Introduction
Uterine cervical cancer (UCC) is a global health
problem and one of the most challenging medical issues, due to the
complexity of the pathophysiological mechanisms that are involved
(1). This complexity limits the
ability to determine molecular, biochemical and clinical markers
that allow for adequate classification of precursor lesions. At
present, clinicians cannot specify the evolution of cancer once the
process has been initiated.
Due to the almost constant presence of the human
papillomavirus (HPV) in premalignant and malignant lesions of the
cervix, this infection is among the most prevalent sexually
transmitted diseases. It is well known that variation among the
>100 viruses of the HPV family is a determining factor in the
immune response of the host. HPV types 16 and 18 continue to be the
principal causal agents; however, the effects of co-infection with
several subtypes remain unknown; this phenomenon is currently more
frequently observed (2,3). The routine use of cytology for the
early diagnosis of dysplastic lesions, as well as the
administration of HPV vaccines to women who have not had prior
contact with HPV, have not exhibited a significant impact on the
mortality index generated by the disease (4–6).
Previous studies have revealed that HPV is associated with UCC;
however, its presence is insufficient, and it requires cofactors
for its permanence and for the initiation of carcinogenesis
(7,8).
Numerous studies using transgenic mouse models have
significantly demonstrated the requirement of estrogens in
HPV-driven UCC (5–9). The cervix, particularly the
transformation zone of the uterine ectocervix, which serves as the
site of initiation of tumorigenesis, is an estrogen-responsive
tissue (10). Epidemiological
research has demonstrated the higher risk of developing UCC as a
result of a long-term oral contraceptive use and multiple
pregnancies (11–13).
Steroid hormones achieve their biological effects
through receptors. Current research indicates the existence of two
types of nuclear estrogen receptor (ER): ERα and ERβ, of which
there are several isoforms (14,15).
Investigations using clinical specimens indicated the presence of a
differential ER expression pattern in cervical tumor epithelium
(9). Recently, the role of stromal
ERα in the progression of UCC has gained prominence (16,17).
The signaling complex of prolactin (PRL) and PRL
receptor (PRLR), using both endocrine and local paracrine/autocrine
pathways, has also been implicated in the pathology of UCC
(18–20). Initial studies analyzed the
association between PRL/PRLR and the signaling pathways involved in
proliferation and apoptosis of UCC cell lines. The expression
levels of both PRL and PRLR were revealed to be increased in the
cell lines, and are associated with cell survival; in addition,
treatment with 200 ng/ml human recombinant PRL (hPRL) exerts a
protective effect against etoposide-induced apoptosis (18,19).
It has also been revealed that activation of signal transducer and
activator of transcription 3 (STAT3) is required for the
anti-apoptotic effects of hPRL (20), and PRLR expression is increased in
histopathological samples of patients with UCC compared with in
normal cervical tissues (20).
Extrapituitary sources of PRL in humans include
uterine and brain tissues, lacrimal, sweat and adrenal glands,
immune and mammary epithelial cells, skin fibroblasts, and the
kidneys and ovaries (21). However,
the mechanism by which local PRL contributes to PRL-induced
responses is unclear. Larrea et al evaluated the production
of a 60 kDa PRL isoform by human peripheral mononuclear cells from
healthy subjects and patients with systemic lupus erythematosus
(SLE); immunoreactivity was preferentially detected in subjects
with SLE (22).
Our previous study identified the presence of the 60
kDa PRL in the protein extracts and supernatant of three cell lines
derived from UCC (HeLa, SiHa and C33A). Conversely, this isoform
was absent in the extracts and supernatant of an immortalized
keratinocyte cell line (HaCaT) (18). Following isolation of 60 kDa PRL,
its bioactivity was tested. Using the Nb2 cell line as a model
system to analyze the effects of hPRL on cell mitogenesis,
stimulation with 60 kDa PRL was revealed to increase cell growth
(23,24). Local 60 kDa PRL has been revealed to
exert bioactive effects and to phosphorylate STAT3 (25).
The association between estrogen and PRL has been
reported. Estrogen regulates PRL at the transcriptional level,
acting on the specific sequence on its promoter via its function as
a regulatory steroid hormone (26).
Duan et al identified a functional, non-canonical estrogen
responsive element (ERE) and an activator protein 1 (AP-1) site
located in the PRL distal promoter, and suggested that the effects
of ERs, as well as ERE and AP1 transcription factors, on the
regulation of autocrine/paracrine PRL in the human breast are
critical for the progression of breast cancer (27). Furthermore, the role of
cross-regulation of ERα levels by endogenous PRL in increasing
estrogen responsiveness in breast cancer cells has been established
(28).
Ki67 is a well-known cell proliferation marker,
which has been proposed as a useful tool to distinguish between
dysplastic and non-dysplastic lesions (29). In addition, the overexpression of
B-cell lymphoma 2 (Bcl-2) has been reported in premalignant and
malignant lesions of the cervix, and is associated with the
development of invasive cervical disease (30,31).
Based on these findings, the present study evaluated
the co-expression of ERs and PRLR in UCC samples and precursor
lesions. In addition, the association between estrogen and PRL in
cancer was analyzed by determining the effects of 17β-estradiol
(E2) and 60 kDa PRL stimulation on the cell survival and metabolism
of UCC cell lines, and of HaCaT cells transduced with HPV 16 and 18
E6/E7 oncogenes.
Materials and methods
Reagents
A polyclonal antibody against ERα (MC-20; cat. no.
sc-542) and monoclonal antibodies against PRL (E-9; cat. no.
sc-48383) and ERβ (B-3; cat. no. sc-373853) were obtained from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Monoclonal
antibodies against Ki67 (30-9; cat. no. 790-4286) and Bcl-2 (124
cat. no. 790-4464) were obtained from Ventana Medical Systems, Inc.
(Tucson, AZ, USA). Deparaffinization, antigen retrieval, cell
conditioning and immunohistochemical staining were conducted using
BenchMark ULTRA instrument and BenchMark ULTRA reagents, solutions,
kits and accessories from Ventana Medical Systems, Inc. µMACS™
Protein G MicroBeads MultiMACS™ Protein G kit, which was used for
the isolation of 60 kDa PRL, was provided by Miltenyi Biotec GmbH
(Bergisch Gladbach, German). E2 and MTT were purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany), and cisplatin (cat.
no. 479306) was purchased from Sigma-Aldrich; Merck KGaA.
Cell culture
HeLa, SiHa and C-33A cancer cell lines were obtained
from American Type Culture Collection (Manassas, VA, USA). The
non-tumorigenic keratinocyte HaCaT cell line was kindly provided by
Dr Petra Boukamp from the German Cancer Research Center (DKFZ,
Heidelberg, Germany). SiHa is a cell line derived from a grade II
squamous cell carcinoma of the cervix that expresses HPV 16; this
cell line is adherent and has a doubling time of 26 h. HeLa is cell
line derived from an adenocarcinoma of the cervix, which is
positive for HPV 18, and has a doubling time of 24 h. The C-33A
cell line was used because it is derived from a carcinoma of the
cervix and does not express HPV; this cell line has a doubling time
of 29 h. The HaCaT cell line is a line of immortalized
keratinocytes; this cell line is adherent, HPV-negative and has a
doubling time of 29 h.
Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% of charcoal stripped fetal bovine serum,
penicillin G (10,000 U/ml), streptomycin (10,000 µg/ml) and
amphotericin B (250 µg/ml) was used to grow all cells. Media,
charcoal stripped serum, penicillin G and streptomycin were
obtained from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA,
USA). Cells were stored in a water-jacketed incubator at 37°C in an
atmosphere containing 5% CO2; cells were grown without
exceeding 80% confluence.
Tissue samples
The present study was approved by the Ethical
Investigation and Biosecurity Committee of the University Center of
Health Sciences at the University of Guadalajara (Reference Number
C.I. 093/13 CUCS; Guadalajara, Mexico). Written informed consent
was obtained from all patients included in the present study.
Tissue samples were obtained from the Department of Pathology at
the Hospital Civil Fray Antonio Alcalde (Guadalajara, Mexico). The
sample collection period ran between August 2013 and February 2017.
After collection, tissues were fixed in 4% formalin and embedded in
paraffin. A total of 52 specimens were examined, which were
selected according to tissue integrity, and were classified by
experienced pathologists. According to the proportion of epithelial
thickness that presents mature and differentiated cells, 13
biopsies were characterized as cervical intraepithelial neoplasia
(CIN) I, 12 as CIN II and 12 as CIN III. Of 15 UCC cases, 11 were
diagnosed as epidermoid and 4 were characterized as
adenocarcinomas. The mean age for patients with CIN was 28.16
years, and was 33 years for patients with cancer.
Automated immunohistochemistry
Serial sections (4 µm) from the formalin-fixed
paraffin-embedded blocks were used for the detection of ERα, ERβ,
PRLR, Ki67 and Bcl-2 using immunohistochemical methods. ERα, ERβ
and PRLR antibodies were used at a concentration of 1:50 in 100 µl
of a 1X concentrated solution of Tris-buffered saline with 0.1%
Tween-20 (TBS-T); Ki67 and Bcl-2 antibodies were obtained
prediluted by Ventana Medical Systems, Inc., and were optimized for
use on Ventana staining platforms. Slides were loaded into the
BenchMark ULTRA instrument (cat. no. N750-BMKU-FS 05342716001;
Ventana Medical Systems, Inc.). Sections were stained with
hematoxylin and eosin and underwent Ventana OptiView DAB IHC
detection; the OptiView Amplification kit was used in conjunction
with the OptiView DAB IHC Detection kit (both Ventana Medical
Systems, Inc.) to increase staining intensity. The entire process
lasted ~2.5 h. As a negative control, the primary antibody was
omitted, and breast cancer tissues obtained from the Department of
Pathology at the Hospital Civil Fray Antonio Alcalde (Guadalajara,
Mexico) were used as a positive control.
Specimens were analyzed under an optical microscope
(Carl Zeiss AG, Oberkochen, Germany) and digital image files were
obtained at ×40 magnification. For digital evaluation, five fields
from each lesion were selected, and for digital analysis, the cell
counter function was used on Image-pro Plus 6.0®
software (Media Cybernetics, Inc., Rockville, MD, USA), in which
the color intensity of positive objects (brown) was manually preset
for each pattern (pixel per pixel) based on the
hue-saturation-intensity histogram. The samples in which optical
density was very high for the expression of the protein were
characterized as intense staining, whereas samples in which the
expression of the protein was positive, regardless of density, were
characterized as positive staining.
Isolation and purification of 60 kDa
PRL
The 60 kDa PRL present in the UCC cell supernatants
was isolated using a magnetic bead kit (Protein G MicroBeads
MultiMACS™), according to the manufacturer's protocol. The presence
of correctly separated PRL was determined using polyacrylamide gel
electrophoresis and silver nitrate staining.
After isolation, 60 kDa PRL was purified and
concentrated using 50 kDa molecular cut-off filters
(Amicon® Ultra 0.5 ml centrifugal filters). Filtration
at 4°C was performed under the following conditions: Filtration
phase, 14,000 × g for 30 min; recovery phase, 1,000 × g for 2 min.
Quantification of 60 kDa PRL was conducted using a Thermo
Scientific NanoDrop 2000c Spectrophotometer (NanoDrop Technologies;
Thermo Fisher Scientific, Inc., Wilmington, DE, USA).
Hormone stimulation
E2 was added to cells at a concentration of 10 nM,
and the autocrine 60 kDa PRL was added at a concentration of 200
ng/ml. Cisplatin (1 µg/ml) was used as a cell death control, since
it is a common chemotherapeutic agent administered to patients with
UCC. After applying the stimuli, cells were stored in a
water-jacketed incubator at 37°C in an atmosphere containing 5%
CO2. Cell proliferation and mitochondrial function were
evaluated after 72 h. Apoptosis was detected after 48 h of
stimulation.
Cell proliferation
The cellular proliferation of HeLa, SiHa and C33A
cells was assessed using the xCELLigence platform (Roche Applied
Science, Penzberg, Germany), which measures in real time the number
of cells attached to the bottom of a modified 96-well plate. Once
the optimal conditions to study the behavior of the cell lines were
determined, the effects of stimulation with E2 and 60 kDa PRL were
analyzed separately or in combination. Briefly, cells at a density
of 0.25×104/well were introduced into the xCELLigence
reading station, and after 4 h they were stimulated. The
proliferation rate was assessed in real time every 30 min over a
period of 72 h. HaCaT cells were also included as a negative
control of neoplasia. Cisplatin stimulation was used as a cell
death control. Three independent experiments were performed with
three replicates in each case.
Apoptosis detection
HeLa, SiHa, C33A and HaCaT cells were seeded at a
density of 2.5×106 cells/well in 1.5 ml charcoal
stripped fetal bovine serum in 6-well plates. After 24 h, they were
stimulated with E2, 60 kDa PRL or both. In addition, the effects of
these hormones on cisplatin-induced death were evaluated. The
percentage of positive cells was determined in 48 h by flow
cytometry using an Attune® Acoustic Focusing Cytometer
(Thermo Fisher Scientific, Inc.) and the Annexin V-FLUOS staining
kit (cat. no. 11988549001; Roche Applied Science), according to the
manufacturer's protocol. Results were analyzed using FlowJo V10-1r5
(FlowJo, LLC, Ashland, OR, USA) and reported as geometric mean
fluorescence intensity. Each sample was tested three times in three
independent experiments.
MTT assay
MTT reduction is one of the most useful methods for
measuring cell metabolism through the evaluation of mitochondrial
activity (32). Cells were
stimulated with E2, 60 kDa PRL or a combination of both, during 72
h. Following stimulation, MTT was added at a proportion of
one-tenth of the final medium volume, and cells were incubated for
4 h at 37°C in an atmosphere containing 5% CO2. The
supernatant was removed from the well and was replaced with
HCl-isopropanol solution, in order to dissolve the formed crystals.
The results were analyzed by spectrophotometry and absorbance at
570 nm was measured.
HPV 16 and HPV 18 E6 and E7
cloning
E6 and E7 open reading frames were amplified from
genomic DNA obtained from the biopsies of patients infected with
HPV 16 or HPV 18 in a previous study (33). Polymerase chain reaction (PCR) was
performed using the Expand High Fidelity PCR system kit (cat. no.
11 732 650 001; Roche Applied Science) using the following primer
pairs: HPV 16 E6, forward 5′-CAGACATTTTATGCACCAAA-3′, and reverse
5′ CTCCATGCATGATTACAGC 3′; HPV 16 E7, forward
5′-TAGAGAAACCCAGCTGTAATCA-3′, and reverse
5′-AGGATCAGCCATGGTAGATTAT-3′; HPV 18 E6, forward
5′-AATACTATGGCGCGCTTTGA-3′, and reverse
5′-TTGCCTTAGGTCCATGCATACT-3′; and HPV 18 E7, forward
5′-CGCAGAGAAACACAAGTATAAT-3′ and reverse 5′-GATCAGCCATTGTTGCTTA-3′.
The four amplified products were independently cloned into a pGEM-T
Easy cloning vector (cat. no. A1360; Promega Corporation, Madison,
WI, USA) and were sequenced with M13 forward and reverse primers
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
using the BigDye Terminator Cycle Sequencing kit (Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
an ABI PRISM 310 Genetic Analyzer (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The cloned ORF sequences were
corroborated with the reference sequences reported in GenBank (HPV
16, accession no. K02718; HPV 18, accession no. AY262282;
http://www.ncbi.nlm.nih.gov/genbank/); only HPV 16 E6
exhibited a substitution described in the AF402678 HPV 16 sequence
(268T>G). Finally, the ORFs were subcloned into a pLVX-Puro
lentiviral expression vector (cat. no. 632164; Clontech
Laboratories, Inc., Mountain View, CA, USA) using EcoRI
restriction.
Lentivirus production and HaCaT cell
infection
To produce the lentivirus, Lenti-X 293T cells (cat.
no. 632180; Clontech Laboratories, Inc.) were used. The cells were
cultured in DMEM supplemented with 10% fetal bovine serum and
penicillin/streptomycin (10,000 U/ml and 10,000 µg/ml,
respectively); cells (4×106) were seeded in a 100 mm
tissue culture plate, incubated for 24 h and independently
transfected with pLVX-Puro empty vector, pLVX-HPV 16 E6, pLVX-HPV
16 E7, pLVX-HPV 18 E6 or pLVX-HPV 18 E7 vectors, using Lenti-X HTX
Packaging system from the Lenti-X Lentiviral Expression system
(Clontech Laboratories, Inc.). After 48 h post-transfection,
infectious viral particles were collected from the cell
supernatant, and the presence of the virus was determined using
Lenti-X GoStix (Clontech Laboratories, Inc.). HaCaT keratinocytes
were individually infected with 100 µl each viral supernatant, and
selection of transduced cells was conducted with 1 µg/ml puromycin.
Following RNA extraction from the established cell lines, E6 and E7
expression levels were determined with reverse
transcription-quantitative PCR (RT-qPCR).
RNA isolation and RT-qPCR
Total RNA was extracted from the cell lines and
biopsies using the RNeasy Plus Mini kit (Cat. 74136; QIAGEN Mexico,
S. de R.L. de C.V., San Ángel, Mexico), according to the
manufacturer's protocol. RNA was quantified by measuring the
absorbance at 260/280 nm. Subsequently, 5 µg total RNA was used to
generate cDNA using the Transcriptor First Strand cDNA Synthesis
kit (Roche Applied Science) primed with Oligo dT, according to the
manufacturer's protocol. cDNA was used to confirm E6 and E7
expression using the LightCycler® FastStart DNA Master
PLUS SYBR Green I kit on a LightCycler 2.0 instrument (Roche
Applied Science), according to the manufacturer's protocol. Primers
used to amplify E6 and E7 were the as those used for cloning. To
normalize qPCR reactions, β-actin-expression was assessed using the
following primer sequences: Forward, 5′-TCCGCAAAGACCTGTACG-3′ and
reverse, 5′-AAGAAAGGGTGTAACGCAACTA-3′. Thermocycling conditions
were as follow: One denaturation step at 95°C for 10 min, followed
by an amplification program of 35 cycles consisting of the
following steps: 95°C for 10 sec, 60°C for 10 sec and 72°C for 12
sec, followed by a final extenstion step at 72°C for 10 min.
Relative expression was calculated using the 2−ΔΔCq
algorithm (34).
Statistical analysis
Statistical assessment was conducted using SPSS
Statistics 20 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 6
(GraphPad Software, Inc., La Jolla, CA, USA) statistical software.
Data obtained from three independent tests were analyzed using
one-way analysis of variance with Bonferroni correction in SPSS.
The data are presented as the means ± standard error of the mean.
P≤0.05 was considered to indicate a statistically significant
difference.
Results
Expression of ERα, ERβ and PRLR in
cervical epithelia
To analyze the expression levels of ERα, ERβ and
PRLR, immunohistochemistry was performed on premalignant lesions
and UCC tissues. The mean age for patients with dysplasia was 28.16
years, and 33 years for patients with cancer. Three squamous cell
carcinoma samples (20%) did not express ERα. Of the samples
negative for PRLR expression, there were two UCC samples (13.3%),
two CIN III samples (16.6%) and three CIN I samples (23.1%). All of
the samples were positive for ERβ (Table I).
 | Table I.Expression of ERs, PRLR, Ki67 and
Bcl-2 in intraepithelial lesions and in cervical cancer
tissues. |
Table I.
Expression of ERs, PRLR, Ki67 and
Bcl-2 in intraepithelial lesions and in cervical cancer
tissues.
|
| Histopathological
diagnosis |
|---|
|
|
|
|---|
|
|
|
|
| Invasive
carcinom |
|---|
|
|
|
|
|
|
|---|
| Positive protein
expression | CIN I | CIN II | CIN III | Adenocarcinoma | Squamous cell
carcinoma |
|---|
| ERα (%) | 100 | 100 | 100 | 100 | 80.0 |
| ERβ (%) | 100 | 100 | 100 | 100 | 100 |
| PRLR (%) | 76.9 | 100 | 83.4 | 100 | 86.7 |
| Ki67 (%) | 84.6 | 91.6 | 100 | 100 | 100 |
| Bcl-2 (%) | – | – | 83.3 | 75.0 | – |
| Total (n) | 13 | 12 | 12 | 4 | 11 |
A total of 10 representative samples from each of
the four groups were selected to analyze the co-expression of the
receptors, using five similar fields for each lesion. The magnified
views of dysplastic and neoplastic epithelium are presented in
Fig. 1A; ERα nuclear expression was
most noticeable in the neoplastic tissue. The mean optical
densities of ERα positive expression in CIN I (155.7), CIN II
(277.2), CIN III (321.0) and cancer (481.0) samples were gradually
increased as the lesion progressed toward malignancy; however,
there were no significant differences between the CIN III and CIN
II groups. Similarly, as shown in Fig.
1B, a progressive increase in the nuclear positive expression
of ERβ was observed from CIN I to cancer; these increases were
statistically significant in all groups (mean optical densities,
103.7, 206.7, 315.4 and 461.6; P<0.05).
 | Figure 1.Expression of ERα, ERβ and PRLR in
precursor lesions and cervical cancer tissues. (A) ERα, (B) ERβ and
(C) PRLR expression levels were detected using
immunohistochemistry; brown coloration indicated positive staining.
The expression of the receptors increased as the lesion progressed.
Left panels, images shown are representative of five fields
(magnification, ×10; magnification inset images, ×40). The small
boxes represent the magnified regions. Right panels, epithelial
distribution of receptor expression in samples from each
pathological grade. Samples in which cellular optical density was
very high for the expression of the protein were characterized as
intense staining, whereas positive samples regardless of density
were characterized as positive staining. *P<0.05 (analysis of
variance). CIN, cervical intraepithelial neoplasia; ER, estrogen
receptor; PRLR, prolactin receptor. |
As shown in Fig. 1C,
in UCC samples, PRLR expression was increased compared with in the
dysplastic epithelium and there was a significant increase in
positive expression from CIN I to cancer (mean optical densities,
184.2, 420.0, 622.0 and 942.1; P<0.05). Immunostaining was
mainly diffusely localized in the cytoplasm; however, in five
cancer samples (50%) it was also evident at the nuclear level.
Expression of Ki67 and Bcl-2 in
cervical epithelia
The present study detected Ki67 and Bcl-2 protein
expression, in order to evaluate the proliferative and
anti-apoptotic nature of the premalignant lesions and UCC tissues.
Nuclear Ki67 positive expression was considerably higher in
neoplastic epithelium (mean optical density: 394.10 in UCC vs.
69.0, 153.50 and 193.80 in CIN I, CIN II and CIN III, respectively;
P<0.05); however, it cannot be confirmed that the expression
pattern gradually increased as the degree of dysplasia advanced to
cancer, since there were no statistically significant differences
between the CIN II and CIN III groups (Fig. 2A).
Only five samples (8.8%) were positive for Bcl-2
(three adenocarcinomas and two CIN III samples), which is an
intracellular membrane protein that prevents apoptotic cell death
(30). The immunostaining of Bcl-2
was cytoplasmic and was localized predominantly in the basal layer.
The images presented in Fig. 2B are
examples of the negative Bcl-2 staining detected in most
lesions.
Evaluation of cell proliferation in
real time
To determine whether exogenous E2 and 60 kDa PRL,
individually or in combination, are required for the proliferation
of UCC, analyses were performed on HeLa, SiHa, C33A and HaCaT cells
using the xCELLigence system RTCA biosensor instrument. The cell
index curves revealed several important details about the kinetics
of initial adhesion and cell survival. Only stimulation with 60 kDa
PRL had a significant effect on SiHa cell proliferation (P=0.04;
Fig. 3). In addition, in SiHa
cells, E2 alone and E2 combined with PRL tended to increase
cisplatin-induced death; however, only treatment with a combination
of both hormones reached statistical significance (P=0.03; Fig. 3). The proliferation of HeLa and C33A
cells was not significantly affected in response to any of the
hormones. Both SiHa and HeLa cells began to proliferate at ~33 h,
whereas C33A cell proliferation began at 11 h. Conversely, in HaCaT
cells, E2 and 60 kDa PRL significantly increased the proliferative
index (P=0.015 for E2, P=0.003 for PRL and P=0.01 for the
combination; Fig. 3). In addition,
the combination decreased cisplatin-induced cell death, and
proliferation began before 11 h.
Evaluation of apoptosis
Annexin V-FITC/PI staining was examined by flow
cytometry to investigate the induction of apoptosis by E2 and 60
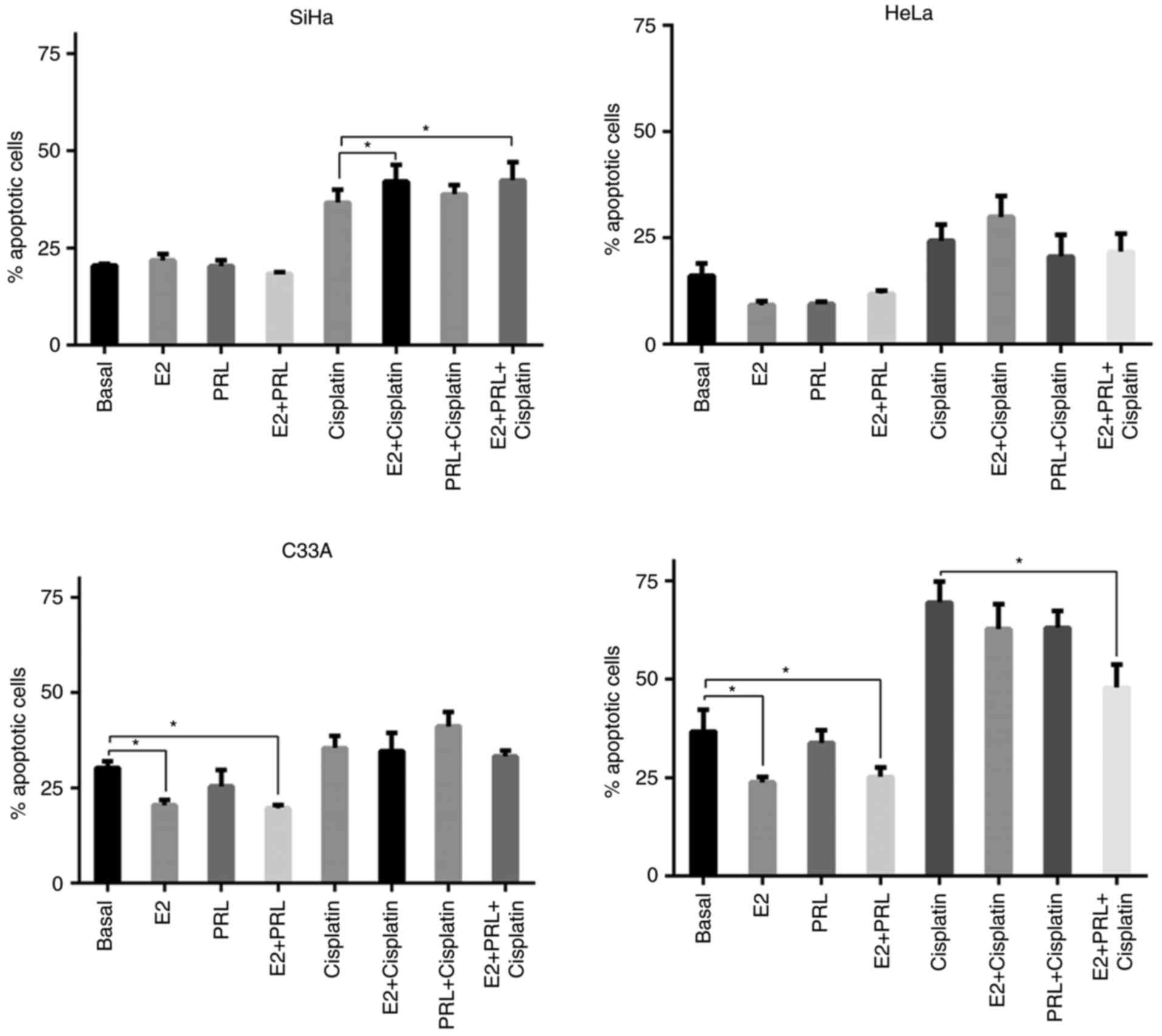
kDa PRL in SiHa, HeLa, C33A and HaCaT cells (Fig. 4). Cell apoptosis was defined as
Annexin V+ or Annexin V+/PI+.
Hormones did not have a direct impact on the
percentage of apoptosis in SiHa and HeLa cells. Only E2 stimulation
increased the percentage of apoptosis in SiHa cells in the presence
of cisplatin, either when it was used individually (P=0.006) or in
association with 60 kDa PRL (P=0.035). Conversely, C33A cells
experienced a significant reduction in the apoptotic index in
response to E2 (P=0.024) and combination treatment (P=0.006). HaCaT
cells exhibited similar behavior to C33A in response to E2
(P=0.007) and combination treatment (P=0.006). However, only in the
HaCaT cell line did combination treatment confer a protective
effect against the cytotoxic effects of cisplatin (P=0.01).
The proportion of necrotic cells in the UCC and
HaCaT cell lines was also compared. E2 combined with 60 kDa PRL
reduced necrosis in SiHa (P=0.003), HeLa (P=0.04) and C33A
(P=0.006) cells. Conversely, the necrosis index in HaCaT cells was
not affected by these treatments (data not shown).
Determination of cellular
metabolism
To understand the possible roles of estrogen and 60
kDa PRL in cervical carcinoma, mitochondrial activity was assessed
by the MTT assay. The energetic metabolism of HeLa and SiHa cells
was significantly increased 72 h after stimulation with E2 (P=0.003
and P=0.052, respectively). The same effect was observed in HeLa
cells in response to 60 kDa PRL and combination treatment (P=0.004
and P=0.007, respectively). Treatment of C33A with the hormones had
no significant effect, whereas in HaCaT cells it produced a
decrease in mitochondrial activity (P=0.033, P=0.02 and P=0.05 for
E2, 60 kDa PRL and combination treatment, respectively. Notably, in
HeLa and SiHa cells, 60 kDa PRL exhibited a tendency to modulate
the effects produced by E2; however, only in SiHa cells did it
reach statistical significance (P=0.05). This modulation was not
evident in C33A and HaCaT cells (Fig.
5A).
 | Figure 5.Effects of stimulation with E2 (10
nM) and 60 kDa PRL (200 ng/ml) on the metabolism of cervical cancer
cells, and on HaCaT cells transduced with E6/E7 viral oncogenes
from HPV 16 and 18 during 72 h. (A) Effect of stimulation on HeLa,
SiHa, C33A and control HaCaT cells. Hormones produced an increase
in the metabolism of HeLa cells, but only E2 had the same effect on
SiHa cells. (B) Evaluation of E6 and E7 oncogene expression in
HaCaT cells transduced with pLVX vector alone, E6 or E7 from HPV 16
and HPV 18. Relative expression was calculated using HPV 16 E6
expression as calibrator and β-actin expression as a reference
gene; the same approach was used to calculate the relative
expression of E7, using E7 from HPV 16 expression as calibrator.
(C) Effects of hormonal stimulation on HaCaT cells transduced with
E6/E7 viral oncogenes from HPV 16 and HPV 18. Hormones increased
cellular metabolism in HaCaT cells transduced with HPV 18 E6 and
HPV 16 E7 viral oncogenes. E2 accelerated mitochondrial function in
HaCaT cells transduced with HPV 16 E6. Graphs show the results of
experiments performed in triplicate, repeated at least three times.
*P≤0.05 (ANOVA test). 18 E6 and 18 E7, HaCaT cells transduced with
E6 and E7 from HPV 18, respectively; 16 E6 and 16 E7, HaCaT cells
transduced with E6 and E7 from HPV 16, respectively; pLVX, HaCaT
cells transduced with empty vector; E2, 17β-estradiol; PRL,
prolactin. |
In the present study, HaCaT cells were transduced
with the viral oncogenes HPV 18 and 16 E6 and E7 followed by
hormonal stimulation. As shown in Fig.
5B, the mRNA expression levels of HPV 16 and 18 E6 and E7
oncogenes were increased in response to transduction. Stimulation
with E2 and 60 kDa PRL resulted in increased cell metabolism in
HaCaT cells transduced with the HPV 18 E6 viral oncogene (P=0.01
and P=0.042, respectively; Fig.
5C). The modulating effects of 60 kDa PRL on estrogen were
evident (P=0.003; Fig. 5C). No
change from the baseline was observed in HaCaT cells transduced
with the HPV 18 E7.
In HaCaT cells transduced with the E6 and E7
oncogenes of HPV 16, E2 stimulation produced a significant increase
in cellular metabolism (P=0.02 and P=0.002; respectively). Only in
cells transduced with HPV 16 E7, did 60 kDa PRL exhibit an E2-like
effect (P=0.001; Fig. 5C). In
addition, modulatory effects of PRL on E2 in HaCaT cells transduced
with HPV 16 E6 and E7 were demonstrated (P=0.02 and P=0.001,
respectively). Notably, HaCaT transduced only with PLVX viral
particles only exhibited a similar behavior to untransduced HaCaT
cells; hormonal stimulation decreased cellular metabolism.
Discussion
Previous studies have suggested that estrogen
exposure may be a critical factor for the development of UCC
(5–10); however, the participation of
estrogen and its role in the control of certain processes, such as
proliferation, apoptosis, migration and metastasis, remains to be
elucidated.
The cervical carcinogenic effects of estrogen can be
explained by analyzing its possible synergism with the infection of
epithelial cells by HPV, which is the primary risk factor for the
development of this type of cancer. HPV 16 contains a transcription
enhancer that is activated by cellular elements other than the
viral E2 protein, which resides on a 232-bp segment that contains
one binding site for steroid receptors (35). In the genome of HPV 16, seven
regions of high resemblance to ER sequences have been described
(36,37). Epidemiological studies have revealed
an elevated risk of UCC among HPV-positive women who had used an
oral contraceptive medication containing estrogen (11–13).
In this study, a gradual and significant increase in
the expression of ERα and ERβ was detected as the degree of
dysplasia progressed to the tumor phenotype. The role of ERs in the
pathogenesis of UCC is inconclusive due to a large number of
contradictory results. For example, in vivo studies have
significantly demonstrated the requirement of ERs in HPV-associated
cancer of the cervix (6,38,39),
whereas the analysis of cervical samples remains open to question
(9,17,40).
Chung et al (2008) revealed that K14E7
transgenic mice expressing HPV16 E7 develop UCC after prolonged
treatment with physiological levels of exogenous estrogen and ERα
serves a crucial role in this outcome (38). In addition, it has been observed
that inhibition of ERα is effective in treating and preventing UCC
in mice (39). In contrast to
ERα-sufficient HPV transgenic mice, ERα-deficient HPV transgenic
mice fail to develop UCC when treated with estrogen; notably,
estrogen-treated ERα-null HPV transgenic mice do not develop any
aspect of the progressive disease leading to UCC (6,38).
Immunohistochemistry has also been used to
investigate the prognostic significance of ERα; a previous study
reported that the positive expression of ERα in UCC is 68.18%,
which is significantly higher compared with in the normal cervix
(35%) and in chronic cervicitis (50%). Furthermore, ERα in
carcinoma was revealed to be associated with HPV infection, the
depth of infiltration and the presence of lymphatic metastasis
(40). These results demonstrated
that cervical neoplastic cells express ERα and suggested that
estrogens could regulate some tumor functions via this
receptor.
Previous studies have also revealed that ERα in UCC
is decreased to undetectable levels, at the protein and mRNA level,
compared with the precursor lesions (8,17).
Furthermore, another study did not detect ERα protein expression in
any of the analyzed invasive cervical carcinoma samples; however,
significant mRNA expression levels of ERα were detected in the same
samples. In this previous study, immunopositivity for ERβ protein
was detected in 70.6% of UCC samples; however, the staining was
almost exclusively cytoplasmic (41). ERβ was not detected in UCC by den
Boon et al (17). Therefore,
evidence of the presence of ERs in human cervical neoplasms remains
limited in nature and inconclusive.
The physiological interplay between estrogens and
PRL has been well tested; however, the impact in cancer has been
poorly explored. E2 directly induces extrapituitary PRL gene
expression in breast cancer cells, and a functional noncanonical
ERE and an AP1 site are located in the PRL distal promoter, which
E2 can act (42,43). In the present study, similar to that
observed for ERα and ERβ, PRLR expression was increased in UCC. In
10 samples from each group (CIN I, II, III and UCC) ERs and PRLR
expression was evidenced in the same histopathological sections,
leading to the hypothesis that these receptors are
co-expressed.
It has previously been reported that PRLR expression
is significantly increased in UCC compared with in normal tissue
and precursor lesions (19). The
internalization of PRLR and its translocation to the nucleus has
been the subject of intense debate, due to conflicting results
(44). The present experiment is in
favor of the nuclear translocation of PRL complexed with its
receptor, due to evidence of the presence of PRLR in the nucleus in
50% of UCC samples.
Ki67 is a nuclear and nucleolar protein that is
expressed only in the active phases of the cell cycle
(G1, S, G2 and M phases) but not in the
resting phases (G0 and early G1). In the
present study, Ki67 expression in the samples was directly
associated with severity of the cervical lesions; this finding was
similar to the results of a previous study, which detected Ki67 in
~100% of UCC specimens (43).
Although overexpression of Ki67 is correlated with high cellular
proliferation (45), in a
systematic review, Kisser and Zechmeister-Koss (2015) concluded
that evaluation of Ki67 could not be recommended for the triage of
women with atypical squamous cells of indeterminate significance or
low-grade squamous intraepithelial lesions cytology, due to
insufficient high-quality evidence (46).
In the present study, low Bcl-2 expression was
evidenced. Although it has been suggested that Bcl-2 expression is
strongly associated with the development of invasive cervical
disease (30,31), other studies have reported that
Bcl-2 positivity confers a better 5-year survival rate and
prognosis (47). Shukla et
al (2014) reported that Bcl-2 expression is much lower in UCC
compared with in premalignant lesions (48).
Our previous study discovered the presence of a PRL
isoform with a molecular weight of ~60 kDa in the protein extracts
and supernatants of HeLa, SiHa and C33A cells, which was absent in
HaCaT cells (18). Due to the
relevance that has recently been conferred to local PRL in the
pathophysiology of some tumors, its bioactivity was initially
determined (25). Subsequently,
cells were stimulated with this PRL isoform, in order to evaluate
the effects of its use separately and in combination with E2 on the
mechanisms underlying cell survival and metabolism.
The present study revealed that E2 and 60 kDa PRL
did not modulate the proliferation of most cell lines derived from
UCC; only in SiHa cells did stimulation with 60 kDa PRL have a
significant effect. Nair et al reported that treatment with
10−9 mol/l E2 enhances cellular proliferation of C33A,
Caski, HT3 and C4I cells; however, it was suggested that local
estrogen formed by high aromatase activity may be responsible for
this outcome (49). Differences
between the two studies may have been reported due to the different
methods used to measure proliferation. In addition, the effects
induced by estrogens vary depending on cell type, the used
concentration and the time of application. In the present study,
SiHa and HeLa cells entered the proliferative phase at ~33 h,
whereas in HaCaT cells, proliferation began earlier. In HaCaT
cells, treatment with the hormones increased proliferative
index.
Previous studies have supported the
pro-proliferative, anti-apoptotic and pro-metastatic roles of PRL
in the progression of some tumor types. The particular PRL response
depends on factors such as the intensity of the expression of PRLR
and other molecules acting as collaborators (43,50–52).
In UCC, hPRL does not affect proliferation; however, it decreases
etoposide-induced cell death; this effect is mediated by the
phosphorylation of STAT3 (18,20).
Recently, 60 kDa PRL, which was isolated from the supernatant of
HeLa cells, was revealed to induce both responses (25).
The combination of E2 and 60 kDa PRL was evaluated
in the present study, in order to determine if it has any impact on
the mechanisms of programmed cell death. In addition, the present
study aimed to determine if the presence of these hormones modifies
the response of tumor cells to cisplatin treatment. Only in C33A
cells, did the use of E2 separately and with 60 kDa PRL produce a
decrease in the percentage of apoptosis. Notably, this cell line is
not infected by HPV; therefore, this may explain the different
behavior in comparison to HeLa and SiHa cells, in which no effects
were shown. Regarding the impact on cisplatin-induced cell death,
in SiHa cells, cotreatment with E2 and 60 kDa PRL resulted in
increased sensitivity of these cells to the chemotherapeutic agent,
whereas in HaCaT cells, this cotreatment had a protective
effect.
Previous studies have reported the synergism between
estrogen and PRL in the promotion of breast cancer (26–28).
Conversely, in the present study, a synergistic effect of these
hormones on the progression of UCC through the modulation of
proliferation and apoptosis was not identified.
Another critical feature of cancer is the alteration
in cellular metabolism to favor the uncontrolled growth of cells
(53). HeLa cells treated with E2
and 60 kDa PRL, either individually or in combination, exhibited an
increase in mitochondrial activity. In addition, in SiHa cells,
stimulation with E2 had a similar result. In addition, HaCaT cells
were transduced with HPV 16 and 18 viral E6 and E7 oncogenes; HaCaT
cells transduced with HPV 18 E6 possessed similar behavior to HeLa
cells, although E2 + PRL had no effect on HaCaT cells transduced
with HPV 18 E6. Similar to SiHa cells, E2 stimulation increased the
metabolism of HaCaT cells transduced with HPV 16 E6/E7
oncogenes.
The effects of E2 on SiHa and HeLa cells, as well as
in HaCaT cells transduced with E6 oncogene of HPV 18 and with E6
and E7 oncogenes of HPV 16 can be explained by the important role
of this hormone within mitochondria. Estrogens have been reported
to exert direct and indirect effects on mitochondrial function, and
co-localization of ERα and/or ERβ with the mitochondria has been
reported (52). Estrogens can
regulate mitochondrial morphology, biogenesis, bioenergetics and
mitochondrial genome transcription, among other functions, in a
cell-specific manner (54–56).
The progression of UCC has been associated with an
increase in viral oncogene expression in HPV-positive lesions in
patients (57). Estrogens can
transactivate viral oncogenes; ERα activated by estradiol can bind
to responsive elements within the long control region of the HPV
genome and can increase E6 and E7 transcription (36,58,59).
This can profoundly affect cell biology, for example by inducing
metabolic reprogramming. Zeng et al demonstrated that E6/E7
expression induces aerobic glycolysis, also known as the ‘Warburg
effect’. This induction is mediated by c-MYC, which elevates
hexokinase-II, the rate-limiting enzyme responsible for the first
step in the glycolytic pathway (60). In addition, it has been reported
that O-linked GlcNAcylation (O-GlcNAc) and O-GlcNAc transferase are
markedly increased in HPV-induced cervical neoplasms and mouse
embryonic fibroblasts transduced with HPV 16 oncogene E6 or E6/E7
(61). It has been proposed that
O-GlcNAc is a nutrient sensor that modulates some cellular pathways
in response to metabolic regulation (62). Hypoxia in cancer has been associated
with the metabolic shift towards aerobic glycolysis, and the
ability of the E7 oncoprotein to enhance the activity of
hypoxia-inducible factor 1α has been demonstrated (63,64).
The present results suggested that the relationship of viral
oncogenes E6/E7 with estrogen and PRL may influence metabolic
pathways in UCC.
The hypothesis that PRL may regulate metabolic genes
is more difficult to explain. Previous studies have suggested that
PRL regulates citrate production of prostate epithelial cells, and
AP-1 mediates PRL regulation of mitochondrial aspartate
aminotransferase expression, which is an important protein in the
metabolic pathway for citrate production in the prostate (65,66).
Estrogens and PRL can cooperate in various
physiological processes; however, they can also have antagonistic
functions (67). In the present
study, it was revealed that in SiHa and HeLa cells, and in HaCaT
cells transduced with the viral oncogene E6 of HPV 18 and the viral
oncogenes E6/E7 of HPV 16, 60 kDa PRL tended to negatively modulate
the effects of E2 on metabolism. One possible explanation for this
result is the differential roles of PRL stimulation on ERα and ERβ
transcription; PRL upregulates the mRNA expression levels of both
ERs in a context- and dose-dependent manner via the Janus kinase
2-STAT5 pathway. Either STAT5a or STAT5b stimulates ERα
transcription, whereas only STAT5b stimulates ERβ (68,69).
Different roles have been postulated for both ERs in various cancer
models (70). It is unknown if in
UCC 60 kDa PRL regulates the effects of estrogen through ERβ. It
would be interesting to evaluate in future studies the role of 60
kDa PRL in the modulation of ERs in UCC, and the potential impact
on the pathophysiology of the disease.
The MTT assay has been extensively used to measure
cell proliferation and survival capacities. In the present study,
the different findings obtained from the xCELLIGENCE platform and
the MTT assay may seem contradictory. Other studies have also
reported differences in cell number determination using
metabolism-based assays and other methods (71–73).
For example, Wang et al demonstrated a significant
difference in the determination of the antiproliferative activity
of green tea polyphenols when using MTS-based assays in comparison
to ATP- and DNA-based methods and the trypan blue assay (73). The present results coincided with
those of other studies, with regards to the need for careful
selection of the methods used for in vitro evaluation of
cell proliferation.
As aforementioned, several studies have analyzed the
association between estrogens and PRL with regards to cancer
progression and metabolism; however, to the best of our knowledge,
this is the first study to evaluate this hormonal correlation in
UCC, particularly using an isoform of PRL that is secreted by tumor
cells.
In conclusion, the present study revealed that ERs
and PRLR are co-expressed in UCC and their precursor lesions; in
addition, they are associated with the severity of injury. However,
the individual and combined stimulation of estrogen and PRL under
the conditions used in the present study did not exhibit a
significant impact on proliferation/apoptosis in UCC cell lines.
However, cellular metabolism, as evaluated through mitochondrial
succinate dehydrogenase enzyme activity, was elevated in HeLa cells
treated with both hormones individually or in combination, and in
SiHa cells stimulated with E2. In the HaCaT cell line transduced
with HPV 16 E7 and 18 E6 viral oncogenes, E2 and 60 kDa PRL
individually increased cellular metabolism; however, although only
in HaCaT cells transduced with HPV 16 E7 did the combination affect
metabolism. Furthermore, E2 accelerated mitochondrial function in
HaCaT cells transduced with HPV 16 E6.
Some histopathological specimens used in the present
study had no information available regarding HPV infection; this
information may assist further in interpretation of the
immunohistochemical findings. In addition, no information regarding
clinical variables was available, which may have allowed for
analysis of the correlation between these data and the
histopathological expression of hormone receptors. A further
prospective study with more appropriate and accurate data should be
conducted, in order to understand how HPV infection modulates
hormone receptors to initiate and maintain the process of cervical
carcinogenesis.
Acknowledgements
The authors would like to thank the histopathology
technicians at the Pathological Anatomy Service of the Civil
Hospital of Guadalajara Fray Antonio Alcalde (Guadalajara, México)
for their assistance.
Funding
The present study was partially supported by the
Consejo Nacional de Ciencia y Tecnología (grant no. 222205) and the
Fondo de Investigación en Salud-IMSS (grant no.
FIS/IMSS/PROT/PRIO/15/046 to LFJ-S).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ARL, ARDA, IGRL and CAI performed the experiments.
JRDR and EILP were involved in sample collection and sample
processing, and provided immunohistochemical assistance. PCOL and
LFJS assisted in flow cytometry data analysis and DNA cloning.
JGMB, SDTA, MMB and JFMV assisted in the interpretation of results
and statistical analysis, and reviewed and edited the manuscript.
ALPS designed the study, interpreted the results and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Investigation and Biosecurity Committee of the University Center of
Health Sciences at the University of Guadalajara (Reference Number
C.I. 093/13 CUCS; Guadalajara, Mexico). Written informed consent
was obtained from all patients included in the present study.
Patient consent for publication
Written informed consent was obtained from all
patients included in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen Q, Cao HZ and Zheng PS: LGR5 promotes
the proliferation and tumor formation of cervical cancer cells
through the Wnt/β-catenin signaling pathway. Oncotarget.
5:9092–9105. 2014.PubMed/NCBI
|
|
2
|
Dijkstra MG, Snijders PJ, Arbyn M,
Rijkaart DC, Berkhof J and Meijer CJ: Cervical cancer screening: On
the way to a shift from cytology to full molecular screening. Ann
Oncol. 25:927–935. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Armstrong EP: Prophylaxis of cervical
cancer and related cervical disease: A review of the
cost-effectiveness of vaccination against oncogenic HPV types. J
Manag Care Pharm. 16:217–230. 2010.PubMed/NCBI
|
|
4
|
Cunningham MS, Skrastins E, Fitzpatrick R,
Jindal P, Oneko O, Yeates K, Booth CM, Carpenter J and Aronson KJ:
Cervical cancer screening and HPV vaccine acceptability among rural
and urban women in Kilimanjaro Region, Tanzania. BMJ Open.
5:e0058282015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Son J, Park JW, Lambert PF and Chung SH:
Requirement of estrogen receptor alpha DNA-binding domain for HPV
oncogene-induced cervical carcinogenesis in mice. Carcinogenesis.
35:489–496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chung SH, Franceschi S and Lambert PF:
Estrogen and ERalpha: Culprits in cervical cancer? Trends
Endocrinol Metab. 21:504–511. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chung SH, Shin MK, Korach KS and Lambert
PF: Requirement for stromal estrogen receptor alpha in cervical
neoplasia. Horm Cancer. 4:50–59. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kwasniewska A, Postawski K,
Gozdzicka-Jozefiak A, Kwasniewski W, Grywalska E, Zdunek M and
Korobowicz E: Estrogen and progesterone receptor expression in
HPV-positive and HPV-negative cervical carcinomas. Oncol Rep.
26:153–160. 2011.PubMed/NCBI
|
|
9
|
Ramachandran B: Functional association of
oestrogen receptors with HPV infection in cervical carcinogenesis.
Endocr Relat Cancer. 24:R99–R108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Remoue F, Jacobs N, Miot V, Boniver J and
Delvenne P: High intraepithelial expression of estrogen and
progesterone receptors in the transformation zone of the uterine
cervix. Am J Obstet Gynecol. 189:1660–1665. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marks M, Gravitt PE, Gupta SB, Liaw KL,
Kim E, Tadesse A, Phongnarisorn C, Wootipoom V, Yuenyao P,
Vipupinyo C, et al: The association of hormonal contraceptive use
and HPV prevalence. Int J Cancer. 128:2962–2970. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marks MA, Gupta S, Liaw KL, Tadesse A, Kim
E, Phongnarisorn C, Wootipoom V, Yuenyao P, Vipupinyo C, Rugpao S,
et al: Prevalence and correlates of HPV among women attending
family-planning clinics in Thailand. BMC Infect Dis. 15:1592015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roura E, Travier N, Waterboer T, de
Sanjosé S, Bosch FX, Pawlita M, Pala V, Weiderpass E, Margall N,
Dillner J, et al: The influence of hormonal factors on the risk of
developing cervical cancer and pre-cancer: Results from the EPIC
cohort. PLoS One. 11:e01470292016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gupte AA, Pownall HJ and Hamilton DJ:
Estrogen: An emerging regulator of insulin action and mitochondrial
function. J Diabetes Res. 2015:9165852015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bronowicka-Kłys DE, Lianeri M and
Jagodziński PP: The role and impact of estrogens and xenoestrogen
on the development of cervical cancer. Biomed Pharmacother.
84:1945–1953. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamaguchi H and Sakai R: Direct
interaction between carcinoma cells and cancer associated
fibroblasts for the regulation of cancer invasion. Cancers.
7:2054–2062. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
den Boon JA, Pyeon D, Wang SS, Horswill M,
Schiffman M, Sherman M, Zuna RE, Wang Z, Hewitt SM, Pearson R, et
al: Molecular transitions from papillomavirus infection to cervical
precancer and cancer: Role of stromal estrogen receptor signaling.
Proc Natl Acad Sci USA. 112:E3255–E3264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lopez-Pulido EI, Muñoz-Valle JF, Del
Toro-Arreola S, Jave-Suárez LF, Bueno-Topete MR, Estrada-Chávez C
and Pereira-Suárez AL: High expression of prolactin receptor is
associated with cell survival in cervical cancer cells. Cancer Cell
Int. 13:1032013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ascencio-Cedillo R, López-Pulido EI,
Muñoz-Valle JF, Villegas-Sepúlveda N, Del Toro-Arreola S,
Estrada-Chávez C, Daneri-Navarro A, Franco-Topete R, Pérez-Montiel
D, García-Carrancá A, et al: Prolactin and prolactin receptor
expression in cervical intraepithelial neoplasia and cancer. Pathol
Oncol Res. 21:241–246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Arellano Ramirez A, Lopez-Pulido EI,
Martinez-Neri PA, Chávez Estrada C, González Lucano R,
Fafutis-Morris M, Aguilar-Lemarroy A, Muñoz-Valle JF and
Pereira-Suárez AL: STAT3 activation is required for the
antiapoptotic effects of prolactin in cervical cancer cells. Cancer
Cell Int. 15:832015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Trott JF, Horigan KC, Gloviczki JM, Costa
KM, Freking BA, Farmer C, Hayashi K, Spencer T, Morabito JE and
Hovey RC: Tissue-specific regulation of porcine prolactin receptor
expression by estrogen, progesterone, and prolactin. J Endocrinol.
202:153–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Larrea F, Martinez-Castillo A, Cabrera V,
Alcocer-Varela J, Queipo G, Cariño C and Alarcón-Segovia D: A
bioactive 60-kilodalton prolactin species is preferentially
secreted in cultures of mitogen-stimulated and nonstimulated
peripheral blood mononuclear cells from subjects with systemic
lupus erythematosus. J Clin Endocrinol Metab. 82:3664–3669. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gout PW, Beer CT and Noble RL:
Prolactin-stimulated growth of cell cultures established from
malignant Nb rat lymphomas. Cancer Res. 40:2433–2436.
1980.PubMed/NCBI
|
|
24
|
Noble RL, Beer CT and Gout PW: Evidence in
vivo and in vitro of a role for the pituitary in the growth of
malignant lymphomas in Nb rats. Cancer Res. 40:2437–2440.
1980.PubMed/NCBI
|
|
25
|
De Arellano Ramirez A, Leal Riera A,
Lopez-Pulido EI, González-Lucano LR, Macías Barragan J, Del Toro
Arreola S, García-Chagollan M, Palafox-Sánchez CA, Muñoz-Valle JF
and Pereira-Suárez AL: A 60 kDa prolactin variant secreted by
cervical cancer cells modulates apoptosis and cytokine production.
Oncol Rep. 39:1253–1260. 2018.PubMed/NCBI
|
|
26
|
Giacomini D, Páez-Pereda M, Stalla J,
Stalla GK and Arzt E: Molecular interaction of BMP-4, TGF-beta, and
estrogens in lactotrophs: Impact on the PRL promoter. Mol
Endocrinol. 23:1102–1114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Duan R, Ginsburg E and Vonderhaar BK:
Estrogen stimulates transcription from the human prolactin distal
promoter through AP1 and estrogen responsive elements in T47D human
breast cancer cells. Mol Cell Endocrinol. 281:9–18. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gutzman JH, Miller KK and Schuler LA:
Endogenous human prolactin and not exogenous human prolactin
induces estrogen receptor alpha and prolactin receptor expression
and increases estrogen responsiveness in breast cancer cells. J
Steroid Biochem Mol Biol. 88:69–77. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aslani Sari F, Safaei A, Pourjabali M and
Momtahan M: Evaluation of Ki67, p16 and CK17 markers in
differentiating cervical intraepithelial neoplasia and benign
lesions. Iran J Med Sci. 38:15–21. 2013.PubMed/NCBI
|
|
30
|
Kamaraddi S, Nayak A, Honnappa S and
Swarup A: Expression of Bcl-2 marker in premalignant lesions of
cervical cancer. Int J Reprod Contracept Obstet Gynecol. 5:965–969.
2016. View Article : Google Scholar
|
|
31
|
Ter Harmsel B, Smedts F, Kuijpers J,
Jeunink M, Trimbos B and Ramaekers F: BCL-2 immunoreactivity
increases with severity of CIN: A study of normal cervical
epithelia, CIN, and cervical carcinoma. J Pathol. 179:26–30. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sánchez NS and Königsberg M: Using yeast
to easily determine mitochondrial functionality with
1-(4,5-dimethylthiazol-2-yl)-3,5-diphenyltetrazolium bromide (MTT)
assay. Biochem Mol Biol Educ. 34:209–212. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Flores-Miramontes MG, Torres-Reyes LA,
Alvarado-Ruiz L, Romero-Martínez SA, Ramírez-Rodríguez V,
Balderas-Peña LM, Vallejo-Ruíz V, Piña-Sánchez P, Cortés-Gutiérrez
EI, Jave-Suárez LF, et al: Human papillomavirus genotyping by
linear array and next-generation sequencing in cervical samples
from Western Mexico. Virol J. 12:1612015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chong T, Chan WK and Bernard HU:
Transcriptional activation of human papillomavirus 16 by nuclear
factor I, AP1, steroid receptors and a possibly novel transcription
factor, PVF: A model for the composition of genital papillomavirus
enhancers. Nucleic Acids Res. 18:465–470. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mitrani-Rosenbaum S, Tsvieli R and
Tur-Kaspa R: Oestrogen stimulates differential transcription of
human papillomavirus type 16 in SiHa cervical carcinoma cells. J
Gen Virol. 70:2227–2232. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Elson DA, Riley RR, Lacey A, Thordarson G,
Talamantes FJ and Arbeit JM: Sensitivity of the cervical
transformation zone to estrogen-induced squamous carcinogenesis.
Cancer Res. 60:1267–1275. 2000.PubMed/NCBI
|
|
38
|
Chung SH, Wiedmeyer K, Shai A, Korach KS
and Lambert PF: Requirement for estrogen receptor alpha in a mouse
model for human papillomavirus-associated cervical cancer. Cancer
Res. 68:9928–9934. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chung SH and Lambert PF: Prevention and
treatment of cervical cancer in mice using estrogen receptor
antagonists. Proc Natl Acad Sci USA. 106:19467–19472. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fan DM, Tian XY, Wang RF and Yu JJ: The
prognosis significance of TGF-β1 and ER protein in cervical
adenocarcinoma patients with stage Ib~IIa. Tumour Biol.
35:11237–11242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
López-Romero R, Garrido-Guerrero E,
Rangel-López A, Manuel-Apolinar L, Piña-Sánchez P, Lazos-Ochoa M,
Mantilla-Morales A, Bandala C and Salcedo M: The cervical malignant
cells display a down regulation of ER-α but retain the ER-β
expression. Int J Clin Exp Pathol. 6:1594–1602. 2013.PubMed/NCBI
|
|
42
|
Clevenger CV, Gadd SL and Zheng J: New
mechanisms for PRLr action in breast cancer. Trends Endocrinol
Metab. 20:223–229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lim JH, Kim TY, Kim WH and Park JW: CAML
promotes prolactin-dependent proliferation of breast cancer cells
by facilitating prolactin receptor signaling pathways. Breast
Cancer Res Treat. 130:19–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Perrot-Applanat M, Gualillo O, Buteau H,
Edery M and Kelly PA: Internalization of prolactin receptor and
prolactin in transfected cells does not involve nuclear
translocation. J Cell Sci. 110:1123–1132. 1997.PubMed/NCBI
|
|
45
|
Kanthiya K, Khunnarong J, Tangjitgamol S,
Puripat N and Tanvanich S: Expression of the p16 and Ki67 in
cervical squamous intraepithelial lesions and cancer. Asian Pac J
Cancer Prev. 17:3201–3206. 2016.PubMed/NCBI
|
|
46
|
Kisser A and Zechmeister-Koss I: A
systematic review of p16/Ki-67 immuno-testing for triage of low
grade cervical cytology. BJOG. 122:64–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Aletra C, Ravazoula P, Scopa C, Kounelis
S, Sotiropoulou G, Kourounis G, Ladopoulos I and Bonikos D:
Expression of bcl-2 and bax in cervical intraepithelial neoplasia
and invasive squamous cell carcinoma of the uterine cervix. Eur J
Gynaecol Oncol. 21:494–498. 2000.PubMed/NCBI
|
|
48
|
Shukla S, Dass J and Pujani M: p53 and
bcl2 expression in malignant and premalignant lesions of uterine
cervix and their correlation with human papilloma virus 16 and 18.
South Asian J Cancer. 3:48–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nair HB, Luthra R, Kirma N, Liu YG,
Flowers L, Evans D and Tekmal RR: Induction of aromatase expression
in cervical carcinomas: Effects of endogenous estrogen on cervical
cancer cell proliferation. Cancer Res. 65:11164–11173. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Llovera M, Pichard C, Bernichtein S, Jeay
S, Touraine P, Kelly PA and Goffin V: Human prolactin (hPRL)
antagonists inhibit hPRL-activated signaling pathways involved in
breast cancer cell proliferation. Oncogene. 19:4695–4705. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Doll F, Pfeilschifter J and Huwiler A:
Prolactin upregulates sphingosine kinase-1 expression and activity
in the human breast cancer cell line MCF7 and triggers enhanced
proliferation and migration. Endocr Relat Cancer. 14:325–335. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen KE, Bustamante K, Nguyen V and Walker
AM: Involvement of miR-106b in tumorigenic actions of both
prolactin and estradiol. Oncotarget. 8:36368–36382. 2017.PubMed/NCBI
|
|
53
|
DeBerardinis RJ, Lum JJ, Hatzivassiliou G
and Thompson CB: The biology of cancer: Metabolic reprogramming
fuels cell growth and proliferation. Cell Metab. 7:11–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Klinge CM: Estrogenic control of
mitochondrial function and biogenesis. J Cell Biochem.
105:1342–1351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ivanova MM, Radde BN, Son J, Mehta FF,
Chung SH and Klinge CM: Estradiol and tamoxifen regulate NRF-1 and
mitochondrial function in mouse mammary gland and uterus. J Mol
Endocrinol. 51:233–246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Klinge CM: Estrogens regulate life and
death in mitochondria. J Bioenerg Biomembr. 49:307–324. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fontecha N, Basaras M, Hernáez S, Andia D
and Cisterna R: Assessment of human papillomavirus E6/E7 oncogene
expression as cervical disease biomarker. BMC Cancer. 16:8522016.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
de Villiers EM: Relationship between
steroid hormone contraceptives and HPV, cervical intraepithelial
neoplasia and cervical carcinoma. Int J Cancer. 103:705–708. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Matos A, Castelão C, da Silva Pereira A,
Alho I, Bicho M, Medeiros R and Bicho MC: Epistatic interaction of
CYP1A1 and COMT polymorphisms in cervical cancer. Oxid Med Cell
Longev. 2016:27698042016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zeng Q, Chen J, Li Y, Werle KD, Zhao RX,
Quan CS, Wang YS, Zhai YX, Wang JW and Youssef M: LKB1 inhibits
HPV-associated cancer progression by targeting cellular metabolism.
Oncogene. 36:1245–1255. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zeng Q, Zhao RX, Chen J, Li Y, Li XD, Liu
XL, Zhang WM, Quan CS, Wang YS, Zhai YX, et al: O-linked
GlcNAcylation elevated by HPV E6 mediates viral oncogenesis. Proc
Natl Acad Sci USA. 113:9333–9338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Erickson JR, Pereira L, Wang L, Han G,
Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, et
al: Diabetic hyperglycaemia activates CaMKII and arrhythmias by
O-linked glycosylation. Nature. 502:372–376. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bodily JM, Mehta KP and Laimins LA: Human
papillomavirus E7 enhances hypoxia-inducible factor 1-mediated
transcription by inhibiting binding of histone deacetylases. Cancer
Res. 71:1187–1195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hoppe-Seyler K, Bossler F, Braun JA,
Herrmann AL and Hoppe-Seyler F: The HPV E6/E7 oncogenes: Key
factors for viral carcinogenesis and therapeutic targets. Trends
Microbiol. 26:158–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Costello LC and Franklin RB: Testosterone
and prolactin regulation of metabolic genes and citrate metabolism
of prostate epithelial cells. Horm Metab Res. 34:417–424. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Franklin RB, Zou J, Ma J and Costello LC:
Protein kinase C alpha, epsilon and AP-1 mediate prolactin
regulation of mitochondrial aspartate aminotransferase expression
in the rat lateral prostate. Mol Cell Endocrinol. 170:153–161.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
McMurray RW: Estrogen, prolactin, and
autoimmunity: Actions and interactions. Int Immunopharmacol.
1:995–1008. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Telleria CM, Zhong L, Deb S, Srivastava
RK, Park KS, Sugino N, Park-Sarge OK and Gibori G: Differential
expression of the estrogen receptors alpha and beta in the rat
corpus luteum of pregnancy: Regulation by prolactin and placental
lactogens. Endocrinology. 139:2432–2442. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Frasor J and Gibori G: Prolactin
regulation of estrogen receptor expression. Trends Endocrinol
Metab. 14:118–123. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Helguero LA, Faulds MH, Gustafsson JA and
Haldosén LA: Estrogen receptors alfa (ERalpha) and beta (ERbeta)
differentially regulate proliferation and apoptosis of the normal
murine mammary epithelial cell line HC11. Oncogene. 24:6605–6616.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Shappell NW: Ergovaline toxicity on Caco-2
cells as assessed by MTT, alamarBlue, and DNA assays. In Vitro Cell
Dev Biol Anim. 39:329–335. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Quent VM, Loessner D, Friis T, Reichert JC
and Hutmacher DW: Discrepancies between metabolic activity and DNA
content as tool to assess cell proliferation in cancer research. J
Cell Mol Med. 14:1003–1013. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang P, Henning SM and Heber D:
Limitations of MTT and MTS-based assays for measurement of
antiproliferative activity of green tea polyphenols. PLoS One.
5:e102022010. View Article : Google Scholar : PubMed/NCBI
|