Introduction
Lung cancer is a highly prevalent primary pulmonary
malignancy that originates in the bronchial mucosa and has a very
high mortality rate (1). Non-small
cell lung cancer accounts for ~80% of all lung cancer cases
(2). To date the primary treatment
of intermediate- and advanced-stage lung cancer is chemotherapy or
radiotherapy. However, the effects of these treatments are limited
with a low survival rate and serious side-effects often accompany
such treatments (3). Thus, the
identification of new agents that produce minimal side-effects and
have a good treatment efficacy is a potential mean to improve the
effects of therapy and overall patient survival.
It is well known that different parts of mulberry
(Morus) trees have pharmaceutical use. The root bark (cortex
mori) is widely used in traditional Chinese medicine. Cortex mori
contains a variety of chemical components, including flavonoids
(4), alkaloids (5), coumarin (6) and stilbenes (7–9).
Morusin is a flavone that was first isolated from the root bark of
Morus alba L. (10).
Flavones exhibit many kinds of medical functions, such as antitumor
and antioxidant activities. Research has revealed that morusin has
anti-leukemic (11,12), anti-bacterial (13), anti-convulsive (14), anti-HIV (15) and anti-Alzheimer's (16,17)
functions, though the main part of the effect of morusin lies in
its antitumor activity. Early in 1989, Yoshizawa et al
reported the inhibitory effect of morusin on tumor promotion by
teleocidin. These results indicated that morusin inhibited the
specific binding of ([3H]TPA
(12-O-tetradecanoyl-phorbol-13-acetate) to a particulate
fraction of mouse skin and inhibited teleocidin-induced activation
of protein kinase C. Thus, morusin is a novel antitumor agent
(18). Fujiki et al reported
that morusin inhibited tumor promotion in two-stage mouse skin
carcinogenicity tests and may be used for the future
chemoprevention of cancer (19). A
recent study indicated that morusin inhibited cell proliferation
and tumor growth by downregulating c-Myc in human gastric cancer
(20). Furthermore, other studies
have illustrated that morusin exerted a strong inhibitory effect on
the growth of HT-29 rectosigmoid adenocarcinoma cells via the
activation of caspases and the mitochondrial pathway of cell
apoptosis (21,22). Additionally, morusin suppressed the
signal transducer and activator of transcription 3 (STAT3) and
nuclear factor-κB (NF-κB) signaling pathways, which modulate the
expression of proteins involved in the invasion process in human
SK-Hep1 hepatocellular carcinoma cells (23). In addition, blockage of the STAT3
signaling pathway by morusin induced apoptosis and inhibited
invasion in human pancreatic tumor cells (AsPC-1, BxPC-3, MIA
PaCa-2, and PANC-1) (24). Morusin
can also exert inhibitory effects on stem cell growth and migration
in human cervical cancer (25).
Furthermore, our group has investigated the effects of morusin on
the growth and pathological changes of H22 hepatocarcinoma cells
transplanted into a mouse model. This result indicated that morusin
upregulated the expression of caspase-3 and suppressed NF-κB
signaling (26). Our group also
observed that morusin downregulated the expression of NF-κB,
upregulated caspase-3, caspase-9, p-ERK1/2 and p-JNK as well as
induced the apoptosis of human hepatoma Bel-7402 cells via the
mitochondrial and MAPK pathways (27). Although A549 cells have been
researched extensively, the study of the tumor angiogenesis-related
genes COX-2 and VEGF has been limited. In the present study, we
examined the inhibitory effects of morusin on the growth and
migration of human A549 lung cancer cells. Our results indicated
that morusin induced mitochondrial membrane permeability changes
and downregulated angiogenesis-related genes in the A549 cells. The
present study indicated that morusin was a potential drug candidate
for the chemotherapy of lung cancer.
Materials and methods
Materials
Morusin (2-[2,4-dihydroxyphenyl]-5-hydroxy-8,
8-dimethyl-3-[3-methyl-1-2-butenyl]-4H,8H-benzo[l,2-b;
3,4-b']dipyran-4-one; C25H24O6;
purity ≥98%) was purchased from Chengdu Ruifensi Biotechnology Co.,
Ltd. (Chengdu, China). Morusin was dissolved in DMSO and diluted in
water. A final concentration of <0.1% DMSO was used in the
experiments.
Cell line
The human lung cancer cell line A549 was purchased
from Nanjing KeyGen BioTech Co., Ltd. (Nanjing, China). The A549
cells were cultured in RPMI-1640 medium supplemented with 10% fetal
bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), 100 IU/ml penicillin and 100 IU/ml streptomycin
(Beyotime Institute of Biotechnology, Haimen, China). The cells
were maintained at 37°C in a humidified atmosphere of 5%
CO2.
MTT assay
MTT colorimetric assays were performed to evaluate
the cytotoxic effect of morusin on the A549 cells (28). Firstly, the cells were seeded in
96-well plates at a 1×105 cells/well; the control group
was treated with DMSO + PBS, and the other groups were treated with
morusin at 1, 10 and 30 µg/ml concentrations for 48 h at 37°C.
Following the exposure period, the cells were incubated with 20 µl
MTT (0.5 mg/ml) for an additional 4 h. The formazan crystals were
dissolved in 150 µl DMSO. The absorbance was assessed with a
spectrophotometer (SpectraMax M5; Molecular Devices, San Jose, CA,
USA) at 495 nm. Secondly, the cells were seeded in 96-well plates
at a specific density and treated with morusin at 1, 10 and 30
µg/ml concentrations at 37°C for 24, 48 and 72 h, and the remaining
steps were performed as above-described. The inhibition ratio was
calculated as follows: (Acon -
Aexp)/Acon × 100%, where Aexp and
Acon are the absorbance values of the treated and
untreated cells, respectively.
Wound-healing assay
A wound-healing assay was used to assess cell
migration. The A549 cells were seeded into 6-well plates and
incubated for 24 h. A ‘wound’ was made by manually scraping the
monolayer in the middle of the each well with a 10-µl pipette tip.
Cells were added with fresh morusin-containing medium or medium
along (control), and the cells were incubated for another 24 h.
After incubation, the cells were imaged via inverted microscopy
(AMG, Mill Creek, WA, USA) to determine the wound-healing rate.
Annexin V-FITC/PI double staining
A549 cells were treated with morusin for 24 h, and
then examined via flow cytometry using a commercial Annexin
V-FITC/PI Apoptosis Detection kit (Nanjing KeyGen BioTech).
Following treatment, the cells were collected and washed twice in
PBS, then resuspended in Annexin-binding buffer. The samples were
subsequently stained with Annexin V-fluorescein isothiocyanate
(FITC) and propidium iodide (PI), and then incubated for 15 min in
the dark. The cells were analyzed by flow cytometry
(Becton-Dickinson, San Jose, CA, USA).
Mitochondrial membrane potential (∆ψ)
assay
∆ψ was evaluated using the probe JC-1, which
reversibly changes color from green, at 535 nm, to orange, at 590
nm, as ∆ψ increases. JC-1 has both a monomer and polymer state. It
exists in monomer and green fluorescent can be detected in the form
of FL-1 channel with FITC when the cells are in high concentration.
It exists in polymer and red fluorescent can be detected in the
form of FL-2 channel with PE. In brief, the cells were harvested,
washed, resuspended in PBS, and stained with JC-1 for 15–20 min at
37°C in the dark. The resuspended solutions were then analyzed by
flow cytometry.
Determination of antioxidant
activities
The cells were treated with or without different
concentrations of morusin for 24 h. The SOD, GSH and T-AOC
concentrations were detected with the appropriate T-SOD, GSH-PX and
T-AOC commercial kits (Jiancheng Institute of Biotechnology, Co.,
Ltd, Nanjing, China), according to the manufacturer's
instructions.
Real-time PCR analysis
Total RNA was extracted from the cells using
TRIzol® reagent (Life Technologies; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
The concentration of total RNA was quantified by the absorbance at
260 nm using a NanoDrop spectrophotometer (Thermo Fisher
Scientific, Inc.). Reverse transcription was performed with Prime
Script™ RT reagent kit (Takara Bio, Inc., Otsu, Japan)
and the cDNA was prepared for real-time PCR. Real-time PCR was
performed in triplicate with SYBR Premix Ex Taq™ II RT-PCR kit
(Takara Bio) using ABI 7900HT Fast Real-Time PCR system (Applied
Biosystems Inc., Foster City, CA, USA). The RT-PCR amplification
conditions were as follows: 95°C for 5 min; 40 cycles at 95°C for
15 sec, 60°C for 20 sec and 72°C for 40 sec. The sequences of the
primers are listed in Table I.
 | Table I.The primer sequences of the genes
used in RT-PCR. |
Table I.
The primer sequences of the genes
used in RT-PCR.
| Gene | Primer sequences
(5′ to 3′) |
|---|
| COX-2 | Forward:
AGCATCTACGGTTTGCTGTG |
|
| Reverse:
CCTGTTTAAGCACATCGCAT |
| VEGF | Forward:
GCAGCTTGAGTTAAACGAACG |
|
| Reverse:
GGTTCCCGAAACCCTGAG |
Statistical analysis
Data are presented as the mean ± standard deviation
(SD). Statistical significance was tested using one-way ANOVA with
Origin 7.5 software (OriginLab, Northampton, MA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Morusin inhibits the growth of human
lung cancer cells
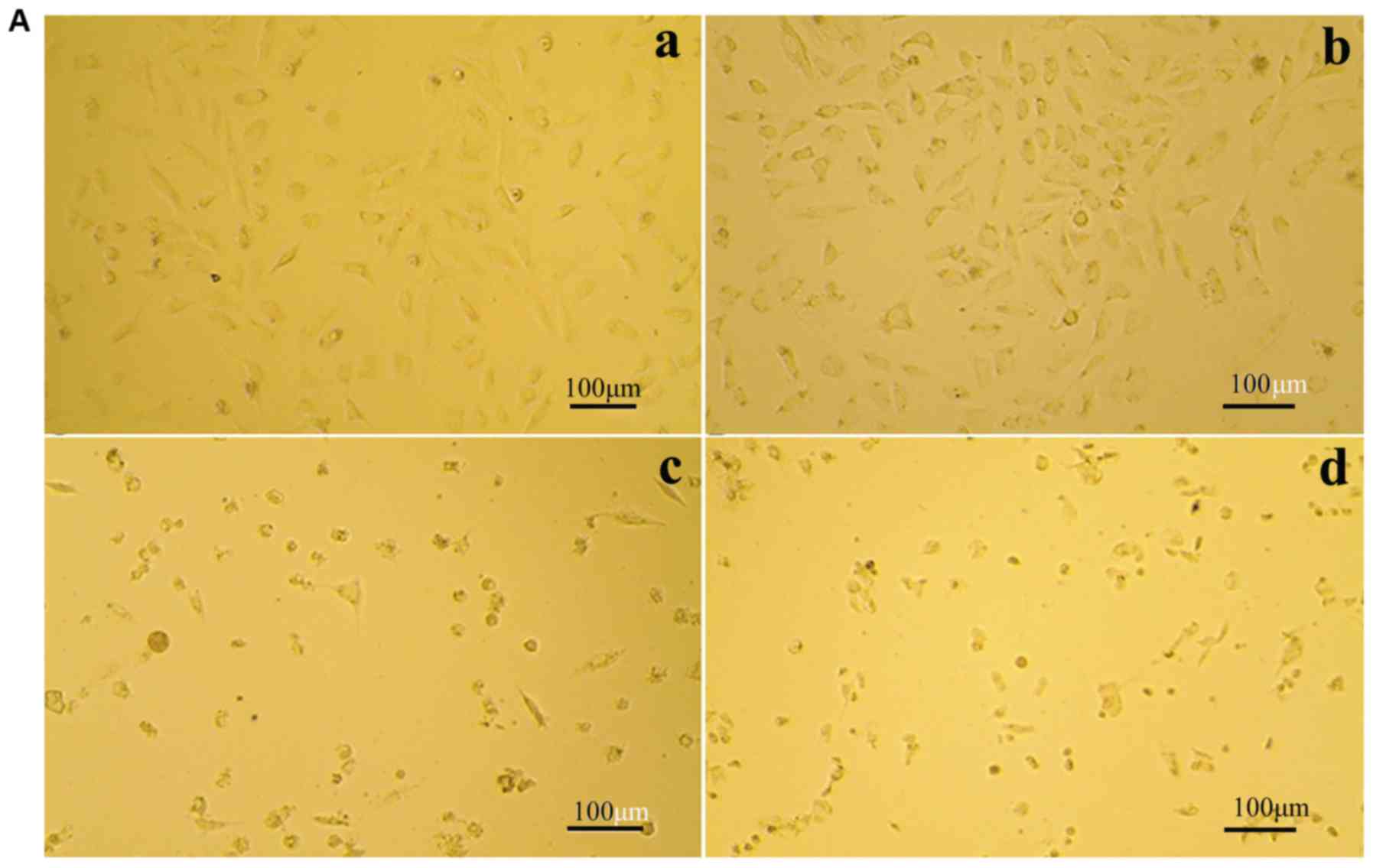
The ability of morusin to inhibit the growth of the
human A549 lung cancer cell line was evaluated. Following treatment
with the various concentrations of morusin (1, 10 and 30 µg/ml) for
24 h, the A549 cells were observed by inverted microscopy (Fig. 1A). The cells of the control group
(Fig. 1A-a) were adherent, shiny
and exhibited clear polygon forms. However, after treatment with 10
or 30 µg/ml morusin (Fig. 1A-b, c and
d), morphological changes were observed, including cell
shrinkage and cavitation, and the numbers of cells were
significantly decreased. Furthermore, the majority of the cells
were floating, and the number of adherent cells had decreased.
The MTT assay was used to determine the cytotoxicity
of morusin against the A549 human lung cancer cells. The results
are displayed in Fig. 1B-e. Morusin
inhibited the growth of A549 cells in a dose-dependent manner. All
the treatment groups exhibited significant differences (P<0.01)
compared with the control group. After treatment with 30 µg/ml
morusin, the viability of the A549 cells was only 3.21%, and the
inhibition rate had reached 96.79%. The results revealed that, when
the morusin concentrations reached 30 µg/ml, the inhibition rate
did not decrease with time. This finding indicated that morusin
played an important role in the inhibition of A549 cells. As
demonstrated in Fig. 1B-f, the
viability of the A549 cells was evaluated after treatment with
different concentrations of morusin for 24, 48 and 72 h, and when
the concentration of morusin was 30 µg/ml, cell viability did not
reduce with time, indicating that the morusin treatment had reached
its maximum effect. At concentrations below 30 µg/ml, and with
increasing duration, morusin markedly decreased the cell survival
rates and increased the inhibition rates of the A549 cells.
Morusin inhibits the migration of A549
cells
As displayed in Fig.
2, A549 cells exhibited wound closure activity, but this
activity was significantly reduced in the morusin-treated groups in
a dose-dependent manner. The degree of wound healing in the
untreated group was greater than that in each of the
morusin-treated groups. These results indicated that morusin
treatment significantly inhibited the migration rates of A549
cells.
Effects of morusin on the apoptosis
and ∆ψ of A549 cells
Annexin V/PI staining was performed to assess
morusin-induced apoptosis. As displayed in Fig. 3, significant increases in the ratio
of apoptotic cells were observed in the cells treated with
different concentrations of morusin (P<0.01), compared with that
of the morusin-untreated cells. The apoptotic rate of the control
group was 7.08%, whereas cells treated with 30 µg/ml morusin
exhibited an apoptotic rate of 70.80%. The changes in the apoptotic
rates in morusin-treated cells were dose-dependent.
The mitochondrion is a key organelle in the process
of apoptosis. The loss of ∆ψ is putatively the initial event that
leads to apoptosis (29). To
elucidate the association between ∆ψ and morusin-induced apoptosis,
we examined the loss of ∆ψ by flow cytometry. As illustrated in
Fig. 4, ∆ψ was high and JC-1 was
mostly in the form of polymers in the control group. Cells were
treated with different concentrations of morusin for 24 h, and
significant decreases in ∆ψ and increases of green fluorescence
were observed in the A549 cells, in comparison with the untreated
cells. Collectively, these results revealed that morusin induced
the loss of ∆ψ, which caused changes in the permeability of the
mitochondria and eventually induced cell apoptosis.
Morusin increases the antioxidant
activities of A549 cells
The levels of antioxidant activity in
morusin-treated A549 cells were examined in the present study. As
shown in Fig. 5, the antioxidant
enzyme levels were increased following treatment with different
concentrations of morusin. The results showed that morusin clearly
and significantly increased the activity levels of SOD, GSH-PX and
T-AOC, in comparison with those of the control group (P<0.01),
in a dose-dependent manner, and thus increased the antioxidant
abilities of the A549 cells.
Morusin reduces the expression of the
VEGF gene and COX-2 gene in A549 cells
VEGF is a key regulator of vascular permeability and
promotes endothelial cell proliferation and migration (30,31).
The RT-PCR results revealed that the expression of VEGF was reduced
by 18, 38 and 56% following treatment of A549 cells with 1, 10 and
30 µg/ml morusin, respectively (Fig.
6A). This indicated that morusin suppressed expression of the
VEGF gene, which is related to angiogenesis, in a dose-dependent
manner. As displayed in Fig. 6B,
the expression of the COX-2 gene was high in the control group.
After the cells were treated with different concentrations of
morusin, the expression of the COX-2 gene was decreased in a
dose-dependent manner. In summary, these results indicated that
morusin inhibited the expression of VEGF and COX-2 genes, and thus
affected tumor angiogenesis in A549 cells.
Discussion
The A549 human lung carcinoma cell line possesses a
vigorously proliferative ability. The dysregulation and indefinite
proliferation of cells is the foundation of the occurrence and
development of tumors. Thus, analysis of the cell cycle in tumors
is often used for anticancer drug screening and the evaluation of
anticancer effects. Certain natural products play important roles
in the development of drugs. For example, flavonoids exhibit a
variety of biological activities that include anti-proliferative
and anti-angiogenic activities. Many studies have demonstrated that
morusin, an isoprenylated flavone from the mulberry tree, exerts
inhibitory effects on cell proliferation. Therefore, morusin is
regarded as a chemopreventive agent. The present study observed the
cytotoxic effect of morusin on the A549 cell line in
vitro.
The MTT assay is one of the most commonly used
methods for assessing cell survival rates. The results of the
present study indicated that morusin significantly inhibited the
proliferation of A549 cells in a concentration-dependent manner.
Within the 1–30 µg/ml concentration range, the cell survival rates
gradually decreased with increasing concentrations and durations of
morusin treatment. Since those concentrations affected cell
viability, the effects observed in subsequent experiments may be
due to the effects of cell viability. Chen et al found that
the survival rate of SK-Hep1 cells was significantly reduced
following treatment with 25–70 µM morusin for 24 h (22). These experimental results were
consistent with those of our study. Additionally, the morphological
changes of the A549 cells were observed by inverted microscopy
following the MTT assays. The results revealed that the numbers of
adherent cells were significantly decreased in the morusin-treated
groups in comparison with that of the control group. In addition,
cell shrinkage and cracking were also observed in morusin-treated
groups. Furthermore, Annexin V-FITC/PI double staining was used to
detect cell apoptosis. The analyses indicated that the cells were
primarily in apoptotic stage following morusin treatment. With
increasing concentrations of morusin, the numbers of apoptotic
cells significantly increased, a result that was consistent with
the results of the MTT assay.
Mitochondria occupy a core position in cell survival
and play a vital role in the cell apoptosis pathway. Mitochondrial
morphology and functions are substantially altered when cells
undergo apoptosis. The loss of ∆ψ can result in an increase in
mitochondrial permeability and the release of cytochrome c.
Cytochrome c and apoptotic proteases are activating factors,
and can combine with caspase-9 to form a polymer complex that
subsequently activates caspase-3. Activated caspase-3 induces the
caspase-mediated apoptotic pathway, subsequently causing apoptosis
(32,33). In summary, morusin induces the loss
of ∆ψ and causes cell apoptosis in human A549 lung carcinoma cells
via the mitochondrial pathway.
Reactive oxygen species (ROS) are a series of
substances produced in the process of cell aerobic metabolism and
have a toxic effect on cells. The overproduction of ROS, or
reductions in ROS scavenging abilities, can cause oxidative stress
in cells. Oxidative stress can lead to increases in tissue lipid
peroxidation, which then causes oxidative damage to the DNA and,
eventually, abnormal protein expression. The results of the present
study revealed that morusin increased the levels of SOD, GSH-PX and
T-AOC. Furthermore, these activities were enhanced with increasing
concentrations of morusin and were significantly increased in
comparison with that of the control group (P<0.01). The results
indicated that morusin increased the ROS scavenging abilities of
the cells and reduced oxidative stress by increasing the activities
of SOD and GSH-PX, as well as the level of T-AOC to achieve its
antitumor effects.
The migration of tumor cells is a key part of tumor
infiltration and migration. Inhibition of the migratory abilities
of tumor cells can reduce the recurrence rate and prolong the
survival of postoperative tumor patients. The results of the
present study demonstrated that the wound-healing rates of the
morusin-treated cells were much slower than those of the untreated
cells, indicating a reduced migratory capacity.
Modern medical research has revealed that tumor
angiogenesis is essential for tumor growth and migration (34). Tumor vasculature can provide
sufficient nutrition for tumor cells. A series of angiogenic
factors exist in the human body, among which COX-2 and VEGF are the
most closely related to tumor angiogenesis (35). COX-2 is a type of induction enzyme
that is only generated by stimulation from associated cytokines,
tumor inducers and tumor genes. Cao et al have reported that
COX-2 is a common feature of neoplasms, particularly those with an
epithelial origin (36), and that
COX-2 inhibitors exhibit good antitumor effects (37). VEGF is an important
angiogenesis-promoting factor that can promote the growth and
migration of tumor cells and is highly expressed during tumor
angiogenesis (38) and in tumor
cells (39). A number of Chinese
herbal components have demonstrated that anti-angiogenic effects
can be achieved by targeting VEGF-induced angiogenesis (40). In the present study, RT-PCR results
indicated that COX-2 and VEGF genes in the morusin-treated A549
cells were significantly decreased in a concentration-dependent
manner. Overall, these results indicated that morusin could inhibit
tumor angiogenesis and affect the migration and invasion of tumor
cells.
In conclusion, morusin significantly inhibited the
proliferation of human A549 lung carcinoma cells and promoted cell
apoptosis, as demonstrated by MTT assays and flow cytometry. In
addition, morusin induced cell apoptosis via the loss of
mitochondrial membrane potential. Furthermore, some antioxidant
abilities were detected, indicating that morusin can enhance
antioxidant capacity. Furthermore, the research further examined
the effects of morusin on the genes that were associated with tumor
angiogenesis. The results indicated that, following treatment with
various concentrations of morusin, the expression levels of the
COX-2 and VEGF genes in the A549 cells were significantly reduced
in a dose-dependent manner. In general, morusin affected the
invasion and migration of tumor cells via the inhibition of tumor
angiogenesis. However, the specific mechanisms remain unclear and
require further research.
Acknowledgements
Not applicable.
Funding
The present study was supported by the earmarked
fund (CARS-18-ZJ0502) from the China Agriculture Research System
and funding from the Priority Academic Program Development of
Jiangsu Higher Education Institutions, P.R. China.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YQZ conceived this study. YL and SW performed most
of the experiments and constructed the database. XLY performed some
experiments, the statistical analysis, and wrote the manuscript.
YQZ revised the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Soochow University.
Consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beadsmoore CJ and Screaton NJ:
Classification, staging and prognosis of lung cancer. Eur J Radiol.
45:8–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:742005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim SY, Gao JJ, Lee WC, Ryu KS, Lee KR and
Kim YC: Antioxidative flavonoids from the leaves of Morus alba.
Arch Pharm Res. 22:81–85. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asano N, Yamashita T, Yasuda K, Ikeda K,
Kizu H, Kameda Y, Kato A, Nash RJ, Lee HS and Ryu KS:
Polyhydroxylated alkaloids isolated from mulberry trees (Morus
alba L.) and silkworms (Bombyx mori L). J Agric Food
Chem. 49:4208–4213. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oh H, Ko EK, Jun JY, Oh MH, Park SU, Kang
KH, Lee HS and Kim YC: Hepatoprotective and free radical scavenging
activities of prenylflavonoids, coumarin, and stilbene from
Morus alba. Planta Med. 68:932–934. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qiu F, Komatsu K, Kawasaki K, Saito K, Yao
XS and Kano Y: A novel stilbene glucoside, oxyresveratrol
3′-O-β-glucopyranoside, from the root bark of Morus
alba. Planta Med. 62:559–561. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Piao SJ, Qiu F, Chen LX, Pan Y and Dou DQ:
New stilbene, benzofuran, and coumarin glycosides from Morus
alba. Helvetica Chimica Acta. 92:579–587. 2009. View Article : Google Scholar
|
|
9
|
Piao SJ, Chen LX, Kang N and Qiu F:
Simultaneous determination of five characteristic stilbene
glycosides in root bark of Morus alba L. (Cortex mori) using
high-performance liquid chromatography. Phytochem Anal. 22:230–235.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nomura T, Fukai T, Yamada S and Katayanagi
M: Studies on the constituents of the cultivated mulberry tree. I.
Three new phenylflavones from the root bark of Morus alba L.
Chem Pharm Bul. 26:1394–1402. 1978. View Article : Google Scholar
|
|
11
|
Ferlinahayati, Syah YM, Juliawaty LD,
Achmad SA, Hakim EH, Takayama H, Said IM and Latip J: Phenolic
constituents from the wood of Morus australis with cytotoxic
activity. Z Naturforsch C. 63:35–39. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suhartati T, Yandri AS, Hadi S and Jhons
FS: Morusin, a Bioactive Compound from the Root Bark of Artocarpus
dadah. Eur J Sci Res. 38:643–648. 2009.
|
|
13
|
Mazimba O, Majinda RRT and Motlhanka D:
Antioxidant and antibacterial constituents from Morus nigra. Afc J
Pharm Pharmaco. 5:751–754. 2011.
|
|
14
|
Guptaa G, Duaa K, Kazmi I and Anwar F:
Anticonvulsant activity of Morusin isolated from Morus alba:
Modulation of GABA receptor. Biomed Aging Pathology. 4:29–32. 2014.
View Article : Google Scholar
|
|
15
|
Luo SD, Nemec J and Ning BM: Anti-HIV
flavonoids from Morus alba. Acta Botanica Yunnanica.
17:89–95. 1995.
|
|
16
|
Cho JK, Ryu YB, Curtis-Long MJ, Kim JY,
Kim D, Lee S, Lee WS and Park KH: Inhibition and structural
reliability of prenylated flavones from the stem bark of Morus lhou
on b-secretase (BACE-1). Bioorg Med Chem Lett. 21:2945–2948. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim JY, Lee WS, Kim YS, Curtis-Long MJ,
Lee BW, Ryu YB and Park KH: Isolation of cholinesterase-inhibiting
flavonoids from Morus lhou. J Agr Food Chem. 59:4589–4596. 2011.
View Article : Google Scholar
|
|
18
|
Yoshizawa S, Suganuma M, Fujiki H, Fukai
T, Nomura T and Sugimura T: Morusin, isolated from root bark of
Morus alba L., inhibits tumour promotion of teleocidin.
Phytotherapy Res. 3:193–195. 1989. View Article : Google Scholar
|
|
19
|
Fujiki H, Suganuma M, Takagi K, Yoshizawa
S, Furuya HS, Yoshizawa S, Nishiwaki S, Kobayashi M, Okuda T,
Nomura T, et al: Sarcophytols A and B, (−)-epigallocatechin gallate
(EGCG), and morusin, anticarcinogenesis radiation Protection.
2:357–362. 1991.
|
|
20
|
Wang F, Zhang D, Mao J, Ke XX, Zhang R,
Yin C, Gao N and Cui H: Morusin inhibits cell proliferation and
tumor growth by down-regulating c-Myc in human gastric cancer.
Oncotarget. 8:57187–57200. 2017.PubMed/NCBI
|
|
21
|
Zhao JL: Morusin induces human colorectal
cancer cell death via apoptosisNational Cheng Kung University;
2003
|
|
22
|
Lee JC, Won SJ, Chao CL, Wu FL, Liu HS,
Ling P, Lin CN and Su CL: Morusin induces apoptosis and suppresses
NF-κB activity in human colorectal cancer HT-29 cells. Biochem
Biophys Res Commun. 372:236–242. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin WL, Lai DY, Lee YJ, Chen NF and Tseng
TH: Antitumor progression potential of morusin suppressing STAT3
and NFκB in human hepatoma SK-Hep1 cells. Toxicol Lett.
232:490–498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim C, Kim JH, Oh EY, Nam D, Lee SG, Lee
J, Kim SH, Shim BS and Ahn KS: Blockage of STAT3 signaling pathway
by morusin induces apoptosis and inhibits invasion in human
pancreatic tumor cells. Pancreas. 45:409–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Guo H, Yang L, Dong L, Lin C,
Zhang J, Lin P and Wan X: Morusin inhibits human cervical cancer
stem cell growth and migration through attenuation of NF-κB
activity and apoptosis induction. Mol Cell Biochem. 379:7–18. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wan LZ, Ma B and Zhang YQ: Preparation of
morusin from Ramulus mori and its effects on mice with transplanted
H22 hepatocarcinoma. Biofactors. 40:636–645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ding B, Lv Y and Zhang YQ: Anti-tumor
effect of morusin from the branch bark of cultivated mulberry in
Bel-7402 cells via the MAPK pathway. Rsc Adv. 6:17396–17404. 2016.
View Article : Google Scholar
|
|
28
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Medthods. 65:55–63. 1983. View Article : Google Scholar
|
|
29
|
Petit PX, Susin SA, Zamzami N, Mignotte B
and Kroemer G: Mitochondria and programmed cell death: Back to the
future. FEBS Lett. 396:7–13. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Prager GW, Lackner EM, Krauth MT, Unseld
M, Poettler M, Laffer S, Cerny-Reiterer S, Lamm W, Kornek GV,
Binder BR, et al: Targeting of VEGF-dependent transendothelial
migration of cancer cells by bevacizumab. Mol Oncol. 4:150–160.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gong C, Xu C, Ji L and Wang Z: A novel
semi-synthetic andrographolide analogue A5 inhibits tumor
angiogenesis via blocking the VEGFR2-p38/ERK1/2 signal pathway.
Biosci Trends. 7:230–236. 2013.PubMed/NCBI
|
|
32
|
Chen LH, Fang J, Li H, Demark-Wahnefried W
and Lin X: Enterolactone induces apoptosis in human prostate
carcinoma LNCaP cells via a mitochondrial-mediated,
caspase-dependent pathway. Mol Cancer Ther. 6:2581–2590. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen M, Guerrero AD, Huang L, Shabier Z,
Pan M, Tan TH and Wang J: Caspase-9-induced mitochondrial
disruption through cleavage of anti-apoptotic BCL-2 family members.
J Biol Chem. 282:33888–33895. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sikora J: Tumor angiogenesis in human lung
adenocarcinoma. Cancer. 76:915–916. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim TJ, Lee YS, Kang JH, Kim YS and Kang
CS: Prognostic significance of expression of VEGF and Cox-2 in
nasopharyngeal carcinoma and its association with expression of
C-erbB2 and EGFR. J Surg Oncol. 103:46–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rundhaug JE, Mikulec C, Pavone A and
Fischer SM: A role for cyclooxygenase-2 in ultraviolet
light-induced skin carcinogenesis. Mol Carcinog. 46:692–698. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cao Y and Prescott SM: Many actions of
cyclooxygenase-2 in cellular dynamics and in cancer. J Cell
Physiol. 190:279–286. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bremnes RM, Camps C and Sirera R:
Angiogenesis in non-small cell lung cancer: The prognostic impact
of neoangiogenesis and the cytokines VEGF and bFGF in tumours and
blood. Lung Cancer. 51:143–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ferrara N and Kerbel RS: Angiogenesis as a
therapeutic target. Nature. 438:967–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Izuta H, Shimazawa M, Tsuruma K, Araki Y,
Mishima S and Hara H: Bee products prevent VEGF-induced
angiogenesis in human umbilical vein endothelial cells. BMC
Complement Altern Med. 9:452009. View Article : Google Scholar : PubMed/NCBI
|