Introduction
DEAD-box proteins, named by the amino acid motif
D-E-A-D (Asp-Glu-Ala-Asp), are members of the largest family of RNA
helicases (1). Moreover, the
DEAD-box proteins are ATP-dependent RNA binding proteins and
RNA-dependent ATPases, which play essential roles in rearranging
RNA-RNA and RNA-protein interactions (2). The DEAD-box RNA helicase 3 (DDX3), is
one highly conserved family member of DEAD-box proteins in all
eukaryotes from yeasts to human beings (3).
The human genome code for two types of functional
DDX3 genes, including DDX3X and its homolog DDX3Y (4). DDX3Y is located on the Y-chromosome,
and plays particular roles of spermatogenesis and male fertility
(5). While DDX3X is located on the
X-chromosome and widely expresses in a broad range of organisms. In
addition, DDX3 is ubiquitously reported to control pleiotropic
physiological events in a variety of tissues (6). Thus, most research pays more attention
to DDX3X (refer DDX3 to DDX3X in following text).
As an RNA helicase, DDX3 protein consists of 662
amino acids. Its crystal structure has been reported by protein
crystallization and X-ray diffraction analysis (7,8).
Accumulating studies have confirmed that DDX3 has the ability to
regulate different steps of RNA metabolism (9,10),
including RNA splicing (11), mRNA
export (12–14), transcription (15) and translation initiation (16,17).
DDX3 manipulates RNAs that range from rRNAs to mRNAs. However, DDX3
is considered to be a nuclear-cytoplasmic shuttling protein through
CRM1-mediated export pathway or Tip-associated protein-dependent
export pathway (13,14). After transporting from the nucleus
to the cytoplasm, DDX3 is prepared for translation, and eventually
destroyed. The translation of DDX3 is controlled by activation of
elF3 and cap-dependent translation or inhibition of elF4 (16,17).
At the same time, DDX3 is involved in many biological processes,
such as stress response (18), cell
apoptosis (19), cell cycle
regulation (20) and embryogenesis
(21). A loss of DDX3 induces an
early embryonic lethality in mice (21).
In recent years, DDX3 is getting increasing
attention due to its essential roles in cancer progression
(22). Accordingly, DDX3 may be a
new potential target for cancer biotherapy. However, the roles of
DDX3 in cancer development are rather complicated. As a
‘double-edged sword’ gene, DDX3 either promotes cancer progression
or acts as a tumor suppressor in some cancer types. In the present
study, we summarize and discuss the dual roles of DDX3 and
DDX3-mediated signaling pathways in multiple cancers. In addition,
we also analyze the interplaying causes for the controversial roles
of DDX3 in different types of cancer and sum up the potential
anticancer drugs targeting DDX3.
DDX3 role in cancer: Tumor suppressor or
oncogene
The biological roles of DDX3 in cancer development
are conflicting. Its tumor promoting and suppressing effects have
been broadly reported. Notably, the dual roles of DDX3 are reported
not only in different types of cancer but also in the same type of
cancer. The divergent roles of DDX3 in multiple cancer progression
(Table I) are discussed in the
specific cancer as follows.
 | Table I.Summary of DDX3 roles in cancer. |
Table I.
Summary of DDX3 roles in cancer.
| Cancer type | DDX3 roles | Signaling
pathways | (Refs.) |
|---|
| Hepatocellular
carcinoma | Dual roles | Oncogene: Act as a
cellular transforming gene in hepatocarcinogenesis | (25) |
|
|
| Tumor suppressor:
Upregulate cyclin D1 and downregulate p21 | (20) |
|
|
| Tumor suppressor:
Upregulate p21 in a manner independent of p53 | (15) |
|
|
| Tumor suppressor:
Activate the tumor-suppressive miRNAs | (26) |
| Breast cancer | Oncogene |
HIF-1α/DDX3/E-cadherin pathway | (27–32) |
| Lung cancer | Dual roles | Oncogene: Activate
Wnt signaling | (35) |
|
|
| Tumor suppressor:
p53/DDX3/p21 pathway | (33) |
|
|
| Tumor suppressor:
MDM2/Slug/E-cadherin pathway | (34) |
| Colorectal
cancer | Dual roles | Oncogene: Activate
the β-catenin/ZEB1 signaling pathway | (38) |
|
|
| Oncogene: Activate
the DDX3/β-catenin/TCF4 pathway | (40) |
|
|
| Oncogene: Activate
Wnt signaling through CK1ε/Dvl2 axis | (37) |
|
|
| Oncogene: DDX3
increases cell aggressiveness via a KRAS/HIF-1α/YAP1/SIX2
cascade | (39) |
|
|
| Tumor suppressor:
DDX3/Snail/E-cadherin pathway | (36) |
| Oral squamous cell
carcinoma | Dual roles | Oncogene: Ketorolac
salt downregulates DDX3 expression and inhibits the proliferation
of oral cancer cells | (42) |
|
|
| Oncogene: High DDX3
expression is associated with poorer survival in smokers | (43) |
|
|
| Tumor suppressor:
Low expression of DDX3 may predict poor prognosis in non-smoker
patients with oral cancer | (41) |
| Brain cancer | Oncogene | Glioblastoma: DDX3
induces EMT process though DDX3/snail/E-cadhein axis | (47) |
|
|
| Medulloblastoma:
Mutant DDX3 in combination with mutant β-catenin strengthen
transactivation of a TCF promoter | (18,50,51) |
| Natural
killer/T-cell lymphoma | Oncogene | Mutant DDX3X
increases the phosphorylation of ERK and elevates the nuclear level
of RelB to promote cell proliferation | (52) |
| Chronic myeloid
leukemia | Oncogene | Patients with
mutated DDX3 exhibits a shorter survival. | (53,54) |
| Ewing sarcoma | Oncogene | Konckdown of DDX3
by RK-33 treatment in Ewing sarcoma cells leads to decreased
tumorigenic activity | (45) |
| Prostate
cancer | Oncogene | Inhibition of DDX3
by RK-33 treatment in the aggressive prostate cancer cells
decreases proliferation and induces cell cycle arrest. | (46) |
| Pancreatic ductal
adenocarcinoma | Oncogene | Positive DDX3
expressions are associated with poor clinical outcome | (48) |
| Gallbladder
cancer | Oncogene | High DDX3
expression is correlated with poor prognosis | (49) |
Hepatocellular carcinoma
DDX3 has been shown to be essential for the
replication of hepatitis B virus (HBV) and hepatitis C virus (HCV)
which are two types of hepatitis virus usually linked to
hepatocellular carcinoma (HCC) (23,24).
DDX3 acts as diverse roles in HCC development. DDX3 overexpression
was observed in HCC, and it was identified as a cellular
transforming gene in hepatocarcinogenesis (25). In contrast, Chang et al found
that a decreased expression level of DDX3 is present in
HBV-positive HCC patients, but not in the HCV-positive ones
(20). Based on the findings, they
proposed a molecular mechanism of DDX3 where the inhibition of DDX3
upregulates cyclin D1 and downregulates p21waf1/cip1,
and thereby promotes S phase entry to facilitate tumor cell growth.
Other research demonstrated that DDX3 inhibits cell colony
formation ability of HCC HuH-7 cells by upregulating
p21waf1/cip1 in a p53-independent manner (15). A recent study supports DDX3 as a
tumor suppressor in HCC, since DDX3 downregulation promotes stem
cell-like properties and tumorigenesis by silencing the
tumor-suppressive miRNAs in HepG2 cells (26). In addition, the decrease of DDX3
correlates with poor prognosis of HCC patients.
Breast cancer
DDX3 exerts oncogenic roles in breast cancer. DDX3
upregulation can increase the ability of cell growth, proliferation
and epithelial-mesenchymal-like transformation (EMT) in normal
breast epithelial MCF10A cells (27). Similarly, HIF-1α induces the
transcriptional activation of DDX3 via binding to the HIF
responsive element located in the DDX3 promoter region in MCF10A
cells (28). Moreover, DDX3 is
overexpressed in a large series of breast cancer patients (29), and DDX3 upregulation is correlated
with distant metastases of breast cancer (30). While DDX3 knockdown decreases tumor
volume and metastasis in vivo (31). In addition, a combination treatment
using DDX3 and PARP inhibitors induces cooperative therapeutic
effects in BRCA1-proficient breast cancer (32). Thus, DDX3 drives breast cancer
carcinogenesis and it can be a potential treatment target for
breast cancer.
Lung cancer
DDX3 has dual roles, inhibiting or promoting
carcinogenesis, in lung cancer. DDX3 seems to have antitumor
activity in human papillomavirus (HPV)-positive lung cancer. HPV E6
decreases DDX3 transcription to synergistically suppress p21
expression, thereby low level of DDX3 leads to a poor relapse-free
survival (RFS) in early-stage lung cancer and non-small cell lung
cancer patients (33,34). Furthermore, loss of DDX3 suppressed
E-cadherin by MDM2/Slug axis to promote tumor progression and
metastasis in HPV-infected lung cancer cells (34).
However, DDX3 is also reported to overexpress in
lung cancer, and DDX3 upregulation is related with shorter survival
for lung cancer patients (35).
While DDX3 downregulation decreases colony formation in lung A549
cells. In addition, a small molecule inhibitor RK-33 by targeting
DDX3 can efficiently inhibit lung cancer growth (35).
To date, DDX3 roles are variable in lung cancer. It
is still likely that DDX3 should be considered as a drug target for
lung cancer therapy.
Colorectal cancer
DDX3 functions in colorectal cancer are
controversial. Several studies showed that DDX3 acts as a tumor
suppressor in colorectal cancer. Knockdown of DDX3 increases cancer
progression through Snail/E-cadherin pathway in colon cancer cells
(36). In addition, colorectal
cancer patients with low DDX3 expression have poor prognosis and
more frequent distant metastasis (36).
In contrast, several groups obtain different
conclusions of the role of DDX3 in colorectal cancer (37–40).
In KRAS-mutated colon cancer cells, DDX3 promotes cell invasion and
the nodule formation of xenograft lung tumor due to β-catenin/TCF
activation through the CK1ε/Dvl2 axis (37). In addition, DDX3 enhances KRAS
transcription and activates the following β-catenin/ZEB1 signaling
to promote KRAS-mediated tumor invasion (38). Accordingly, the colorectal tumor
growth and metastasis mediated by KRAS gene mutations seem to
require β-catenin/TCF activation by DDX3. While in KRAS-wild-type
colorectal cancer, DDX3 promotes cell aggressiveness through the
KRAS/HIF-1α/YAP1/SIX2 cascade (39). Inhibition of DDX3 by a small
inhibitor RK-33 reduces cell proliferation and causes a G1 arrest
in colorectal cancer cells (40).
In addition, a high DDX3 expression leads to poor clinical overall
survival (OS) for colorectal cancer patients (37–40).
The conflicting role of DDX3 in colorectal cancer
needs to be further explored. So far, it is inaccurate to predict
survival outcomes of colorectal cancer patients only using the
protein DDX3 due to its multiple functions of DDX3 in cancer
development. It seems more feasible to jointly analyze several
proteins, including DDX3 and its associated molecules, with
colorectal cancer progression and prognosis.
Oral squamous cell carcinoma
Notably, DDX3 has also been found to play divergent
roles in oral squamous cell carcinoma (OSCC). A low/negative DDX3
expression is correlated with a poorer OS, notably in non-smoker
patients with OSCC (41). This
review demonstrates that DDX3 acts as a tumor suppressor, and it is
probable to be an independent survival predictor in non-smoker
patients with oral cancer.
Conversely, different conclusions claim that DDX3 is
an oncogene to promote the progression of OSCC (42,43).
DDX3 expression is not correlative to survival in head and neck
squamous cell carcinomas on the whole (43). However, a high DDX3 expression is
associated with poorer survival in smokers. There are multiple
reasons for explaining the different effects of DDX3 in oral
cancer. One possible key factor is that smoker patients more often
have HPV-negative tumors and non-smokers more frequently have
HPV-positive tumors. DDX3 is increased in response to cigarette
smoke exposure, whereas it has different roles in virus infection.
Furthermore, DDX3 mutations have been found in head and neck
cancers (44), which is partially
responsible for the complexity of DDX3 roles.
Other cancers
DDX3 is also usually increased in the Ewing sarcoma
(45), prostate cancer (46), glioblastoma (47), pancreatic ductal adenocarcinoma
(48) and gallbladder cancer
(49), in which a positive DDX3
expression is linked to a poor clinical outcome. However, the
molecular mechanism of DDX3 exerting oncogenic functions in these
cancers requires further study and discussion. Inhibition of DDX3
expression by inhibitor RK-33 leads to decreased tumorigenic
activity of Ewing sarcoma cells and xenografts (45). Importantly, an in vivo
combination treatment of RK-33 and radiation obtain synergistic
inhibiting effects on prostate tumor growth (46). RK-33 alters cellular proteome,
particularly proteins related to DNA damage repair, protein
translation and proteasome function.
Exceptionally, the mutant DDX3 has been confirmed to
have oncogenic functions in medulloblastoma (18,50,51),
natural killer/T-cell lymphoma (52) and chronic lymphocytic leukemia
(53,54). Mutations of DDX3 are identified in
majority of medulloblastoma through whole-exome hybrid capture and
deep sequencing. The mutant helicase domains of DDX3, in
combination with a mutant β-catenin, strengthen transactivation of
a TCF promoter and enhance cell viability in medulloblastoma
(50). DDX3 mutants exhibit less
RNA-unwinding activity and loss of depressing effects on cell cycle
progression in NK cells, while the wild-type DDX3 inhibited the
phosphorylation of ERK and reduced the nuclear level of RelB to
inhibit cell proliferation (52).
However, lymphocytic leukemia patients with DDX3 mutations have a
poor prognosis and a shorter survival (53). Notably, DDX3 mutants are more
frequently detected in cases relapsed after therapy (54). Therefore, the mutation of DDX3 is an
essential factor leading to tumorigenesis. DDX3 has potential to be
a new therapeutic target for natural killer/T-cell lymphoma.
Multifunctions of DDX3-mediated
signaling pathways
The double-edged functions of DDX3 are partially due
to multiple DDX3-mediated cell signaling pathways in different
cancers. We summarize several DDX3-involved signaling pathways to
promote or inhibit carcinogenesis and tumor development (Figs. 1–4).
Cross-talk between different signaling pathways also contributes to
the complexity of DDX3-modulated cellular activities.
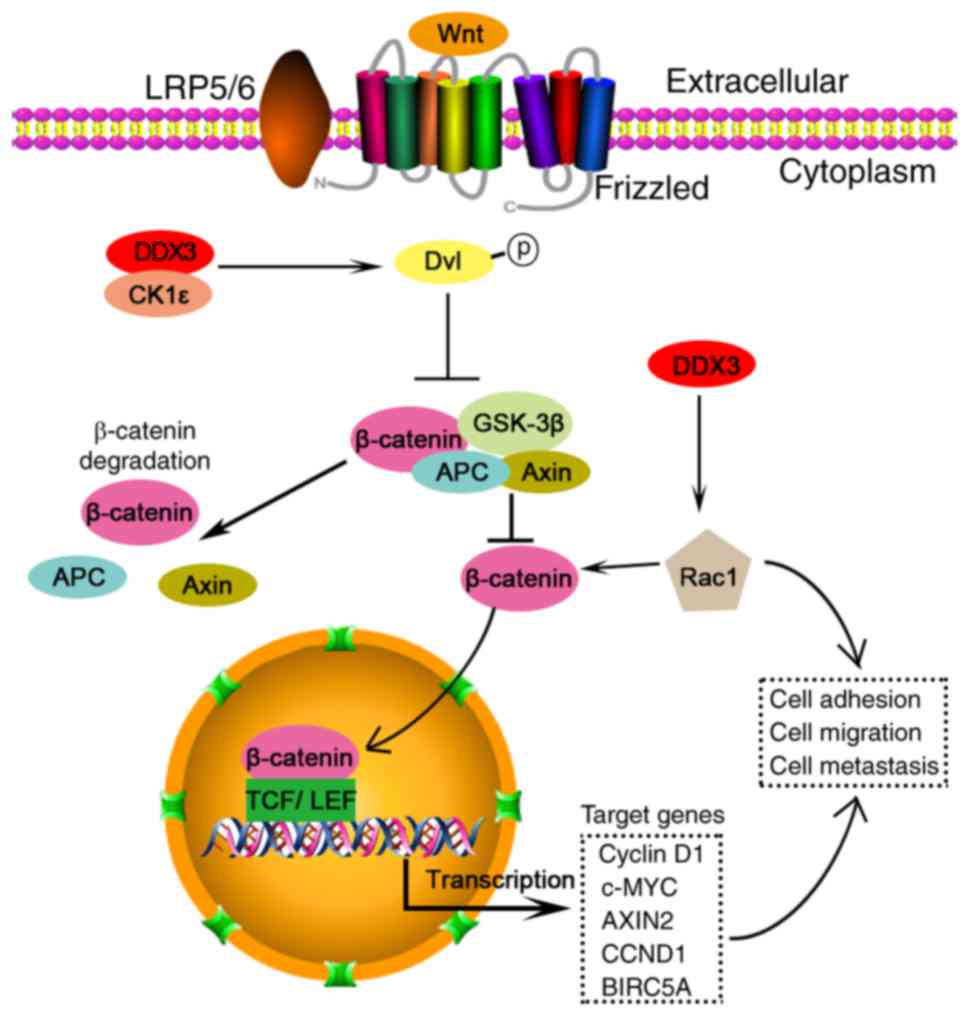 | Figure 1.DDX3 acts as an oncogene through
Wnt/β-catenin pathway in colorectal cancer cells. DDX3 interacts
with CK1ε to form a complex, which phosphorylates dishevelled
(Dvl). Dvl phosphorylation inhibits the generation of a complex of
axin, GSK3β, APC and β-catenin. The complex induces β-catenin
degradation. In contrast, Dvl phosphorylation increases free
β-catenin, which translocates from cytoplasm to nucleus. The
nucleus β-catenin could interact with two major transcription
factors, the T-cell factor (TCF) and lymphocyte enhancer factor
(LEF), and regulate multiple gene transcription, such as cyclin D1,
c-MYC, AXIN2, CCND1 and BIRC5A. In contrast, DDX3 elevates Rac1
expression and thereby increase β-catenin stability and its
signaling. Rac1 and/or β-catenin can regulate cell adhesion,
migration and metastasis. |
DDX3-mediated signaling pathways to promote cancer
progression
Wnt/β-catenin pathway
The DDX3/Wnt/β-catenin signaling pathway plays a
pivotal role in tumor invasion. DDX3 directly binds to CK1ε to
stimulate its kinase activity, and further promotes phosphorylation
of the scaffold protein dishevelled (Dvl), thus facilitating
β-catenin translocation into nucleus in a Wnt-dependent manner
during the development of mammalian cells (55). Similarly, DDX3 acts as a subunit of
CK1ε and phosphorylates Dvl2 to activate β-catenin/TCF signaling,
and finally enhances tumor invasion in colorectal cancer (37). In addition, DDX3 increases protein
levels of both Rac1 and β-catenin, and thereby promotes Wnt
signaling to modulate cell adhesion, migration and metastasis
(56). DDX3 mutations accompanied
with mutant β-catenin increase transactivation of a TCF promoter
and augment cell viability in medulloblastoma (50). Overall, these findings provide new
insight into inhibiting cancer cell migration by targeting
DDX3/Wnt/β-catenin pathway.
DDX3/Snail/E-cadherin pathway
The EMT process, along with E-cadherin
downregulation and Snail upregulation, accelerates the invasion and
metastasis of cancer cells (57,58).
Although DDX3 is related to the Snail regulator GSK3β (59,60),
DDX3 has the opposite effect on EMT in breast cancer and colorectal
cancer cells. DDX3 knockdown downregulates Snail levels and
decreases cell proliferation and migration in breast cancer MCF-7
cells (47). However, the
inhibition of DDX3 induces the EMT process, with upregulation of
Snail and decrease of E-cadherin in colorectal cancer DLD-1 and
HCT116 cells (36).
HIF-1α/DDX3/E-cadherin pathway
DDX3 promotes oncogenesis in breast cancers through
HIF-1α/DDX3/E-cadherin pathway (28,29).
Under hypoxic conditions, HIF-1α binds to the HRE located in the
DDX3 promoter region to induce the transcriptional activation of
DDX3. Subsequently, upregulation of DDX3 represses E-cadherin
expression, thus inducing the EMT process to facilitate breast
cancer invasion.
KRAS/HIF-1α/YAP1/SIX2 cascade
In KRAS wild-type colorectal cancer, DDX3 increases
cell aggressiveness via a DDX3/KRAS/ROS/HIF-1α/DDX3 cascade
feedback loop. ROS generation by DDX3-mediated KRAS expression
promotes YAP1 transcription through increasing HIF-1α bound to YAP1
promoter. Finally the YAP1/SIX2 axis results in the DDX3-induced
cell invasiveness (39).
DDX3-modulates signaling pathway to inhibit
tumorigenesis
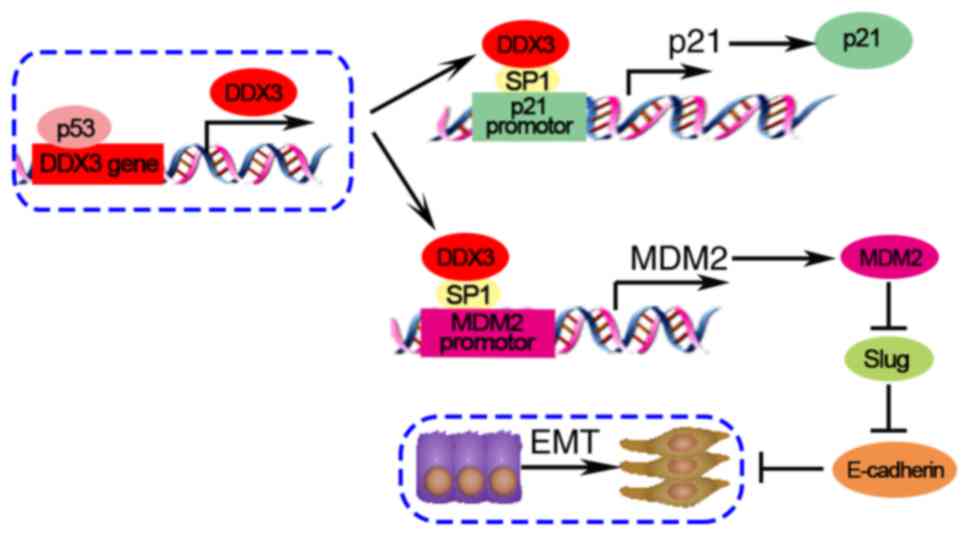
MDM2/Slug/E-cadherin pathway
DDX3 plays a tumor suppressor role in non-small cell
lung cancer cells through the MDM2/Slug/E-cadherin pathway
(34). DDX3 loss is induced by p53
knockdown and HPV E6 overexpression in lung cancer A549 cells. DDX3
loss inhibits MDM2 transcription via reducing SP1 binding to MDM2
promoter. A previous study has demonstrated MDM2 improves
E-cadherin expression via mediating degradation of Slug in lung
cancer cells. While knockdown of MDM2 upregulates Slug and
downregulates E-cadherin to promote tumor malignancy (61). In conclusion, in low DDX3-expressing
cancer cells, DDX3 loss may enhance the tumor progression and
metastasis through the MDM2/Slug/E-cadherin signaling axis.
p53/DDX3/p21 pathway
DDX3 interacts with Sp1 to upregulate the promoter
activity of p21 regardless of p53 status in Huh-7 human
hepatocellular cell line (15).
DDX3 overexpression elevates p21 and decreases cyclin D1, leading
to S phase arrest (20). In
addition, DDX3 is regulated by p53, which enhances p21
transcription (33). Overall, DDX3
has functions as a tumor suppressor through the p53/DDX3/p21
signaling pathway.
DDX3/tumor-suppressive miRNA
pathway
Accumulating research has demonstrated that
microRNAs (miRNAs) exhibited tumor suppressive activity in cancer
progression (62,63). DEAD-box RNA helicase can involve in
miRNA biogenesis (64). DDX3 is
hypothesized to interact with transcription factors on promoter
regions of tumor-suppressive miRNAs, such as miR-200b, miR-200c,
miR-122 and miR-145, which possibly activates the expression of
these miRNAs, thus, suppressing cancer stem cell properties and
tumorigenesis in liver cells (26).
Moreover, DDX3 interacts with Drosha/DGCR8 complex (miRNA
microprocessor) as a novel RNA binding protein and affects
pri-miRNA processing activity, and eventually promotes mature miRNA
expression level to control cancer development (65). The DDX3-mediated miRNA regulation is
promising to pave new strategy to explore its multiple
functions.
Factors contributing to functional
divergence of DDX3
Briefly, as an oncogene or tumor suppressor, DDX3
has a double-edged function in cancer occurrence and progression.
Notably, even though in the same type of cancer, DDX3 roles are
controversial. We discuss below the possible factors to confer
many-sided abilities for DDX3.
Viral infection
DDX3 has been demonstrated to be an important
component of the innate immune response against viral infections
(66). DDX3 has a central place in
virus-associated cancers for its proverbial function in viral
replication. For instance, DDX3 expression level is decreased in
HBV-positive, but not in the HCV-positive HCC patients (20). More importantly, DDX3 has been shown
to promote HCV virus replication through its interaction with HCV
core protein, but it interferes with HBV replication by binding to
the HBV polymerase and inducing IFN-β production (66). Therefore, DDX3 exerts specific
functions on different types of virus infection in HCC
development.
Although DDX3 has some relation with HPV infection,
the molecular mechanism of HPV interaction with DDX3 is little
known in HPV-infected cervical cancer, lung cancer (33,34)
and OSCC (41,43). However, the interaction of DDX3 with
virus protein was discovered to involve innate immune response
against virus infection by targeting with TBK1 to induce IFN-β
production (67,68). Moreover, DDX3 has been demonstrated
to regulate NF-κB signal pathway (69,70).
DDX3-modulated immune response signaling still needs to be
clarified in the virus infection-associated cancers.
Gender differences
DDX3 is located on chromosome X, and it is
preferentially mutated in males. DDX3 can escape X-inactivation,
which may protect females from complete functional loss by a single
gene mutation (20,71). Moreover, DDX3 expression is closely
associated with living habits (41), including smoking, alcohol
consumption and other habits, which are more frequent in males than
in females. For instance, a lower DDX3 level is more frequently
present in male patients with HCC or OSCC than that in females
(20,41).
Other protein interactions
DDX3 interacts with variable proteins to involve in
different signaling pathways, which also determines its function as
tumor suppression or oncogenic role in cancer progression. For
example, DDX3 exerts tumor suppression potentials by downregulating
Snail in colorectal cancer DLD-1 and HCT116 cells, while it
interacts with CK1ε to activate β-catenin/TCF signaling to promote
cancer progress in colorectal cancer cells (37,47).
It is likely more reasonable to jointly consider multiple
DDX3-associated proteins to analyze their influences on tumors and
the patients OS.
Cancer subtypes
DDX3 has dissimilar expression and biological
function in the specific histological subtypes of lung cancer
(34,35). DDX3 is overexpressed in some
histopathologic subtypes of lung cancer, including small cell
carcinoma, adenocarcinoma and squamous cell carcinoma, in which
DDX3 functions as an oncogene (35). In contrast, DDX3 is considered to be
a tumor suppressor in non-small cell lung cancer (34). However, the reason for this
diversity needs further study. A different subtype of colorectal
cancer cells has different sensitivity to the DDX3 inhibitor RK-33
(40). Thus, it is necessary to
develop precision medicine and therapy for various subtypes of
cancer patients based on the specific functions of DDX3.
Subcellular localization
The subcellular localization of DDX3 may shed light
on controversial roles of DDX3 in different cancers. As a
nuclear-cytoplasmic shuttling protein, DDX3 may have
multifunctional roles in transcript regulation and protein
synthesis to regulate various cellular processes. The nuclear
localization of DDX3 is altered in cutaneous squamous cell
carcinoma when compared with normal skin (15). In addition, DDX3 shows a less
nuclear expression and more obvious cytoplasmic expression in OSCC
tissues compared with the tissues adjacent to OSCC (41). Alteration of subcellular
localization from nuclear to cytoplasm perhaps leads to complete
loss of function of DDX3.
DDX3 mutation
DDX3 mutation is detected in several cancers,
including medulloblastoma, natural killer/T-cell lymphoma and
chronic lymphocytic leukemia (50–54).
Obviously, the wild-type and mutant DDX3 may exhibit different
functions in normal and cancer cells. The loss of DDX3 function by
gene mutations results in tumor pathogenesis through altered RNA
unwinding. Not only the expression level but also the gene mutation
of DDX3 determines its functional divergence of DDX3.
Potential anticancer drugs targeting
DDX3
DDX3 possesses several functional domains with
kinase activities, which is designed as drug targets by docking
small molecular inhibitors (72).
In recent years, more and more novel small molecular inhibitors
targeting DDX3 are developed as potential target chemical drugs for
cancer therapy. We illustrated several chemical compounds by
inhibiting DDX3 kinase activity, and their advantages and
disadvantages are compared. Up to now, at least five types of
compounds, including FE-15, NZ51, ketorolac salt, doxorubicin and
RK-33, have been designed to inhibit DDX3 activity (Table II).
 | Table II.Summary of potential anticancer drugs
inhibiting DDX3. |
FE-15, the first small molecule by inhibiting ATPase
activity of DDX3, was specifically designed to inhibit HIV-1
replication by targeting the RNA binding site of DDX3 (73). However, the main drawbacks of FE-15
are low activity and selectivity. It is difficult for FE-15 to
selectively recognize DDX3 due to the high homology between other
similar RNA helicases.
NZ51, one of ring-expanded nucleosides (REN), has
been identified as a potential DDX3 inhibitor by abrogating the
unwinding activity of DDX3 helicase (74). NZ51 significantly inhibits cell
motility and viability of breast cancer cells by targeting the ATP
binding pocket domain of DDX3 (31). The compound NZ51 stays active in
normoxic and hypoxic cell environments, so NZ51 can kill breast
cancer cells regardless of cellular oxygenation status.
Unfortunately, NZ51 has no significant tumor suppression efficiency
on tumor-bearing mice (31). This
is due to compound degradation of NZ51, which leads to lack of
effective drug delivery in nude mice.
Ketorolac salt is a newly discovered bioactive
compound against DDX3, which can be used as a candidate drug to
treat oral cancer (42). Ketorolac
salt interacts with the P-loop region (Gly 227, Gly 229 and Thr
231) of DDX3 by forming stable hydrogen bonds. It reduces cell
viability by inhibiting the ATPase activity of DDX3 in a
dose-dependent manner in OSCC cells. Compared with other chemical
inhibitor molecules, ketorolac salt is a type of natural bioactive
compound, which has few side effects with better biological
stability. So far, the novel ketorolac salt-derived chemical
compounds need to be further optimized to efficiently inhibit
cancer cells.
A well-known antitumor drug, doxorubicin, has shown
in vitro anticancer activity on OSCC cells by inhibiting
cellular DDX3 ATPase activity (75). In addition, doxorubicin can form
strong hydrogen bonds with DDX3. Although doxorubicin is easily
available for treating cancer, the in vivo anticancer
effects are accompanied by cardiotoxicity. A key issue is wheather
DDX3 ATPase domain is the only drug target of doxorubicin in cancer
cells.
Another small molecular inhibitor RK-33 has been
demonstrated to bind with the ATP-binding domain of DDX3 to block
its helicase activity in Ewing sarcoma, breast, colorectal,
prostate and lung cancer cells (32,35,40,45,46,79).
RK-33 causes cell cycle G1 arrest and cell apoptosis in
DDX3-overexpressing cancer cells. In addition, RK-33 suppresses
DDX3 function by impairing Wnt signaling and inhibiting the
non-homologous end joining (NHEJ) activity. RK-33 has a great many
advantages as follows. Firstly, RK-33 specifically binds to DDX3,
rather than the closely related DEAD-box proteins DDX5 and DDX17.
Secondly, cell sensitivity to RK-33 is correlated with DDX3 protein
expression levels. Therefore, RK-33 is preferentially cytotoxic to
DDX3-overexpressing cancer cells. Thirdly, RK-33 has been reported
to act as a radiosensitizer, which may reduce normal tissue
toxicity by decreasing the radiation dose. However, no structural
analysis exists to support the direct interaction of RK-33 with
DDX3 to date. However, further studies are needed to optimize drug
formulation, dose and delivery.
Among the inhibitors targeting DDX3, the most widely
studied anticancer agent is RK-33, which has favorable preclinical
anticancer activity. Women harboring breast cancer 1 (BRCA1) gene
mutation are at high risk of suffering from breast cancer, due to a
deficiency in homologous recombination to deal with DNA damage
(76). This leads cancer cells to a
greater dependency on the remaining repair pathways to deal with
DNA damage. So BRCA1 mutation carriers are particularly sensitive
to poly(ADP-ribose) polymerase (PARP) inhibitors, which inhibit the
base excision repair (BER), a single-strand break repair pathway
for DNA damage (32,77). However, cell resistance against PARP
inhibitors is widespread (32).
RK-33 has been demonstrated to impair radiation-induced DNA damage
repair by inhibiting NHEJ, a predominant mechanism for
double-strand break repair (35,78).
Therefore, the combination treatment using RK-33 and PARP
inhibitors has potential synergistic anticancer action and induces
synthetic lethality in BRCA1 pro- and deficient cells (32). However, further human clinical
trials are needed to evaluate the safety and efficacy of this
combined treatment.
In contrast, DDX3 inhibitor RK-33 has been tried to
be modify into a novel nanoparticle reagent for cancer. The poly
(lactic-co-glycolic acid) (PLGA) encapsulated RK-33 nanoparticles
are suitable for intravenous injection (79), which is a systemic delivery and
controlled-release drug delivery (80). PLGA nanoparticles loading with RK-33
may facilitate to overcome chemical hydrophobicity of RK-33.
RK-33-PLGA nanoparticles exhibit cytotoxicity to human breast
carcinoma MCF-7 cells in vitro, and a systemic retention of
RK-33 is markedly improved in mice (79).
Reports on chemical molecules designed to increase
DDX3 expression to exert tumor suppression activity are still rare.
Some p53 protein-stabilizing drugs including nutlin and bortezomib
may benefit recovery of DDX3 expression to improve the clinical
outcome of cancer patients (34).
Future perspectives
DDX3 has been confirmed to regulate the occurrence
and development of cancers and viral infection. DDX3 is one of the
inflammatory markers, which is correlated with tumorigenesis.
Multiple factors interplay with DDX3 to confer its versatile
functions.
Studies have shown that the intracellular mRNA and
protein expression of DDX3 are inconsistent in some cancer cells.
These findings suggest that post-translational modification may be
quite important in DDX3 activity. Up to now, few studies have
directly addressed the relationship between DDX3 modification and
cancer progress. So it deserves great research attention in the
future.
DDX3 shows its multiple enzyme activity including
ATPase and RNA helicase. We should distinguish which activity or
which functional domain is required for DDX3 functions to regulate
cancer. Inducing mutation at the target enzyme site by CRISPR-Cas9
and constructing a truncated DDX3 protein may help us achieve this
goal.
DDX3 is involved in multiple tumor-related signaling
pathways. Recently, a combination treatment of DDX3 and PARP
inhibitors induced cooperative effects in breast cancer. This is an
indication that multi-target therapy based on DDX3 may achieve
greater efficacy.
Several compounds have been developed to potentially
decrease DDX3 activity to inhibit tumor growth. However, these
compounds have their shortcomings. Nowadays, ligand-receptor
interaction can be simulated with computer modeling and theoretical
calculation methods. Moreover, 3D structures of DDX3 can provide
useful information for learning its molecular function mechanism
and designing drugs. The novel small molecule inhibitors targeting
DDX3 can be developed and optimized according to the previous
compounds. Nevertheless, the successful development of biomedical
technologies could ultimately translate our understanding of DDX3
functions in cancer into strategies for the inhibition of
cancer.
Acknowledgements
The present study was financially supported by the
grants from the National Key Basic Research Program of China (grant
nos. 2013CB911303 and 2011CB910703), the National Natural Sciences
Foundation of China (grant no. 31470810), the Science and
Technology Department of Sichuan Province (grant no. 2017JY0232),
the Health and Family Planning Commission of Sichuan Province
(grant no. 17ZD045) and the Guangdong Innovative Research Team
Program (grant no. 2011Y073).
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
HBV
|
hepatitis B virus
|
|
HCV
|
hepatitis C virus
|
|
EMT
|
epithelial-mesenchymal-like
transformation
|
|
HIF-1α
|
hypoxia inducible factor-1α
|
|
HRE
|
HIF responsive element
|
|
HPV
|
human papillomavirus
|
|
RFS
|
relapse-free survival
|
|
OS
|
overall survival
|
|
OSCC
|
oral squamous cell carcinoma
|
|
BRCA1
|
breast cancer 1
|
|
NHEJ
|
non-homologous end joining
|
|
PLGA
|
poly(lactic-co-glycolic acid)
|
References
|
1
|
Linder P and Jankowsky E: From unwinding
to clamping - the DEAD box RNA helicase family. Nat Rev Mol Cell
Biol. 12:505–516. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Linder P and Fuller-Pace F: Happy
birthday: 25 years of DEAD-box proteins. Methods Mol Biol.
1259:17–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tarn WY and Chang TH: The current
understanding of Ded1p/DDX3 homologs from yeast to human. RNA Biol.
6:17–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim YS, Lee SG, Park SH and Song K: Gene
structure of the human DDX3 and chromosome mapping of its related
sequences. Mol Cells. 12:209–214. 2001.PubMed/NCBI
|
|
5
|
Kotov AA, Olenkina OM, Godneeva BK,
Adashev VE and Olenina LV: Progress in understanding the molecular
functions of DDX3Y (DBY) in male germ cell development and
maintenance. Biosci Trends. 11:46–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosner A and Rinkevich B: The DDX3
subfamily of the DEAD box helicases: Divergent roles as unveiled by
studying different organisms and in vitro assays. Curr Med Chem.
14:2517–2525. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rodamilans B and Montoya G: Expression,
purification, crystallization and preliminary X-ray diffraction
analysis of the DDX3 RNA helicase domain. Acta Crystallogr Sect F
Struct Biol Cryst Commun. 63:283–286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Högbom M, Collins R, Van den Berg S,
Jenvert RM, Karlberg T, Kotenyova T, Flores A, Karlsson Hedestam GB
and Schiavone LH: Crystal structure of conserved domains 1 and 2 of
the human DEAD box helicase DDX3X in complex with the
mononucleotide AMP. J Mol Biol. 372:150–159. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soto-Rifo R and Ohlmann T: The role of the
DEAD-box RNA helicase DDX3 in mRNA metabolism. Wiley Interdiscip
Rev RNA. 4:369–385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rocak S and Linder P: DEAD-box proteins:
The driving forces behind RNA metabolism. Nat Rev Mol Cell Biol.
5:232–241. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou Z, Licklider LJ, Gygi SP and Reed R:
Comprehensive proteomic analysis of the human spliceosome. Nature.
419:182–185. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fröhlich A, Rojas-Araya B,
Pereira-Montecinos C, Dellarossa A, Toro-Ascuy D, Prades-Pérez Y,
García-de-Gracia F, Garcés-Alday A, Rubilar PS, Valiente-Echeverría
F, et al: DEAD-box RNA helicase DDX3 connects CRM1-dependent
nuclear export and translation of the HIV-1 unspliced mRNA through
its N-terminal domain. Biochim Biophys Acta. 1859:719–730. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yedavalli VS, Neuveut C, Chi YH, Kleiman L
and Jeang KT: Requirement of DDX3 DEAD box RNA helicase for HIV-1
Rev-RRE export function. Cell. 119:381–392. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lai MC, Lee YH and Tarn WY: The DEAD-box
RNA helicase DDX3 associates with export mRNPs as well as TAP and
participates in translational control. Mol Biol Cell. 19:3847–3858.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chao CH, Chen CM, Cheng PL, Shih JW, Tsou
AP and Lee YH: DDX3, a DEAD box RNA helicase with tumor
growth-suppressive property and transcriptional regulation activity
of the p21waf1/cip1 promoter, is a candidate tumor suppressor.
Cancer Res. 66:6579–6588. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee CS, Dias AP, Jedrychowski M, Patel AH,
Hsu JL and Reed R: Human DDX3 functions in translation and
interacts with the translation initiation factor eIF3. Nucleic
Acids Res. 36:4708–4718. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shih JW, Tsai TY, Chao CH and Wu Lee YH:
Candidate tumor suppressor DDX3 RNA helicase specifically represses
cap-dependent translation by acting as an eIF4E inhibitory protein.
Oncogene. 27:700–714. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oh S, Flynn RA, Floor SN, Purzner J,
Martin L, Do BT, Schubert S, Vaka D, Morrissy S, Li Y, et al:
Medulloblastoma-associated DDX3 variant selectively alters the
translational response to stress. Oncotarget. 7:28169–28182. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun M, Zhou T, Jonasch E and Jope RS: DDX3
regulates DNA damage-induced apoptosis and p53 stabilization.
Biochim Biophys Acta. 1833:1489–1497. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang PC, Chi CW, Chau GY, Li FY, Tsai YH,
Wu JC and Wu Lee YH: DDX3, a DEAD box RNA helicase, is deregulated
in hepatitis virus-associated hepatocellular carcinoma and is
involved in cell growth control. Oncogene. 25:1991–2003. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen CY, Chan CH, Chen CM, Tsai YS, Tsai
TY, Wu Lee YH and You LR: Targeted inactivation of murine Ddx3×:
Essential roles of Ddx3× in placentation and embryogenesis. Hum Mol
Genet. 25:2905–2922. 2016.PubMed/NCBI
|
|
22
|
Bol GM, Xie M and Raman V: DDX3, a
potential target for cancer treatment. Mol Cancer. 14:1882015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang H and Ryu WS: Hepatitis B virus
polymerase blocks pattern recognition receptor signaling via
interaction with DDX3: Implications for immune evasion. PLoS
Pathog. 6:e10009862010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Angus AG, Dalrymple D, Boulant S, McGivern
DR, Clayton RF, Scott MJ, Adair R, Graham S, Owsianka AM,
Targett-Adams P, et al: Requirement of cellular DDX3 for hepatitis
C virus replication is unrelated to its interaction with the viral
core protein. J Gen Virol. 9:122–132. 2010. View Article : Google Scholar
|
|
25
|
Huang JS, Chao CC, Su TL, Yeh SH, Chen DS,
Chen CT, Chen PJ and Jou YS: Diverse cellular transformation
capability of overexpressed genes in human hepatocellular
carcinoma. Biochem Biophys Res Commun. 315:950–958. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li HK, Mai RT, Huang HD, Chou CH, Chang
YA, Chang YW, You LR, Chen CM and Lee YH: DDX3 Represses stemness
by epigenetically modulating tumor-suppressive miRNAs in
hepatocellular carcinoma. Sci Rep. 6:286372016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Botlagunta M, Vesuna F, Mironchik Y, Raman
A, Lisok A, Winnard P Jr, Mukadam S, Van Diest P, Chen JH,
Farabaugh P, et al: Oncogenic role of DDX3 in breast cancer
biogenesis. Oncogene. 27:3912–3922. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Botlagunta M, Krishnamachary B, Vesuna F,
Winnard PT Jr, Bol GM, Patel AH and Raman V: Expression of DDX3 is
directly modulated by hypoxia inducible factor-1 alpha in breast
epithelial cells. PLoS One. 6:e175632011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bol GM, Raman V, van der Groep P,
Vermeulen JF, Patel AH, van der Wall E and van Diest PJ: Expression
of the RNA helicase DDX3 and the hypoxia response in breast cancer.
PLoS One. 8:e635482013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Heerma van Voss MR, Schrijver WA, Ter
Hoeve ND, Hoefnagel LD, Manson QF, van der Wall E, Raman V and van
Diest PJ; Dutch distant breast cancer metastases consortium, : The
prognostic effect of DDX3 upregulation in distant breast cancer
metastases. Clin Exp Metastasis. 34:85–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie M, Vesuna F, Botlagunta M, Bol GM,
Irving A, Bergman Y, Hosmane RS, Kato Y, Winnard PT Jr and Raman V:
NZ51, a ring-expanded nucleoside analog, inhibits motility and
viability of breast cancer cells by targeting the RNA helicase
DDX3. Oncotarget. 6:29901–29913. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Heerma van Voss MR, Brilliant JD, Vesuna
F, Bol GM, van der Wall E, van Diest PJ and Raman V: Combination
treatment using DDX3 and PARP inhibitors induces synthetic
lethality in BRCA1-proficient breast cancer. Med Oncol. 34:332017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu DW, Liu WS, Wang J, Chen CY, Cheng YW
and Lee H: Reduced p21WAF1/CIP1 via alteration of
p53-DDX3 pathway is associated with poor relapse-free survival in
early-stage human papillomavirus-associated lung cancer. Clin
Cancer Res. 17:1895–1905. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu DW, Lee MC, Wang J, Chen CY, Cheng YW
and Lee H: DDX3 loss by p53 inactivation promotes tumor malignancy
via the MDM2/Slug/E-cadherin pathway and poor patient outcome in
non-small-cell lung cancer. Oncogene. 33:1515–1526. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bol GM, Vesuna F, Xie M, Zeng J, Aziz K,
Gandhi N, Levine A, Irving A, Korz D, Tantravedi S, et al:
Targeting DDX3 with a small molecule inhibitor for lung cancer
therapy. EMBO Mol Med. 7:648–669. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Su CY, Lin TC, Lin YF, Chen MH, Lee CH,
Wang HY, Lee YC, Liu YP, Chen CL and Hsiao M: DDX3 as a strongest
prognosis marker and its downregulation promotes metastasis in
colorectal cancer. Oncotarget. 6:18602–18612. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He TY, Wu DW, Lin PL, Wang L, Huang CC,
Chou MC and Lee H: DDX3 promotes tumor invasion in colorectal
cancer via the CK1ε/Dvl2 axis. Sci Rep. 6:214832016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu DW, Lin PL, Cheng YW, Huang CC, Wang L
and Lee H: 'KRAS-induced tumor invasion in colorectal cancer via
the β-catenin/ZEB1 axis. Oncotarget. 7:22687–22699. 2016.PubMed/NCBI
|
|
39
|
Wu DW, Lin PL, Wang L, Huang CC and Lee H:
The YAP1/SIX2 axis is required for DDX3-mediated tumor
aggressiveness and cetuximab resistance in KRAS-wild-type
colorectal cancer. Theranostics. 7:1114–1132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Heerma van Voss MR, Vesuna F, Trumpi K,
Brilliant J, Berlinicke C, de Leng W, Kranenburg O, Offerhaus GJ,
Bürger H, van der Wall E, et al: Identification of the DEAD box RNA
helicase DDX3 as a therapeutic target in colorectal cancer.
Oncotarget. 6:28312–28326. 2015.PubMed/NCBI
|
|
41
|
Lee CH, Lin SH, Yang SF, Yang SM, Chen MK,
Lee H, Ko JL, Chen CJ and Yeh KT: Low/negative expression of DDX3
may predict poor prognosis in non-smoker patients with oral cancer.
Oral Dis. 20:76–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Samal SK, Routray S, Veeramachaneni GK,
Dash R and Botlagunta M: Ketorolac salt is a newly discovered DDX3
inhibitor to treat oral cancer. Sci Rep. 5:99822015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Heerma van Voss MR, van Kempen PM, Noorlag
R, van Diest PJ, Willems SM and Raman V: DDX3 has divergent roles
in head and neck squamous cell carcinomas in smoking versus
non-smoking patients. Oral Dis. 21:270–271. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Stransky N, Egloff AM, Tward AD, Kostic
AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C,
McKenna A, et al: The mutational landscape of head and neck
squamous cell carcinoma. Science. 333:1157–1160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wilky BA, Kim C, McCarty G, Montgomery EA,
Kammers K, DeVine LR, Cole RN, Raman V and Loeb DM: RNA helicase
DDX3: A novel therapeutic target in Ewing sarcoma. Oncogene.
35:2574–2583. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xie M, Vesuna F, Tantravedi S, Bol GM,
Heerma van Voss MR, Nugent K, Malek R, Gabrielson K, van Diest PJ,
Tran PT and Raman V: RK-33 Radio sensitizes prostate cancer cells
by blocking the RNA helicase DDX3. Cancer Res. 76:6340–6350. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sun M, Song L, Zhou T, Gillespie GY and
Jope RS: The role of DDX3 in regulating Snail. Biochim Biophys
Acta. 1813:438–447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liang S, Yang Z, Li D, Miao X, Yang L, Zou
Q and Yuan Y: The clinical and pathological significance of
nectin-2 and DDX3 expression in pancreatic ductal adenocarcinomas.
Dis Markers. 2015:3795682015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Miao X, Yang ZL, Xiong L, Zou Q, Yuan Y,
Li J, Liang L, Chen M and Chen S: Nectin-2 and DDX3 are biomarkers
for metastasis and poor prognosis of squamous cell/adenosquamous
carcinomas and adenocarcinoma of gallbladder. Int J Clin Exp
Pathol. 6:179–190. 2013.PubMed/NCBI
|
|
50
|
Pugh TJ, Weeraratne SD, Archer TC,
Pomeranz Krummel DA, Auclair D, Bochicchio J, Carneiro MO, Carter
SL, Cibulskis K, Erlich RL, et al: Medulloblastoma exome sequencing
uncovers subtype-specific somatic mutations. Nature. 488:106–110.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Robinson G, Parker M, Kranenburg TA, Lu C,
Chen X, Ding L, Phoenix TN, Hedlund E, Wei L, Zhu X, et al: Novel
mutations target distinct subgroups of medulloblastoma. Nature.
488:43–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jiang L, Gu ZH, Yan ZX, Zhao X, Xie YY,
Zhang ZG, Pan CM, Hu Y, Cai CP, Dong Y, et al: Exome sequencing
identifies somatic mutations of DDX3X in natural killer/T-cell
lymphoma. Nat Genet. 47:1061–1066. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang L, Lawrence MS, Wan Y, Stojanov P,
Sougnez C, Stevenson K, Werner L, Sivachenko A, DeLuca DS, Zhang L,
et al: SF3B1 and other novel cancer genes in chronic lymphocytic
leukemia. N Engl J Med. 365:2497–2506. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ojha J, Secreto CR, Rabe KG, Van Dyke DL,
Kortum KM, Slager SL, Shanafelt TD, Fonseca R, Kay NE and Braggio
E: Identification of recurrent truncated DDX3X mutations in chronic
lymphocytic leukaemia. Br J Haematol. 169:445–448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cruciat CM, Dolde C, de Groot RE, Ohkawara
B, Reinhard C, Korswagen HC and Niehrs C: RNA helicase DDX3 is a
regulatory subunit of casein kinase 1 in Wnt-β-catenin signaling.
Science. 339:1436–1441. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen HH, Yu HI, Cho WC and Tarn WY: DDX3
modulates cell adhesion and motility and cancer cell metastasis via
Rac1-mediated signaling pathway. Oncogene. 34:2790–2800. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen B, Zeng X, He Y, Wang X, Liang Z, Liu
J, Zhang P, Zhu H, Xu N and Liang S: STC2 promotes the
epithelial-mesenchymal transition of colorectal cancer cells
through AKT-ERK signaling pathways. Oncotarget. 7:71400–71416.
2016.PubMed/NCBI
|
|
58
|
Wang Y, Shi J, Chai K, Ying X and Zhou BP:
The role of snail in EMT and tumorigenesis. Curr Cancer Drug
Targets. 13:963–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhou BP, Deng J, Xia W, Xu J, Li YM,
Gunduz M and Hung MC: Dual regulation of Snail by
GSK-3beta-mediated phosphorylation in control of
epithelial-mesenchymal transition. Nat Cell Biol. 6:931–940. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sun M, Song L, Li Y, Zhou T and Jope RS:
Identification of an antiapoptotic protein complex at death
receptors. Cell Death Differ. 15:1887–1900. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang SP, Wang WL, Chang YL, Wu CT, Chao
YC, Kao SH, Yuan A, Lin CW, Yang SC, Chan WK, et al: p53 controls
cancer cell invasion by inducing the MDM2-mediated degradation of
Slug. Nat Cell Biol. 11:694–704. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chen B, Li H, Zeng X, Yang P, Liu X, Zhao
X and Liang S: Roles of microRNA on cancer cell metabolism. J
Transl Med. 10:2282012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chen Y, Fu LL, Wen X, Liu B, Huang J, Wang
JH and Wei YQ: Oncogenic and tumor suppressive roles of microRNAs
in apoptosis and autophagy. Apoptosis. 19:1177–1189. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhao L, Mao Y, Zhao Y and He Y: DDX3X
promotes the biogenesis of a subset of miRNAs and the potential
roles they played in cancer development. Sci Rep. 6:327392016.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Valiente-Echeverría F, Hermoso MA and
Soto-Rifo R: RNA helicase DDX3: At the crossroad of viral
replication and antiviral immunity. Rev Med Virol. 25:286–299.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Soulat D, Bürckstümmer T, Westermayer S,
Goncalves A, Bauch A, Stefanovic A, Hantschel O, Bennett KL, Decker
T and Superti-Furga G: The DEAD-box helicase DDX3X is a critical
component of the TANK-binding kinase 1-dependent innate immune
response. EMBO J. 27:2135–2146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gu L, Fullam A, Brennan R and Schröder M:
Human DEAD box helicase 3 couples IκB kinase ε to interferon
regulatory factor 3 activation. Mol Cell Biol. 33:2004–2015. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang X, Wang R, Luo M, Li C, Wang HX, Huan
CC, Qu YR, Liao Y and Mao X: (DEAD)-box RNA helicase 3 modulates
NF-κB signal pathway by controlling the phosphorylation of PP2A-C
subunit. Oncotarget. 8:33197–33213. 2017.PubMed/NCBI
|
|
70
|
Xiang N, He M, Ishaq M, Gao Y, Song F, Guo
L, Ma L, Sun G, Liu D, Guo D and Chen Y: The DEAD-box RNA helicase
DDX3 interacts with NF-κB subunit p65 and suppresses p65-mediated
transcription. PLoS One. 11:e01644712016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Dunford A, Weinstock DM, Savova V,
Schumacher SE, Cleary JP, Yoda A, Sullivan TJ, Hess JM, Gimelbrant
AA, Beroukhim R, et al: Tumor-suppressor genes that escape from
X-inactivation contribute to cancer sex bias. Nat Genet. 49:10–16.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Backus KM, Correia BE, Lum KM, Forli S,
Horning BD, González-Páez GE, Chatterjee S, Lanning BR, Teijaro JR,
Olson AJ, et al: Proteome-wide covalent ligand discovery in native
biological systems. Nature. 534:570–574. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Radi M, Falchi F, Garbelli A, Samuele A,
Bernardo V, Paolucci S, Baldanti F, Schenone S, Manetti F, Maga G
and Botta M: Discovery of the first small molecule inhibitor of
human DDX3 specifically designed to target the RNA binding site:
Towards the next generation HIV-1 inhibitors. Bioorg Med Chem Lett.
22:2094–2098. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yedavalli VS, Zhang N, Cai H, Zhang P,
Starost MF, Hosmane RS and Jeang KT: Ring expanded nucleoside
analogues inhibit RNA helicase and intracellular human
immunodeficiency virus type 1 replication. J Med Chem.
51:5043–5051. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Botlagunta M, Kollapalli B, Kakarla L,
Gajarla SP, Gade SP, Dadi CL, Penumadu A and Javeed S: In vitro
anti-cancer activity of doxorubicin against human RNA helicase,
DDX3. Bioinformation. 12:347–353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bianchini G, Balko JM, Mayer IA, Sanders
ME and Gianni L: Triple-negative breast cancer: Challenges and
opportunities of a heterogeneous disease. Nat Rev Clin Oncol.
13:674–690. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Tutt A, Robson M, Garber JE, Domchek SM,
Audeh MW, Weitzel JN, Friedlander M, Arun B, Loman N, Schmutzler
RK, et al: Oral poly(ADP-ribose) polymerase inhibitor olaparib in
patients with BRCA1 or BRCA2 mutations and advanced breast cancer:
A proof-of-concept trial. Lancet. 376:235–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Schwertman P, Bekker-Jensen S and Mailand
N: Regulation of DNA double-strand break repair by ubiquitin and
ubiquitin-like modifiers. Nat Rev Mol Cell Biol. 17:379–394. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Bol GM, Khan R, Heerma van Voss MR,
Tantravedi S, Korz D, Kato Y and Raman V: PLGA nanoparticle
formulation of RK-33: An RNA helicase inhibitor against DDX3.
Cancer Chemother Pharmacol. 76:821–827. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Danhier F, Lecouturier N, Vroman B, Jérôme
C, Marchand-Brynaert J, Feron O and Préat V: Paclitaxel-loaded
PEGylated PLGA-based nanoparticles: In vitro and in vivo
evaluation. J Control Release. 133:11–17. 2009. View Article : Google Scholar : PubMed/NCBI
|