signaling Technology, Beverly, MA, USA),
rabbit anti-p21 (cat. no. Ab-106; 1:1,000 dilution; Santa Cruz
Biotechnology, Santa Cruz, CA, USA) and rabbit anti-GAPDH (cat. no.
Ab-5; 1:500,000 dilution; ProteinTech Group, Chicago, IL, USA).
Finally, secondary antibody horseradish peroxidase (HRP)-conjugated
goat anti-rabbit (cat. no. SC2054; Santa Cruz Biotechnology) was
applied in a dilution of 1:5,000 at room temperature for 2 h. The
membranes were processed using enhanced chemiluminescence (ECL)
advance detection reagent.
MTT assay
To determine the viable cell number in U937 cells,
the MTT assay was carried out. The shCon, shICT1 (S1) and shICT1
(S2) cells were firstly grown in a 6-well plate at a density of
3,000 cells/well. At various time-points, each sample was mixed
with 20 µl of 5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide and incubated for 2.5 h. The medium
was then replaced with 100 µl of acidic isopropanol (10% SDS, 5%
isopropanol and 0.01 mol/l HCl) to stop the reaction and read at a
wavelength of 595 nm on a microplate reader (Microplate Reader
3550; Bio-Rad, Hercules, CA, USA).
Flow cytometric analysis of the cell
cycle
The untransfected and transfected U937 cells were
plated at a density of 100,000 cells/well in 12-well tissue culture
plates and incubated for 7 days. After being washed in PBS, the
cells were fixed overnight at 4°C in ice-cold 70% alcohol. The
cells were then stained with 1 ml propidium iodide solution (0.1
mg/ml propidium iodide and 20 µg/ml RNaseA DNase-free) at room
temperature, 30°C. Furthermore, the cells were examined by
fluorescence-activated cell sorting (FACS) based on flow cytometry
(FACSort; Becton-Dickinson, Franklin Lakes, NJ, USA) and the cell
cycle distribution was determined using MultiCycle AV software
(Phoenix Flow Systems, San Diego, CA, USA).
Apoptotic cell death assay
Comparison of the abilities of shCon and shICT1 (S1)
transfection to induce cell apoptosis was assessed using an Annexin
V-APC/7-AAD (BD Pharmingen, San Diego, CA, USA) kit according to
the instructions of the manufacturer. The level of apoptosis was
analyzed on a FACSort flow cytometer. Cells positive for both 7-AAD
and Annexin V-APC were late apoptotic cells. Live cells were
represented by Annexin V-APC and 7-AAD negative staining. 7-AAD
stained cells that were Annexin V-APC negative were considered as
necrotic cells. While negative for 7-AAD and positive for Annexin
V-APC were early apoptotic cells.
PathScan intracellular signaling
array
To detect the potential differences in intracellular
signaling between the ICT1 knockdown and control cells, the
PathScan® intracellular signaling array kit (Cell
Signaling Technology) was used, which allows the monitoring of 18
signaling molecules when phosphorylated or cleaved. Samples were
processed according to the manufacturer's procedures and the signal
intensity was visualized under the LI-COR Odyssey imaging system
(LI-COR Biosciences, Lincoln, NE, USA).
Statistical analysis
All data analyses were performed using SPSS software
version 16.0 (SPSS, Inc., Chicago, IL, USA). Data are expressed as
the mean ± SD of at least 3 independent experiments. Student's
t-test was used to calculate the individual comparisons between
different groups. A P-value of <0.05 was considered to indicate
a statistically significant result.
Results
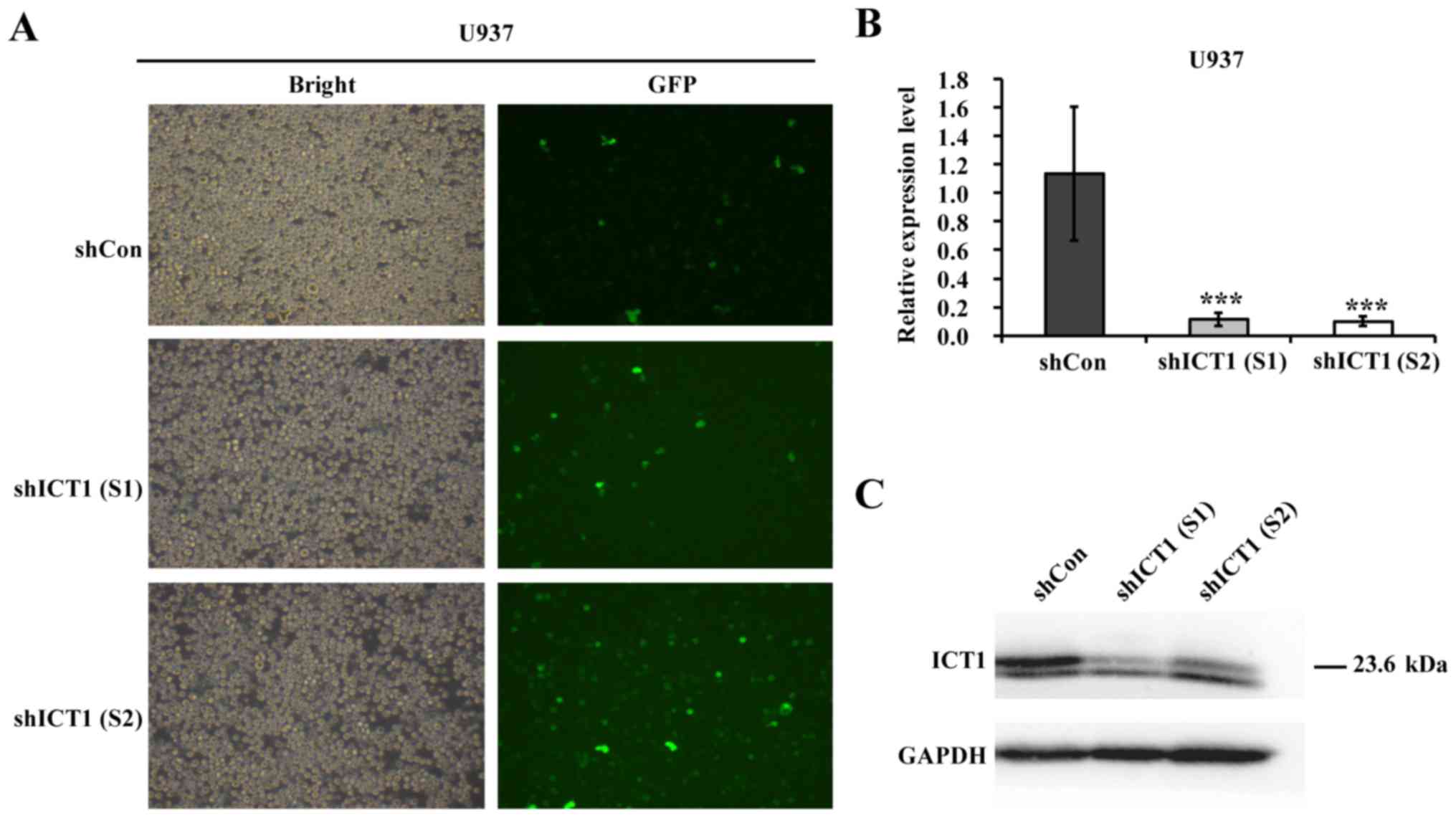
ICT1 stable knockdown by
lentivirus-based shRNA in U937 cells
The recombinant lentiviruses encoding shCon or
shICT1 were constructed to reveal the functional role of ICT1 in
leukemia. The lentiviral vectors also carried a GFP reporter gene
to monitor the infection efficiency. By counting GFP-positive cells
168 h after infection, transfection efficiencies of 90.1, 90.0 and
91.2% were observed in the shCon, shICT1 (S1) and shICT1 (S2)
groups, respectively (Fig. 1A).
As shown in Fig. 1B,
the ICT1 gene expression pattern in the shICT1 (S1) and shICT1 (S2)
cells was lower than that in the shCon cells. Western blotting was
applied to detect whether specific lentivirus-mediated shRNA could
knockdown ICT1 protein level in U937 cells. The knockdown of ICT1
reduced the ICT1 protein level in U937 cells (Fig. 1C).
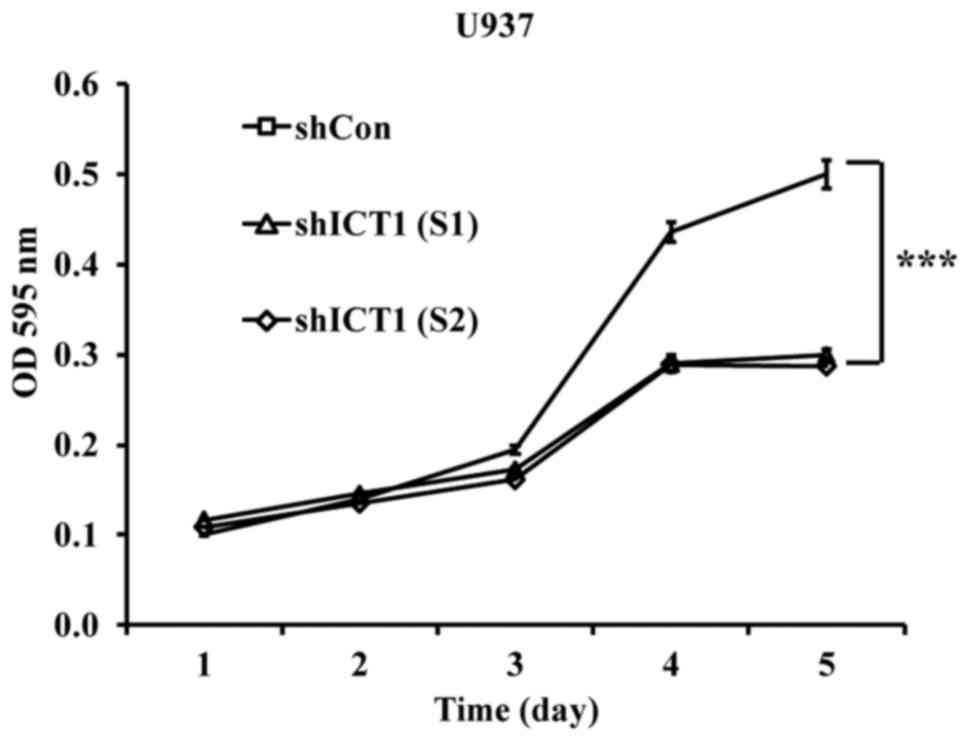
Knockdown of ICT1 inhibits U937 cell
proliferation
As shown in Fig. 2,
the shICT1 (S1)- or shICT1 (S2)-treated cells had a relatively
lower proliferative index from the third day compared with shCon.
On the fourth day, the proliferation was reduced by 33.3% in cells
expressing reduced levels of ICT1 in contrast with the control
(shCon). On the fifth day, in comparison with the control cultures,
proliferation was decreased by 40.2 and 42.6% for shICT1 (S1)- and
shICT1 (S2)-infected U937 cells, respectively.
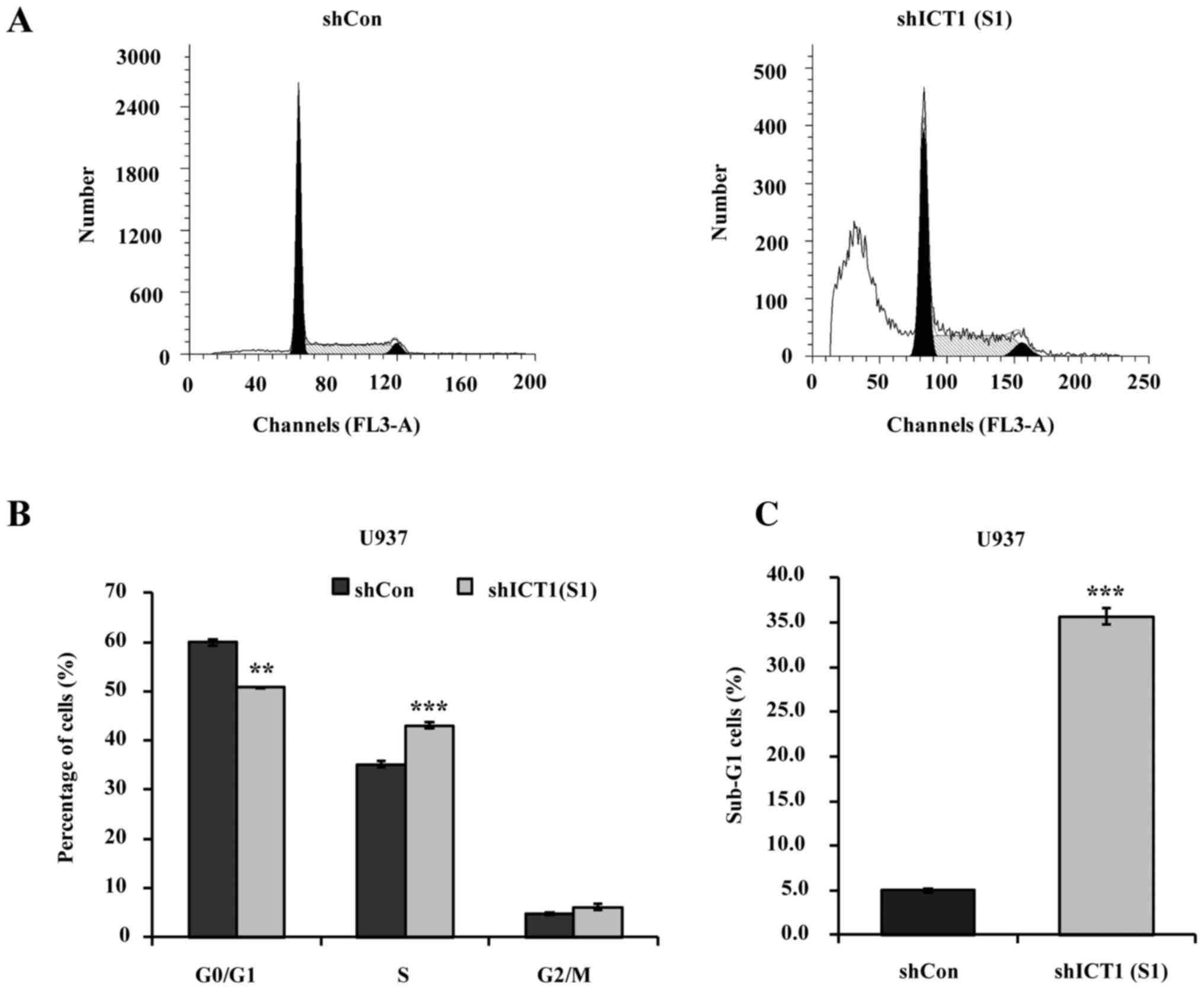
ICT1 knockdown arrests U937 cells in
the S and sub-G1 phases
Flow cytometry was carried out to determine whether
or not deficiency of ICT1 can interfere with cell cycle progression
in U937 cells (Fig. 3A). As shown
in Fig. 3B, the percentage of G0/G1
phase cells decreased from 60.0% in the shCon-treated group to
50.8% in the shICT1 (S1)-treated group. Increased percentage of
S-phase cells occurred in the shICT1 (S1) group with 43.5% compared
with 35.2% in shCon-treated group. There was no statically
significant difference in the G2/M phase between the shCon and
shICT1 (S1) groups.
U937 cells transfected with shICT1 (S1) resulted in
an enhanced sub-G1 (representing apoptotic cells) fraction
(Fig. 3C). The sub-G1 population of
U937 cells represented only 5.02% in shCon, but reached ~35.70%
upon infection with lentiviral vectors expressing shICT1 (S1).
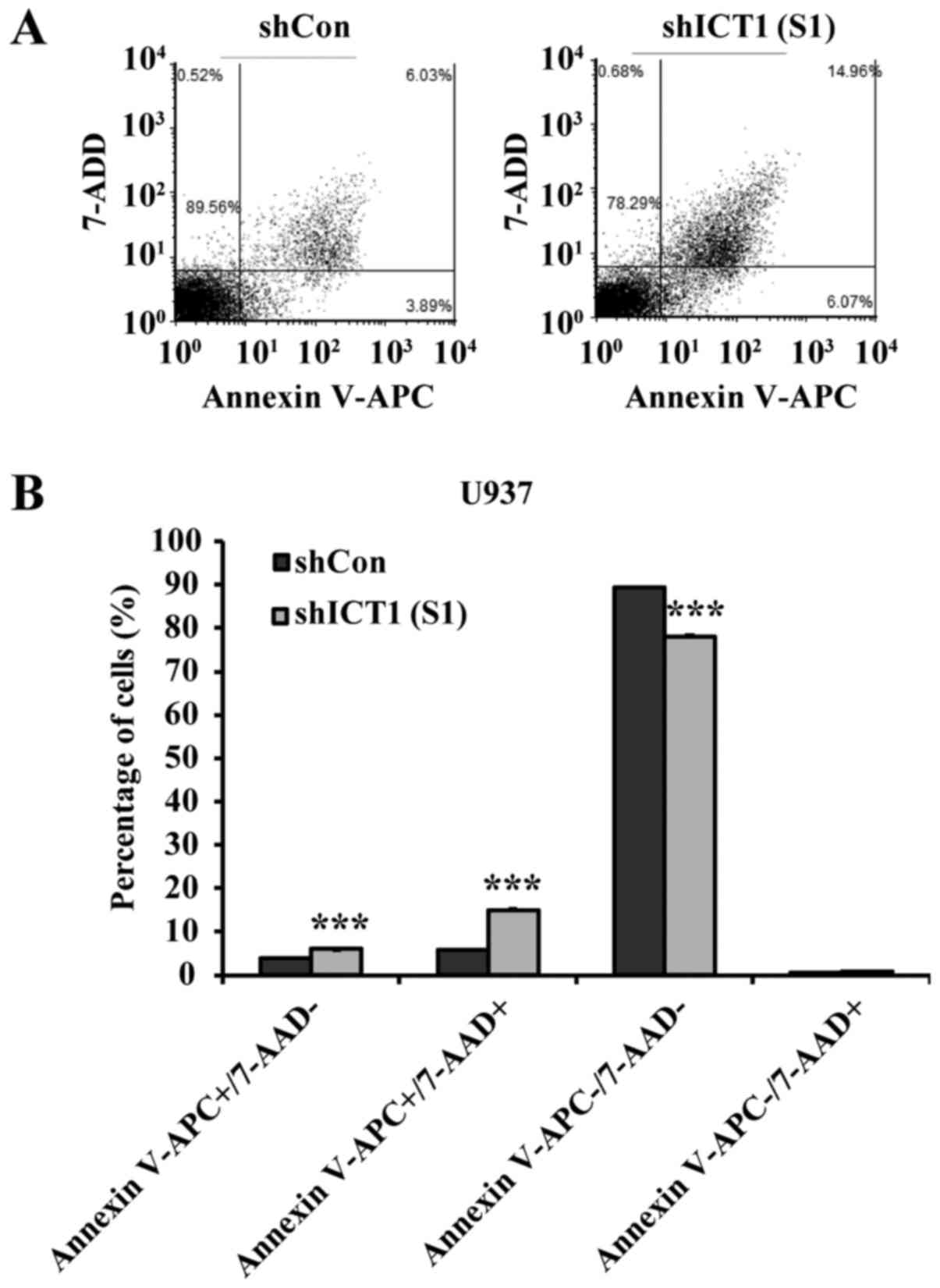
Effect of deficiency on ICT1
expression on the apoptosis in U937 cells
A marked sub-G1 peak was observed in ICT1-deficient
cells. Then Annexin V-APC/7-AAD double labeling was employed to
assess whether U937-cell depletion in ICT1 really undergoes
apoptosis (Fig. 4A). As shown in
Fig. 4B, ICT1 deficiency increased
the level of early apoptosis from 4.0% (shCon-treated cells) to
6.0% [shICT1 (S1)-treated cells] in the. U937 cells treated with
shICT1 (S1) exhibited an increase in late-apoptotic cells compared
with control, varying from 15.1% in cells treated with shICT1 (S1)
to 5.9% in the Lv-shCon treatment. In addition, the proportion of
viable cells was decreased in U937 cells transfected with shICT1
(S1) in comparison with control shCon-transfected cells.
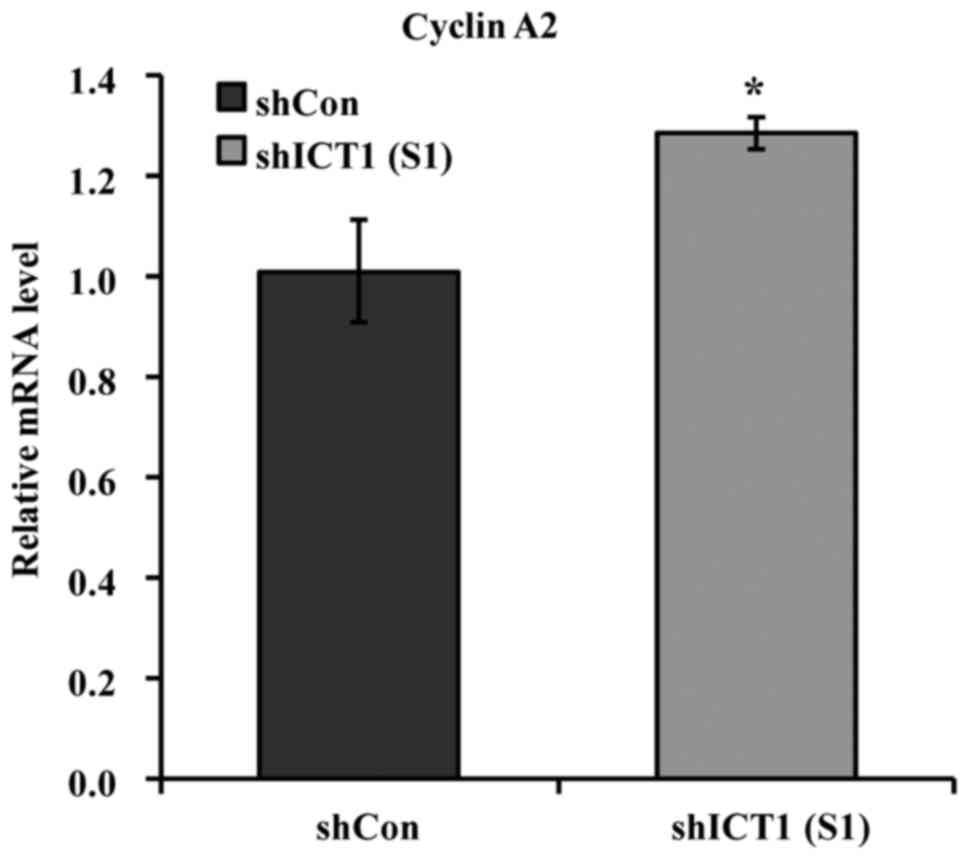
Depletion of ICT1 results in increased
cyclin A2 transcription expression
Using qRT-PCR, we analyzed the cyclin A2 mRNA
expression in U937 cells transfected with shCon and shICT1,
respectively. As observed in Fig.
5, the expression profile of cyclin A2 in the ICT1-knockdown
U937 cells was found to have increased by 27.1% compared with the
shCon-transfected cells.
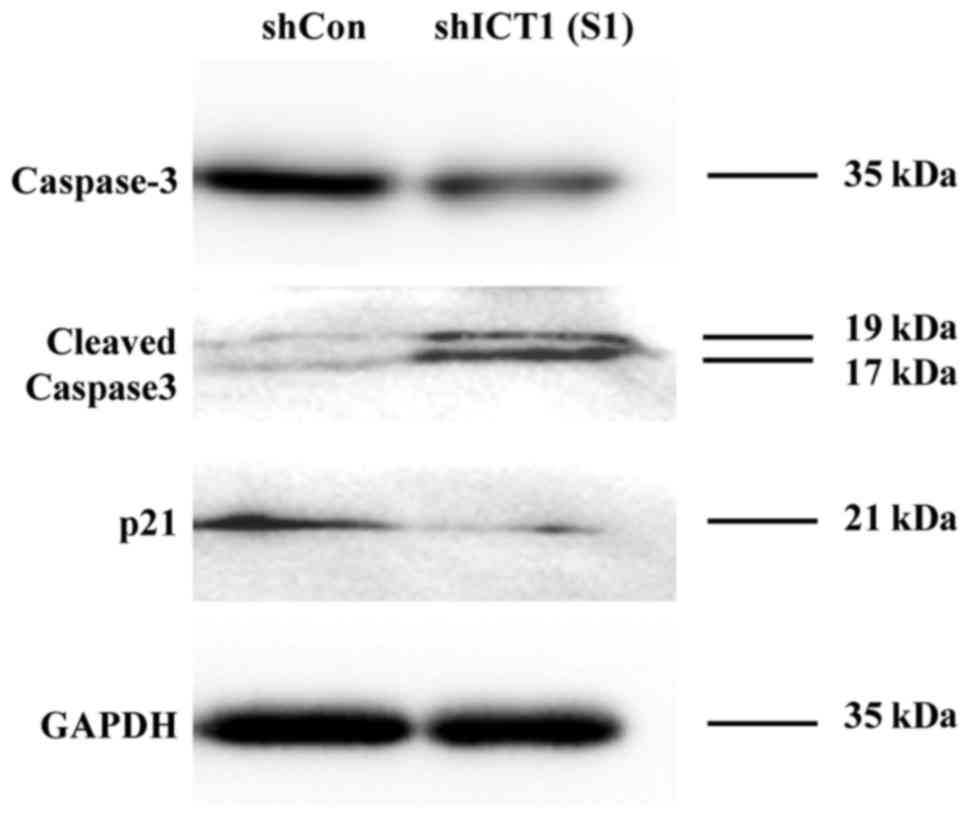
ICT1 downregulation exhibits altered
caspase-3 and p21 protein expression in U937 cells
Active 17 and 19 kDa subunits of caspase-3 were
increased in shICT1 (S1) cells as compared to the controls
(Fig. 6). The pro-caspase3 protein
levels were decreased in U937 cells with ICT1 knockdown compared
with the non-knockdown controls. U937 cells depleted of the ICT1
gene were also found to be decreased in the expression level of p21
protein.
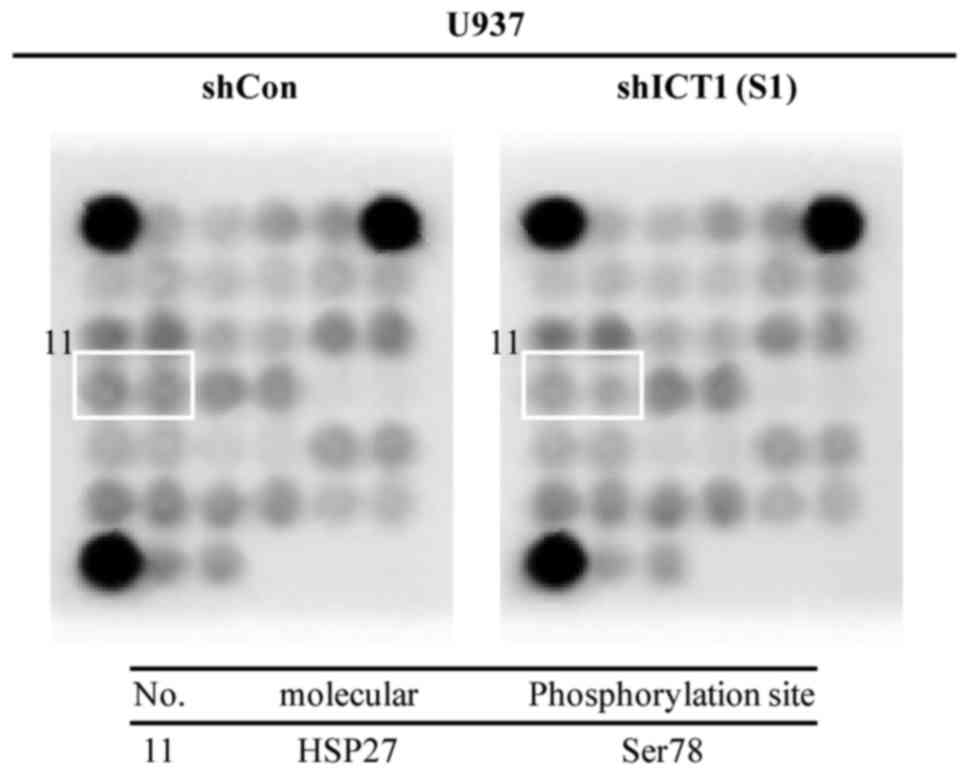
Decreased levels of heat shock protein 27
phosphorylation at Ser78 was correlated with knockdown of ICT in
U937 cells. The expression of 18 phosphorylated or cleaved
signaling molecules was assessed using the PathScan Intracellular
Signaling Array kit. As shown in Fig.
7, Ser78 HSP27 phosphorylation in U937 cells with shICT1 (S1)
transduction was lower than that of the shCon cells. The
phosphorylation level of other signaling molecules was almost
unaltered between the shCon and shICT1 (S1) groups.
Discussion
Immature colon carcinoma transcript 1 (ICT1) is a
component of human mitoribosome that has codon-independent
peptidyl-tRNA hydrolysis activity through a conserved GGQ motif
(13). ICT1 plays a role in the
pathogenesis of colon cancer, hepatoblastoma and glioblastoma
multiforme but its role in leukemia is unclear (16,17).
The present study demonstrated that loss of ICT1 was associated
with the inhibition of leukemia cell proliferation, cell cycle
arrest and apoptosis.
The cell cycle process is a sequence of events in
which one cell divides and then replicates into 2 cells, each
containing the information and components necessary to repeat the
biological process (18,19). Cyclins, including A, B, C, D and E,
are regulatory subunits of cyclin-dependent kinases, forming
different cyclin-CDK complexes to monitor cell cycle progression
(20,21). Cyclin A2, a member of the subclass
of A-type cyclins, is synthesized in somatic cells, promoting
mitotic entry and regulating G1/S transition through CDK1 and CDK2
(22,23). In the present study,
lentivirus-mediated downregulation of the ICT1 gene was able to
accelerate cyclin A2 mRNA expression in U937 cells. Tiwari et
al (24) found that excessive
expression of cyclin A, CDK1 and CDK2 alone could not activate
human β-cells to enter the cell cycle. p21 is a potent inhibitor of
CDKs thus negatively regulating mammalian cell cycle division
(25). In the present study, the
p21 protein level was decreased in U937 cells with ICT1 knockdown.
Thus, the activity of multiple mitotic CDK-cyclin complexes may be
influenced as a consequence of increased cyclin A2 mRNA and
decreased p21 protein levels. S phase-arrest induced by knockdown
of ICT1 may be related to changes in the activity of cyclin/CDK
complexes.
Apoptosis or programmed cell death, a
physiologically cell killing process, is important for suppression
of tumor growth (26,27). Cell apoptosis occurred in HeLa cells
where ICT1 had been knockdown by small interfering RNA, with a
reduction of mitochondrial membrane potential and mass (13). In the present study, apoptotic cell
death in U937 leukemia cells was observed in the ICT1-knockdown
group, which was consistent with previous results (13). Waterhouse et al (28) found that cytochrome c
emancipation and caspase activation was accompanied with the
permeability of the mitochondrial outer membrane during apoptosis.
The effector pro-caspase-3 was thought to be cleaved and activated
in a condition that was promoted by cytochrome c from
mitochondria to monitor cell apoptosis (29). For U937 cells with ICT1 knockdown,
strong cleavage/activation of caspase-3 precursor was obtained
based on increased formation of 17- and 19-kDa fragments. It is
likely that the mitochondrial cytochrome c-mediated
caspase-3 pathway may be activated, inducing apoptosis in
ICT1-knockdown U937 cells.
Overexpression of human chaperone protein HSP27 has
been reported to rescue cells from apoptosis induced by oxidative
stress or cytotoxicity (30,31).
Activity of HSP27 could be modulated by post-translational
modification of phosphorylation (32). At present, 3 phosphorylations sites
on Ser residues at positions 15, 78 and 82 for human HSP27 have
been identified (32,33). Notably, HSP27 acts to protect
mitochondrial integrity and prevent cytochrome c release
(33). The non-phosphorylated
oligomers of HSP27 can directly bind with cytosolic cytochrome
c to protect against apoptosis (33). The present study confirmed that
Ser78 of HSP27 was less phosphorylated in ICT1-knockdown U937 cells
as compared with the control, indicating that more
non-phosphorylated oligomers may have accumulated. Therefore, an
unknown mechanism may exist to impede interaction between
cytochrome c and HSP27, leading to the promotion of
cytochrome c release from mitochondria to the cytosol and
induction of apoptosis by triggering caspases, such as
caspase-3.
In conclusion, the present study demonstrated that
proliferation of leukemia U937 cells was blocked after depletion of
ICT1. Knockdown of ICT1 was found to induce S-phase arrest, and
promote apoptosis possibly via a mitochondrial cytochrome
c-mediated caspase-3 pathway in leukemia U937 cells.
However, the correlation between decreased HSP27 phosphorylation
and increased caspase-3 activation needs further investigation. The
newly identified role of ICT1 in leukemia cells in the present
study may be useful in the development of new therapies for
leukemia.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (nos. 81573772 and 81403263),
and the Science and Technology Developing Project of Shandong
Province (grant no. 2014GSF118141).
References
|
1
|
Mi S, Lu J, Sun M, Li Z, Zhang H, Neilly
MB, Wang Y, Qian Z, Jin J, Zhang Y, et al: MicroRNA expression
signatures accurately discriminate acute lymphoblastic leukemia
from acute myeloid leukemia. Proc Natl Acad Sci USA. 104:pp.
19971–19976. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Golub TR, Slonim DK, Tamayo P, Huard C,
Gaasenbeek M, Mesirov JP, Coller H, Loh ML, Downing JR, Caligiuri
MA, et al: Molecular classification of cancer: Class discovery and
class prediction by gene expression monitoring. Science.
286:531–537. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cortes JE and Kantarjian HM: Acute
lymphoblastic leukemia. A comprehensive review with emphasis on
biology and therapy. Cancer. 76:2393–2417. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Büchner T, Berdel WE, Haferlach C,
Haferlach T, Schnittger S, Müller-Tidow C, Braess J, Spiekermann K,
Kienast J, Staib P, et al: Age-related risk profile and
chemotherapy dose response in acute myeloid leukemia: A study by
the German Acute Myeloid Leukemia Cooperative Group. J Clin Oncol.
27:61–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ziogas DC, Voulgarelis M and Zintzaras E:
A network meta-analysis of randomized controlled trials of
induction treatments in acute myeloid leukemia in the elderly. Clin
Ther. 33:254–279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Radtke S, Zolk O, Renner B, Paulides M,
Zimmermann M, Möricke A, Stanulla M, Schrappe M and Langer T:
Germline genetic variations in methotrexate candidate genes are
associated with pharmacokinetics, toxicity, and outcome in
childhood acute lymphoblastic leukemia. Blood. 121:5145–5153. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang JJ, Cheng C, Devidas M, Cao X,
Campana D, Yang W, Fan Y, Neale G, Cox N, Scheet P, et al:
Genome-wide association study identifies germline polymorphisms
associated with relapse of childhood acute lymphoblastic leukemia.
Blood. 120:4197–4204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Locatelli F, Zecca M, Rondelli R, Bonetti
F, Dini G, Prete A, Messina C, Uderzo C, Ripaldi M, Porta F, et al:
Graft versus host disease prophylaxis with low-dose cyclosporine-A
reduces the risk of relapse in children with acute leukemia given
HLA-identical sibling bone marrow transplantation: results of a
randomized trial. Blood. 95:1572–1579. 2000.PubMed/NCBI
|
|
9
|
McCormick F: Cancer gene therapy: Fringe
or cutting edge? Nat Rev Cancer. 1:130–141. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wierda WG, Cantwell MJ, Woods SJ, Rassenti
LZ, Prussak CE and Kipps TJ: CD40-ligand (CD154) gene therapy for
chronic lymphocytic leukemia. Blood. 96:2917–2924. 2000.PubMed/NCBI
|
|
11
|
Renner AG, Dos Santos C, Recher C, Bailly
C, Créancier L, Kruczynski A, Payrastre B and Manenti S: Polo-like
kinase 1 is overexpressed in acute myeloid leukemia and its
inhibition preferentially targets the proliferation of leukemic
cells. Blood. 114:659–662. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wilda M, Fuchs U, Wössmann W and Borkhardt
A: Killing of leukemic cells with a BCR/ABL fusion gene by RNA
interference (RNAi). Oncogene. 21:5716–5724. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Handa Y, Hikawa Y, Tochio N, Kogure H,
Inoue M, Koshiba S, Güntert P, Inoue Y, Kigawa T, Yokoyama S, et
al: Solution structure of the catalytic domain of the mitochondrial
protein ICT1 that is essential for cell vitality. J Mol Biol.
404:260–273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Richter R, Rorbach J, Pajak A, Smith PM,
Wessels HJ, Huynen MA, Smeitink JA, Lightowlers RN and
Chrzanowska-Lightowlers ZM: A functional peptidyl-tRNA hydrolase,
ICT1, has been recruited into the human mitochondrial ribosome.
EMBO J. 29:1116–1125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akabane S, Ueda T, Nierhaus KH and
Takeuchi N: Ribosome rescue and translation termination at
non-standard stop codons by ICT1 in mammalian mitochondria. PLoS
Genet. 10:e10046162014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie R, Zhang Y, Shen C, Cao X, Gu S and
Che X: Knockdown of immature colon carcinoma transcript-1 inhibits
proliferation of glioblastoma multiforme cells through Gap
2/mitotic phase arrest. Onco Targets Ther. 8:1119–1127.
2015.PubMed/NCBI
|
|
17
|
Aleman MJ, DeYoung MP, Tress M, Keating P,
Perry GW and Narayanan R: Inhibition of Single Minded 2 gene
expression mediates tumor-selective apoptosis and differentiation
in human colon cancer cells. Proc Natl Acad Sci USA. 102:pp.
12765–12770. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mitchison JM: The Biology of The Cell
Cycle. Cambridge University Press; Cambridge, UK: pp. 3131971
|
|
19
|
Murray A and Hunt T: The Cell Cycle: An
Introduction. Q Rev Biol. 2:147–163. 1995.
|
|
20
|
Buckley MF, Sweeney KJ, Hamilton JA, Sini
RL, Manning DL, Nicholson RI, deFazio A, Watts CK, Musgrove EA and
Sutherland RL: Expression and amplification of cyclin genes in
human breast cancer. Oncogene. 8:2127–2133. 1993.PubMed/NCBI
|
|
21
|
Murray AW, Solomon MJ and Kirschner MW:
The role of cyclin synthesis and degradation in the control of
maturation promoting factor activity. Nature. 339:280–286. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chaudhry HW, Dashoush NH, Tang H, Zhang L,
Wang X, Wu EX and Wolgemuth DJ: Cyclin A2 mediates cardiomyocyte
mitosis in the postmitotic myocardium. J Biol Chem.
279:35858–35866. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bendris N, Lemmers B, Blanchard JM and
Arsic N: Cyclin A2 mutagenesis analysis: A new insight into CDK
activation and cellular localization requirements. PLoS One.
6:e228792011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tiwari S, Roel C, Wills R, Casinelli G,
Tanwir M, Takane KK and Fiaschi-Taesch NM: Early and late G1/S
cyclins and Cdks act complementarily to enhance authentic human
β-cell proliferation and expansion. Diabetes. 64:3485–3498. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang MW, Barr E, Lu MM, Barton K and
Leiden JM: Adenovirus-mediated over-expression of the
cyclin/cyclin-dependent kinase inhibitor, p21 inhibits vascular
smooth muscle cell proliferation and neointima formation in the rat
carotid artery model of balloon angioplasty. J Clin Invest.
96:2260–2268. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Strasser A, O'Connor L and Dixit VM:
Apoptosis signaling. Annu Rev Biochem. 69:217–245. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Waterhouse NJ, Goldstein JC, von Ahsen O,
Schuler M, Newmeyer DD and Green DR: Cytochrome c maintains
mitochondrial transmembrane potential and ATP generation after
outer mitochondrial membrane permeabilization during the apoptotic
process. J Cell Biol. 153:319–328. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu X, Zou H, Slaughter C and Wang X: DFF,
a heterodimeric protein that functions downstream of caspase-3 to
trigger DNA fragmentation during apoptosis. Cell. 89:175–184. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huot J, Houle F, Spitz DR and Landry J:
HSP27 phosphorylation-mediated resistance against actin
fragmentation and cell death induced by oxidative stress. Cancer
Res. 56:273–279. 1996.PubMed/NCBI
|
|
31
|
Garrido C, Schmitt E, Candé C, Vahsen N,
Parcellier A and Kroemer G: HSP27 and HSP70: Potentially oncogenic
apoptosis inhibitors. Cell Cycle. 2:579–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yasuda E, Kumada T, Takai S, Ishisaki A,
Noda T, Matsushima-Nishiwaki R, Yoshimi N, Kato K, Toyoda H,
Kaneoka Y, et al: Attenuated phosphorylation of heat shock protein
27 correlates with tumor progression in patients with
hepatocellular carcinoma. Biochem Biophys Res Commun. 337:337–342.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Landry J, Lambert H, Zhou M, Lavoie JN,
Hickey E, Weber LA and Anderson CW: Human HSP27 is phosphorylated
at serines 78 and 82 by heat shock and mitogen-activated kinases
that recognize the same amino acid motif as S6 kinase II. J Biol
Chem. 267:794–803. 1992.PubMed/NCBI
|