Introduction
In normal cells, apoptosis is a genetically
controlled process involving several pathways regulating
development and maintaining homeostasis (1,2). Defects
in apoptotic pathways can lead to pathological conditions such as
malignancy, metastasis and chemotherapy resistance (3). Apoptosis is mediated by two basic
pathways: Extrinsic (death receptor-mediated) and intrinsic
(mitochondrial or Bcl-2-regulated). Initiation of the extrinsic
apoptotic pathway requires the interaction of ligands such as tumor
necrosis factor α (TNF-α) and fatty acid synthase (FAS) with
their transmembrane receptors (4,5). In
particular, the intrinsic pathway maintains the balance between
antiapoptotic and proapoptotic proteins (6). To resist apoptosis, cancer cells either
upregulate the expression of antiapoptotic proteins such as Bcl-2
and Bcl-xL or downregulate the expression of proapoptotic proteins
such as Bax and Bak, and both proteins are regulated by the p53
tumor-suppressor gene (7). Moreover,
in an aggressive malignant phenotype, the overexpressed Bcl-2
protein prevents the release of cytochrome c from the
mitochondrial membrane, which leads to interruption of the
intrinsic apoptotic signaling pathway and prevents apoptotic cell
death (8). Similarly, in many types
of cancer, the overexpression of inhibitor of apoptosis (IAP)
family members is a challenge in chemoresistance (9) and is considered a therapeutic target in
apoptosis-inducing strategies (10).
Breast cancer (BC) is the most commonly diagnosed
cancer and the second leading cause of death among women in the
United States (11). BC is commonly
classified according to the gene expression profile, and the
triple-negative breast cancer (TNBC) subgroup is the most
aggressive and metastatic, representing approximately 10–15% of all
BC cases (12). TNBC is known to be
more common among African-American (AA) patients than Caucasian
American (CA) patients (2). Indeed,
TNBC treatment options are limited because of the absence of the
three characteristic receptors: Estrogen (ER), progesterone (PR)
and human epidermal growth factor (Her2/neu) (13,14).
Although TNBC has initial higher response rates to a variety of
chemotherapy agents (15),
approximately 30% of patients present with a poor prognosis, and
treatment failure leads to a median survival of 1 year (16).
Many studies have demonstrated the medicinal
importance of the polyphenol compound gossypol (GOSS), a minor
constituent of cotton (Gossypium hirsutum L.) seeds
(17–19). GOSS has been used in China as a male
contraceptive, as well as for treating malaria and viral infections
(20,21). GOSS has been suggested to be a potent
anticancer agent against BC (22).
Indeed, the antiproliferative and anti-metastatic effects of GOSS
have been demonstrated in several human cancers, including leukemia
(23), glioma (24), colon (25), prostate (26), adrenal (27) and breast cancer (28–30). The
antiproliferative effect of GOSS is mediated through the induction
of cellular apoptosis (31).
Furthermore, the apoptotic effect of the compound was detected in
different human cells, including multiple myeloma (32,33),
synovial sarcoma (34) pharynx,
tongue and salivary gland (35),
prostate (36–38), colon (39), ovarian (40,41)
gastric (42), leukemia (43,44) and
pituitary (45), in addition to
breast (31,46). In cancer therapy, the combination of
multiple agents is key to overcoming the resistance mechanisms of
the tumor (47), and GOSS has been
found to induce an apoptotic effect in various human cancer cells
in combination with low doses of taxanes (46), doxorubicin (34), dexamethasone (43) and valproic acid (36).
Therefore, the present study was designed to examine
the effect of the natural compound GOSS on two human TNBC cell
lines, MDA-MB-231 (MM-231) and MDA-MB-468 (MM-468), representing
the CA and AA races, respectively (48). In the present study, we investigated
the effect of GOSS on cell viability, proliferation and colony
formation. We hypothesized that GOSS alters the expression of
different apoptosis-related genes that mediate the
antiproliferative effect of GOSS. The present study enhanced our
understanding of events associated with cell death following GOSS
treatment.
Materials and methods
Materials and reagents
GOSS (purity ≥90%), doxorubicin (purity ≥99%), and
cell culture flasks were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Trypsin-EDTA solution and Alamar
Blue® (a solution of resazurin fluorescence dye) were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Dimethyl
sulfoxide (DMSO), penicillin/streptomycin and Dulbecco's
phosphate-buffered saline (DPBS) were obtained from the American
Type Culture Collection (ATCC; Manassas, VA, USA). Dulbecco's
modified Eagle's medium (DMEM), heat-inactivated fetal bovine serum
(FBS), and cell culture plates were purchased from VWR
International (Radnor, PA, USA). An Annexin V-FITC Apoptosis
Detection Kit Plus (cat. no. 68FT-Ann VP-S) was purchased from
RayBiotech (Norcross, GA, USA). A DNA-free™ kit (cat. no. AM1907)
was purchased from Life Technologies, Inc. (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). An iScript™ cDNA Synthesis kit
(cat. no. 170-8890), SsoAdvanced™ Universal SYBR® Green
Supermix and the Human Apoptosis PCR array (SAB Target List) H96
were purchased from Bio-Rad Laboratories (Hercules, CA, USA).
Cell culture
Two TNBC cell models, MM-231 and MM-468, were
purchased from the American Type Culture Collection (ATCC). Both
cell lines were grown in 75-ml tissue culture (TC) flasks at 37°C
in a humidified 5% CO2 incubator and subcultured as
needed with trypsin/EDTA (0.25%). The DMEM contained 4 mM
L-glutamine and was supplemented with 10% heat-inactivated FBS
(v/v) and 1% penicillin/streptomycin salt solution (100 U/ml and
0.1 mg/ml, respectively). The DMEM was supplemented with 2.5%
heat-inactivated FBS.
Cell viability assay
In this study, cells were plated at a density of
5×104 cells/well in 96-well plates and incubated
overnight at 37°C. GOSS was solubilized in dimethyl sulfoxide
(DMSO), and both types of cells were treated for 24 h with the
compound (concentration ranges of 0–100 µM in MM-231 cells and 0–50
µM in MM-468 cells). Control wells were treated with DMSO at the
highest concentration used (<0.1%), and wells treated in the
same manner but without cells were used as blanks. In this study,
Alamar Blue® was used to determine the cell viability as
previously described (49). The
fluorescent fuchsia-reduced resazurin dye was measured at an
excitation/emission wavelength of 530/590 nm using a Synergy HTX
Multi-Mode microplate reader (BioTek Instruments, Inc., Winooski,
VT, USA).
Cell proliferation and clonogenic
assays
The inhibition of cell proliferation by GOSS was
determined for MM-231 and MM-468 TNBC cells based on the
dose-response viability study concentrations (50) using Alamar Blue®. Briefly,
cells were plated at an initial density of 1×104
cells/well in 96-well plates and incubated overnight at 37°C. The
cells were treated for 96 h with GOSS at concentrations ranging
from 0 to 100 µM in MM-231 cells and from 0 to 50 µM in MM-468
cells in a final volume of 200 µl/well. Control cells were exposed
to DMSO at a concentration of <0.1%, and equivalent wells
without cells were used as a blank. Doxorubicin was used as a
positive control at concentrations ranging from 0 to 10 µM in both
cell lines. Proliferation was measured at different intervals up to
96 h for GOSS-treated cells and at 72 h for doxorubicin-treated
cells (51) by adding Alamar
Blue® as previously described (49). The plates were read at an
excitation/emission wavelength of 530/590 nm.
A clonogenic assay was performed to measure the
long-term effect of GOSS on MM-231 and MM-468 TNBC cells. Both cell
lines were seeded and treated similarly to the proliferation study
described above. After either a 1- or a 6-h exposure period, the
GOSS-containing experimental media were replaced by growth media
after washing the cells with DPBS. The cells were allowed to grow
for 7 days, and the colonies formed in both treated and untreated
cells were then evaluated based on the reduction of Alamar
Blue® as previously described in the Cell viability
assay.
Apoptosis assay
The apoptotic effect of GOSS was determined in
MM-231 and MM-468 cells. Briefly, each cell line was seeded at an
initial concentration of 5×105 cells/well in 6-well
plates and incubated overnight at 37°C. To induce apoptosis, cells
were treated for 24 h with GOSS at concentrations ranging from 0 to
100 and from 0 to 50 µM in MM-231 and MM-468 cells, respectively,
in a final volume of 3 ml/well of experimental media. Control cells
were exposed to DMSO at a concentration of <0.1%. After the 24-h
incubation period, treated and control cells from each well were
harvested, pelleted and washed in phosphate-buffered saline (PBS).
According to the manufacturer's protocol, the cell pellets were
resuspended in 500 µl of the provided 1X Annexin V binding buffer
and labeled with 5 µl each of Annexin V-FITC and propidium iodide
(PI). The apoptotic effect was quantified within 5–10 min on a
FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). For
each sample, 1×104 cells were examined and CellQuest
software (BD Biosciences) was used for acquisition and data
analysis.
Reverse transcription-polymerase chain
reaction (RT-PCR) apoptosis array
This experiment was performed based on the data of
the apoptosis assay using the concentrations that did not cause a
considerable necrosis effect in either cell line: 25 µM in MM-231
cells and 20 µM in MM-468 cells. Briefly, two T-75 flasks of
10×106 cells (control and treated for each cell line)
were incubated overnight at 37°C and for an additional 24-h in the
absence or presence of the previously indicated concentrations of
the test compound. The cells from each sample were pelleted and
mixed with 1 ml of TRIzol reagent to isolate total RNA.
Subsequently, 0.2 ml of chloroform was added to each sample,
vortexed, incubated at room temperature (RT) for 2–3 min and
centrifuged for 15 min at 10,000 × g and at 2–8°C. The aqueous
phase was collected and mixed with 0.5 ml of isopropyl alcohol to
precipitate the RNAs. The RNA pellets were then dissolved in ~30–50
µl of Nuclease-free water to measure RNA quantity and quality in
each sample using a NanoDrop spectrophotometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.). Finally, cDNA
representing the control or treated cells was synthesized using the
iScript™ cDNA Synthesis kit according to the manufacturer's
protocol. The obtained cDNA was reconstituted in Nuclease-free
water, and the 96-well apoptosis array was loaded with 10 µl each
of the reconstituted cDNA (2.3 ng) and SsoAdvanced™ Universal
SYBR® Green Supermix (Bio-Rad Laboratories) and then
placed for 5 min in a shaker and centrifuged at 1,000 × g for 1
min. The PCR run was established with 39 cycles of denaturation as
follows: 30-sec activation at 95°C, 10-sec denaturation at 95°C;
20-sec annealing at 60°C; and 31-sec extension at 65°C using a
Bio-Rad CFX96 Real-Time System (Bio-Rad Laboratories). All
real-time PCRs were performed in triplicate for each cell line.
Gene expression was analyzed using the CFX 3.1 Manager software
(Bio-Rad Laboratories) and verified with Student's t-test.
Statistical analysis
Data for this study were analyzed using GraphPad
Prism 6.2 software (GraphPad Software, Inc., San Diego, CA, USA).
All data points were obtained from the average of at least two
independent studies and are expressed as the mean ± SEM. For the
viability assay, IC50 values were determined by
non-linear regression with the R2 best fit and the
lowest 95% confidence interval. In both the viability and apoptosis
assays, the significance of the difference between each control and
its related treatment group was determined using one-way analysis
of variance (ANOVA) followed by Bonferroni's multiple comparison
test. For both the proliferation and clonogenic assays, the
statistical analysis was performed with two-way analysis of
variance (ANOVA) between each exposure period and other(s) followed
by Bonferroni's multiple comparison test. Overall, a difference was
considered significant at *P<0.05 and ****P<0.0001 (as
indicated in the figures and legends). Gene expression was analyzed
using CFX 3.1 Manager software (Bio-Rad Laboratories) and verified
with the Student's t-test.
Results
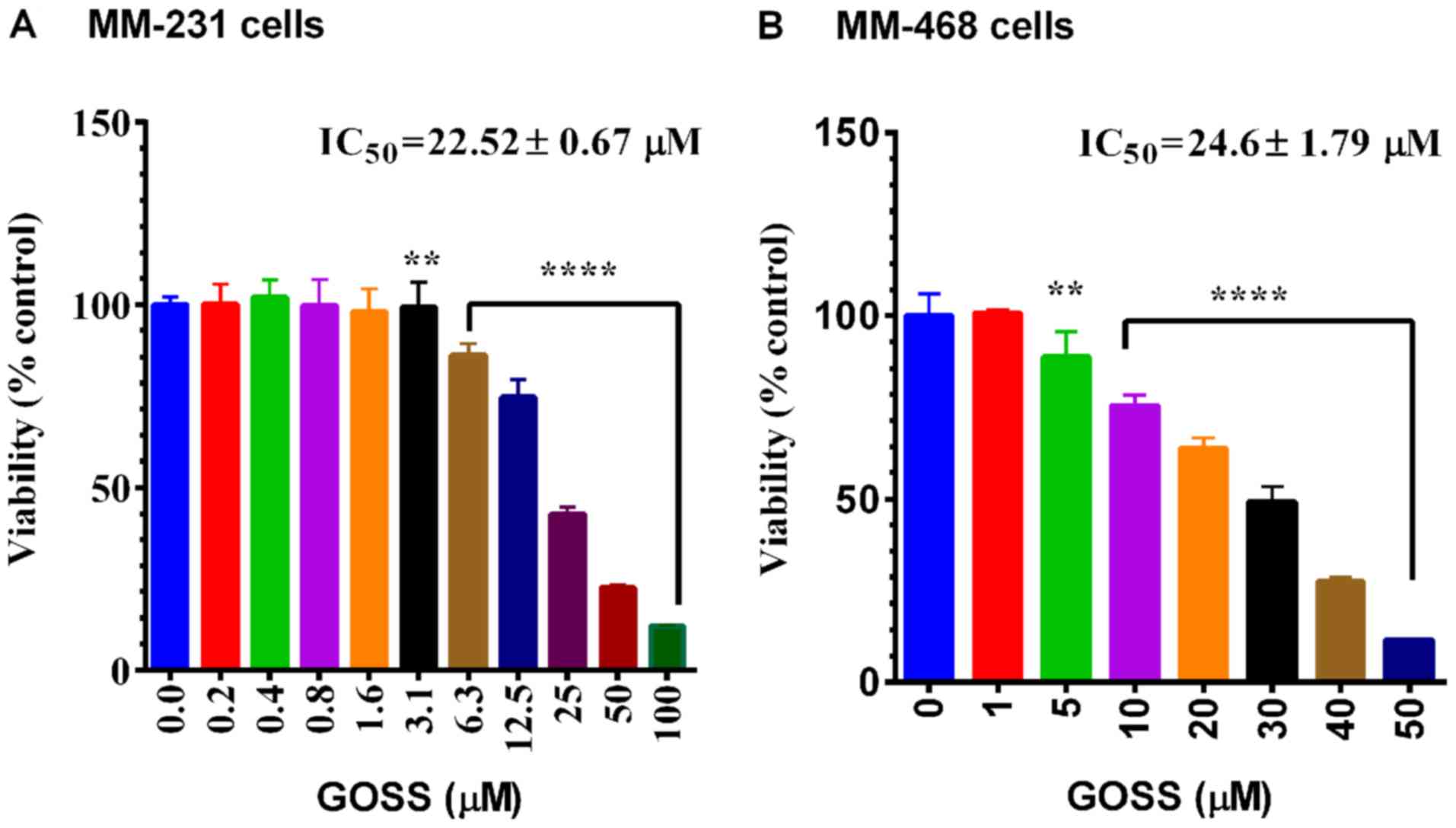
The anticancer effects of GOSS were examined in both
MM-231 and MM-468 cell lines. As indicated in Fig. 1A and B, a highly significant effect
(P<0.0001) of GOSS on cell viability was found in MM-231 and
MM-468 cells at different concentrations of the compound (6.3–100
and 10–50 µM in MM-231 and MM-468, respectively). In both cell
lines, the obtained IC50 did not show a significant
difference in response to the compound (IC50=22.52±0.67
and 24.6±1.79 µM for MM-231 and MM-468 cells, respectively).
To measure the growth inhibitory potency of GOSS in
MM-231 and MM-468 cell lines, both antiproliferative and
clonogenicity assays were performed. In both cell lines, GOSS
inhibited cell proliferation and colony formation in a dose- and
time-dependent manner compared to the control cells (Fig. 2). The inhibition of cell
proliferation, as indicated by the reduction of resazurin and
verified by the decrease in the IC50 values at the
different periods of exposure, is shown in Fig. 2A and C (MM-231 and MM-468 cells,
respectively). Indeed, the IC50 values were reduced
significantly (P<0.0001), from 23.17 to 5.34 µM in MM-231 cells
and from 28.53 to 8.05 µM in MM-468 cells at the 24-vs. 96-h
exposure periods. Furthermore, in both cell lines, GOSS
significantly inhibited cell proliferation (P<0.0001) at the
24-h treatment period vs. other periods, but there was no
significant difference in cell proliferation inhibition between the
72-vs. 96-h treatment period.
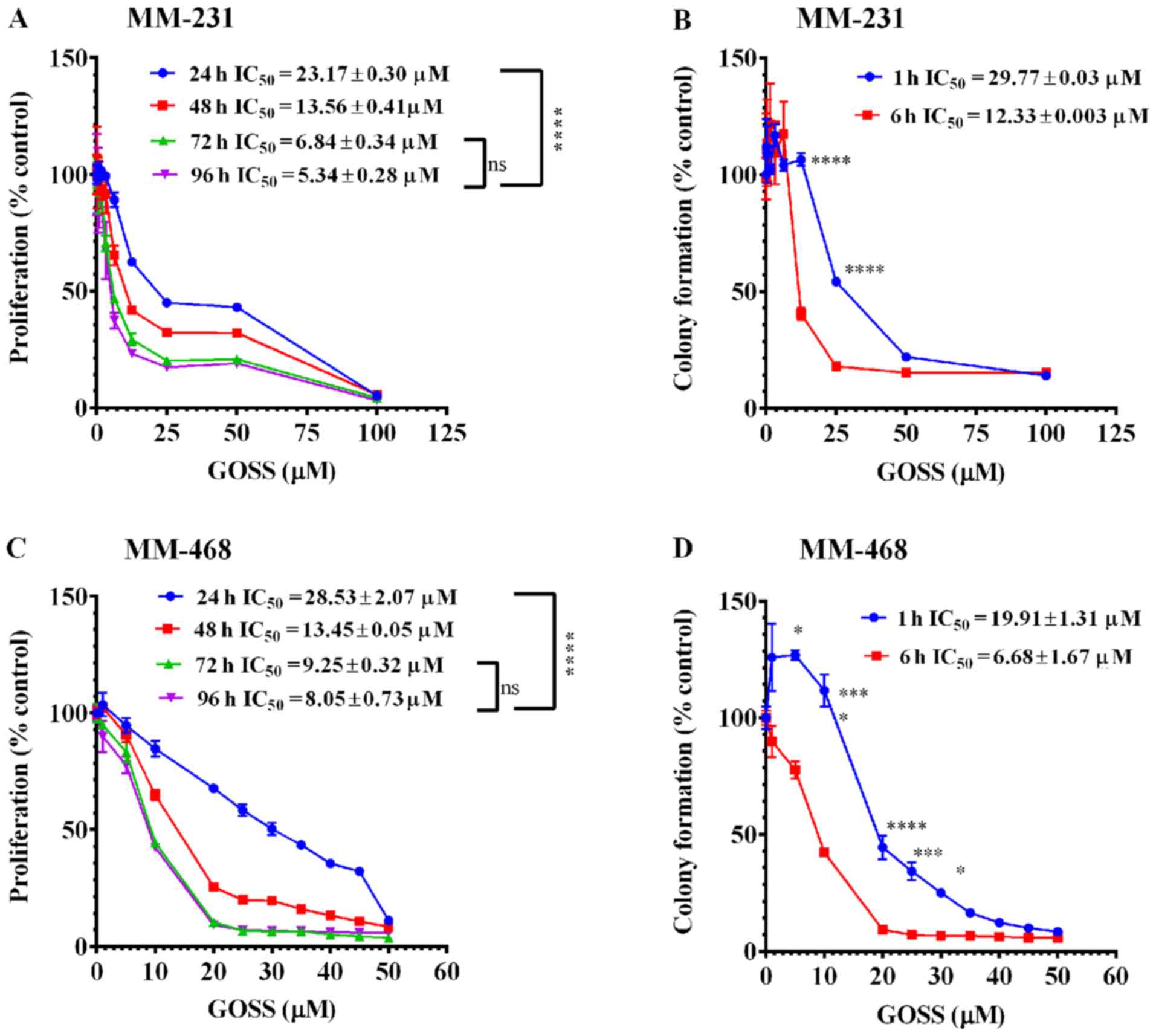 | Figure 2.Effect of GOSS on proliferation and
colony formation in MM-231 and MM-468 TNBC cell lines. In the cell
proliferation study, cells were incubated for 96 h with GOSS at
concentration ranges of 0–100 µM (A) and of 0–50 µM (C) in MM-231
and MM-468 cells, respectively. For the colony formation assay,
MM-231 (B) and MM-468 (D) cells were exposed to similar
concentrations of GOSS for either 1 or 6 h and then washed and
allowed to grow in regular cell culture media for 7 days. In both
assays, each data point represents the mean ± SEM of three
independent experiments, n=4 each. The significance of the
difference between the different exposure periods was calculated
using two-way analysis of variance (ANOVA) followed by Bonferroni's
multiple comparisons test, and ****P<0.0001 indicates a
statistically significant difference between 24-h vs. other
exposure periods, while a non-significant difference was found
between the 72- vs. 96-h exposure periods. Similarly,
****P<0.0001, ***P<0.001 and *P<0.05 indicate a
statistically significant difference in the colony formation
inhibition between the 1- vs. 6-h exposure periods at different
concentrations. GOSS, gossypol; TNBC, triple-negative breast
cancer; MM-231, MDA-MB-231; MM-468, MDA-MB-468; IC50,
half maximal inhibitory concentration; ns, not significant. |
The data also showed that GOSS significantly
inhibited colony formation in both MM-231 and MM-468 cells
(Fig. 2B and D). In MM-231 cells, a
significant delay in colony formation (P<0.0001) was observed
between the 1- vs. the 6-h exposure periods following treatment
with 12.5 and 25.0 µM GOSS (Fig. 2B).
A significant difference was also observed in its counterpart cell
line, MM-468 (P<0.05-P<0.0001), throughout the concentration
range of 5–30 µM, as shown in Fig.
2D.
Nonetheless, the data indicate that GOSS has similar
growth inhibitory potency on both racially distinct cell lines. The
colony formation assessment indicated that after a 6-h treatment
period, GOSS was ~2-fold more effective in MM-468 cells
(IC50=6.68±1.67 µM) than in MM-231 cells
(IC50=12.33±0.003 µM) (Fig. 2B
and D).
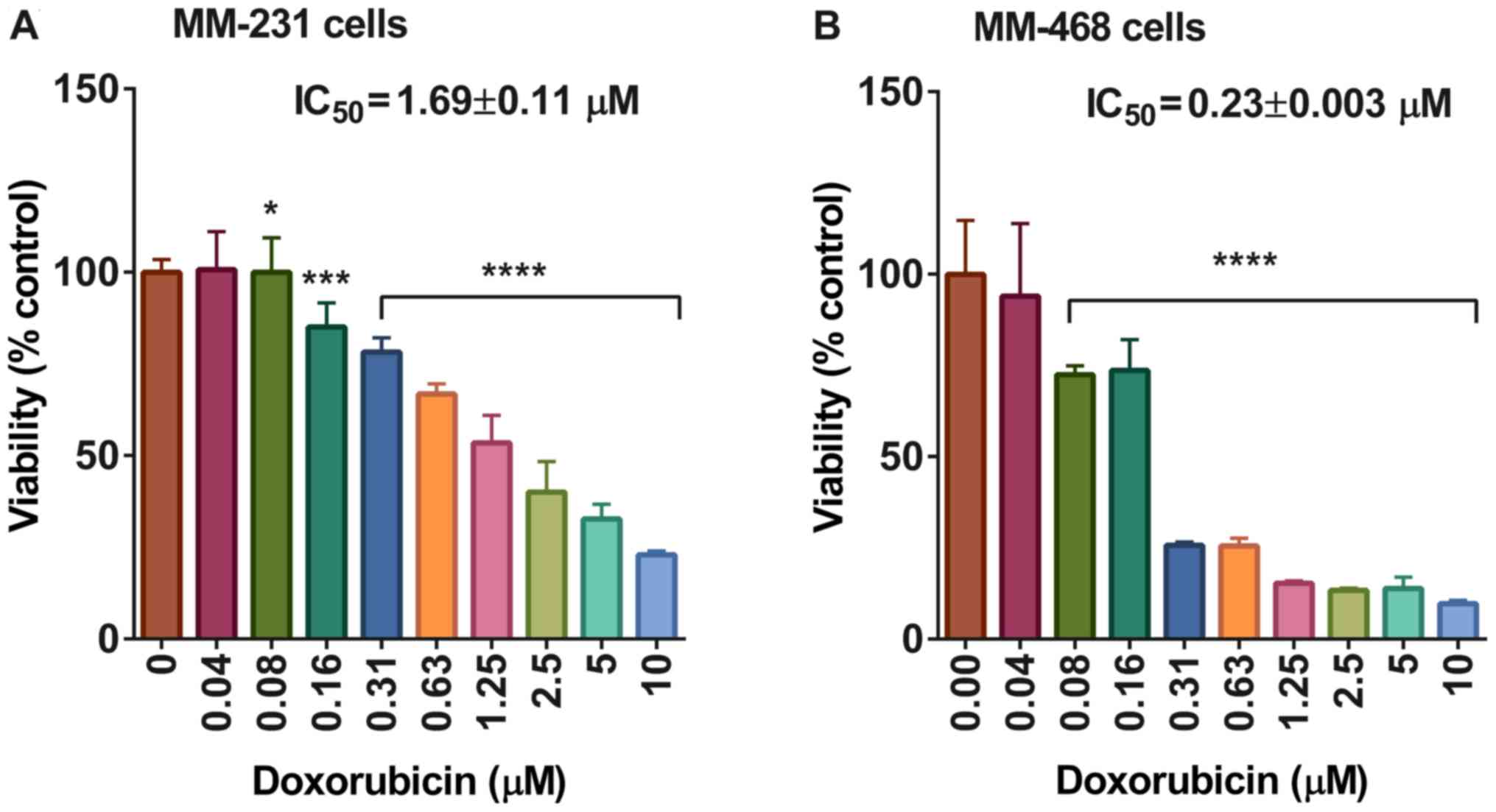
On the other hand, the IC50 value of
cells after 72 h of exposure to doxorubicin was 1.69±0.11 and
0.23±0.003 in MM-231 and MM-468 cells, respectively, with
P<0.05-P<0.0001 (Fig. 3). The
apoptotic effect of GOSS on both MM-231 and MM-468 TNBC cells was
examined to confirm apoptosis mediation of the antiproliferative
effect and colony formation inhibition of GOSS. After 24 h of
exposure, a gradual but significant increase in the number of
apoptotic cells (P<0.0001) was observed in a dose-dependent
manner (Fig. 4A and B). Additionally,
the results indicated that MM-468 cells were more sensitive (almost
2-fold more sensitive) to GOSS than MM-231 cells, and 90% of the
MM-468 cells analyzed were in the apoptotic phase following
treatment with 50 µM GOSS, as shown in Fig. 4B, whereas <60% of MM-231 cells
analyzed exhibited apoptotic effects at 100 µM (Fig. 4A). The obtained data show the strong
apoptotic effect of GOSS on both TNBC cell lines.
Quantitative real-time PCR (qRT-PCR) was performed
in both GOSS-treated cell lines to identify the genes related to
GOSS-induced apoptosis. The profiling of normalized mRNA expression
in both cell lines provided insights into the influence of the
compound on many apoptosis-related genes. In this study, we
identified apoptosis-related genes that were significantly
upregulated/downregulated by the compound. Overall, in both cell
lines, as shown in Fig. 5A and B, the
visually recognized red dots represent the upregulated genes, while
the green dots represent those that were downregulated by GOSS.
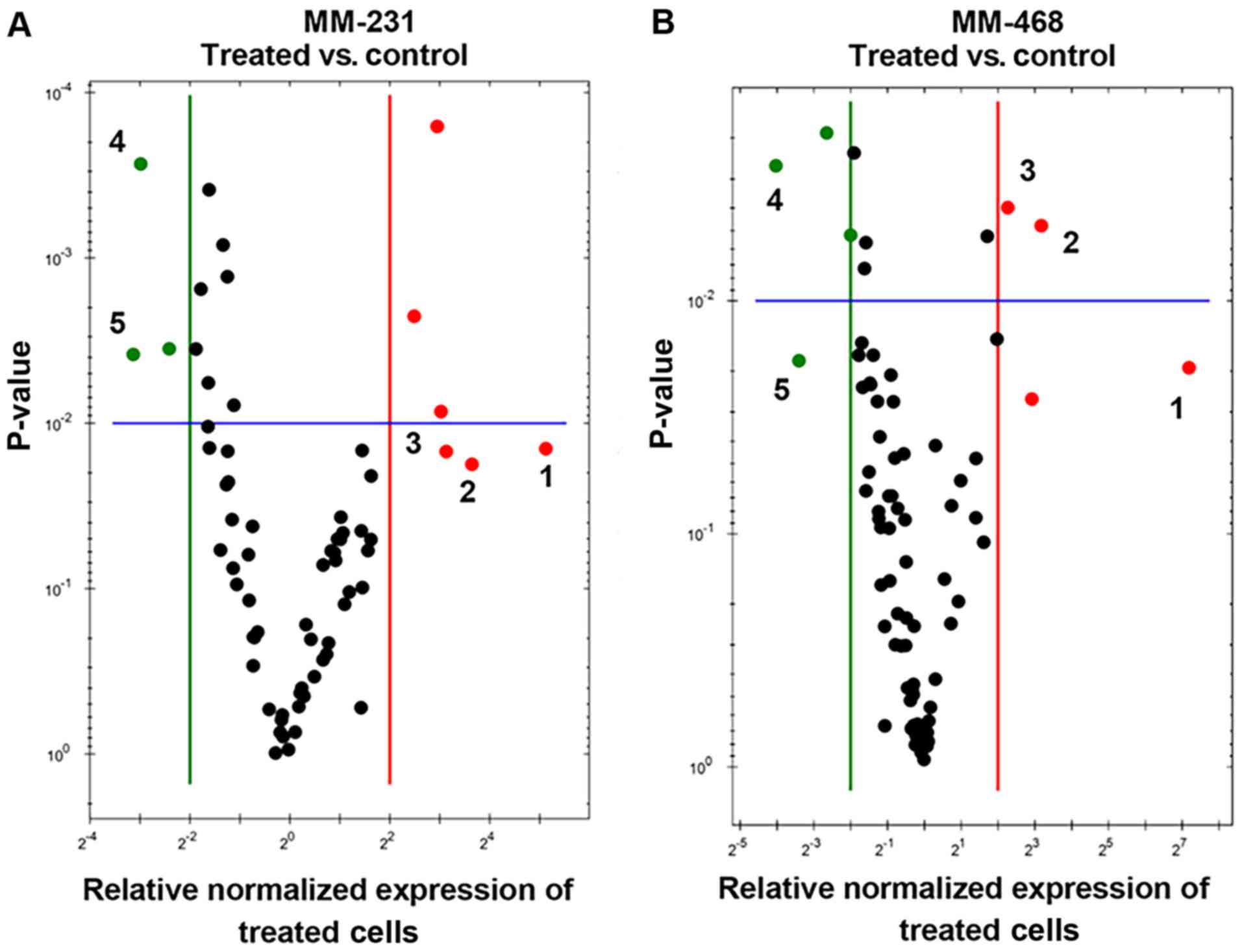 | Figure 5.Scatter plots for MM-231 and MM-468
TNBC cells. Cells were exposed to GOSS for 24 h at a concentration
of 25 µM in MM-231 cells and 20 µM in MM-468 cells and normalized
the mRNA expression of target genes for the control vs. treated
MM-231 (A) and MM-468 (B) cells. Based on the threshold set (green
and red lines), the plot images show the following changes in
apoptosis-related gene expression: Upregulation, red dots;
downregulation, green dots; no change, black dots. In both cell
lines and based on the fold changes, the most upregulated genes are
enumerated from 1–3, while 4–5 refer to the downregulated genes. In
MM-231 cells, GADD45A, TNFRSF9 and BNIP3 were
upregulated, while BIRC5 and DAPK1 were
downregulated. Similarly, in MM-468 cells, TNF, GADD45A and
BNIP3 were upregulated, while BIRC5 and TP73
were downregulated. GOSS, gossypol; TNBC, triple-negative breast
cancer; MM-231, MDA-MB-231; MM-468, MDA-MB-468; GADD45A,
growth arrest and DNA-damage-inducible 45 alpha protein;
TNFRSF9, tumor necrosis factor receptor superfamily 9;
BNIP3, BCL2 interacting protein 3; BIRC5, baculoviral
IAP repeat containing 5; DAPK1, death-associated protein
kinase; TP73, tumor protein 73. |
The specifically impacted genes were quantified and
are presented in Figs. 6 and 7. In MM-231 cells, GOSS significantly
(P<0.05) increased the expression of three genes (GADD45A,
TNFRSF9 and BNIP3) (Fig.
6). Similarly, in MM-468 cells, GADD45A and BNIP3
were upregulated by GOSS treatment (P<0.05-P<0.01) (Fig. 7). Nevertheless, the GADD45A
gene was significantly upregulated in both cell lines; the
fold-increase in MM-231 cells was 33.42 and that in MM-468 cells
was 9.22 (Table I). Moreover, the
measured upregulation of BNIP3 in MM-231 cells was
approximately twice that of its counterpart cell line, MM-468
(Figs. 6 and 7 and Table I).
Two members of the TNF/receptor family of genes were also
increased. TNF was profoundly overexpressed by 159-fold in
MM-468 cells, and an ~12-fold increase in the TNF receptor
TNFRSF9 was found in MM-231 cells. In contrast, GOSS
significantly repressed the mRNA expression of many
apoptosis-related genes (P<0.05-P<0.001). More than 90%
inhibition (≥8-fold decrease) in BIRC5 was observed in both
cell lines, in addition to a 14.79- decrease and an 8.65-fold
decrease in DAPK1 and TP73 in MM-231 and MM-468
cells, respectively (Table I).
Notably, the compound GOSS did not show a significant effect on the
expression of the different caspases (data not shown).
 | Figure 6.Gene expression quantification in
MM-231 cells. Cells were exposed to GOSS for 24 h at a
concentration of 25 µM. Normalized mRNA data indicate a significant
increase in three genes (GADD45A, TNFRSF9 and BNIP3)
in the GOSS-treated MM-231 cells vs. control cells, while two genes
were significantly inhibited (BIRC5 and DAPK1). The
data points represent the mean ± SEM of three independent studies.
The significance of the difference was determined using an unpaired
t-test between the resting vs. treated cells. The difference was
considered significant at *P<0.05, **P<0.01 and
***P<0.001. GOSS, gossypol; MM-231, MDA-MB-231; GADD45A,
growth arrest and DNA-damage-inducible 45 alpha protein;
TNFRSF9, tumor necrosis factor receptor superfamily 9;
BNIP3, BCL2 interacting protein 3; BIRC5, baculoviral
IAP repeat containing 5; DAPK1, death-associated protein
kinase. |
 | Table I.mRNA gene expression changes in
MM-231 and MM-468 TNBC cells. |
Table I.
mRNA gene expression changes in
MM-231 and MM-468 TNBC cells.
|
| Control vs. treated
MM-231 cells | Control vs. treated
MM-468 cells |
|---|
|
|
|
|
|---|
| Target gene | Fold changes | P-value | Target gene | Fold changes | P-value |
|---|
| GADD45A | +33.42 | 0.028 | TNF | +158.98 | 0.023 |
| TNFRSF9 | +12.35 | 0.018 | GADD45A | +9.22 | 0.005 |
| BNIP3 | +8.75 | 0.015 | BNIP3 | +4.84 | 0.004 |
| BIRC5 | −7.96 | 0.0003 | BIRC5 | −24.32 | 0.002 |
| DAPK1 | −14.79 | 0.0012 | TP73 | −8.65 | 0.037 |
Discussion
Apoptosis is a pivotal cellular process that
maintains genomic integrity (52).
Genomic alterations, including the upregulation of genes involved
in DNA synthesis, cell division, proliferation, and cell cycle
progression, have been shown to be key mediators in BC development
and progression. (53,54). In particular, the apoptosis
methylation-mediated silencing of genes is the most important
epigenetic mechanism for regulating normal gene expression
(1).
The present study provides evidence for the
anticancer effect of the natural polyphenol gossypol (GOSS) in two
triple-negative breast cancer (TNBC) cell lines: MDA-MB-231
(MM-231) and MDA-MB-468 (MM-468). However, future studies are
needed to determine the relative toxicity of GOSS in TNBC cells
compared to non-cancer cells. This compound impacted the molecular
apoptotic pathway by altering the mRNA gene expression of specific
apoptosis-related genes (Figs.
5–7 and Table I). Overall, the obtained data are
consistent with those from previous studies (22,29,35). This
study shows similar cytotoxic (Fig. 1A
and B) and antiproliferative (Fig. 2A
and C) effects in both cell lines. Notably, MM-468 cells showed
a higher response during the colony formation (Fig. 2B and D), doxorubicin antiproliferation
(Fig. 3) and apoptotic (Fig. 4) assays. However, doxorubicin was an
extremely potent antiproliferative agent, and the obtained
IC50 values were compatible with those previously
reported (51). However, meager
previous investigations have examined GOSS effects on diet, and a
previous study on women with refractory metastatic breast cancer
(BC) indicated that the maximally tolerated dose of GOSS was 40
mg/day (55).
In normal cells, several molecular pathways control
apoptosis and orchestrate the balance between cell proliferation
and cell death to maintain homeostasis. However, in cancer cells,
triggering the genes involved in these signaling pathways leads to
uncontrolled cell proliferation and tumorigenesis. Therefore,
understanding the mechanisms of apoptosis and targeting the
expression of these apoptotic genes are crucial in the development
of targeted cancer therapy (56). The
profiling of apoptosis-related genes in both MM-231 and MM-468 TNBC
cell lines revealed that GOSS could induce both intrinsic and
extrinsic apoptotic pathways. In the present study, GOSS was found
to upregulate the proapoptotic genes GADD45A, BNIP3, TNF and
TNFRSF9, the critical initiators of the extrinsic apoptotic
pathway. Moreover, the compound was efficiently able to attenuate
the expression of the survivin BIRC5 gene, a well-known
inhibitor of intrinsic apoptosis, as well as two additional
proapoptotic genes, DAPK1 and TP73. Although GOSS
increased GADD45A expression in both cell lines, the gene
was highly upregulated in MM-231 cells (33-fold increase) compared
with MM-468 cells (Table I). The
proapoptotic gene, growth arrest and DNA damage-induced 45 alpha
(GADD45A) is a member of the stress sensor family
GADD45, which normally controls many cellular functions,
including DNA repair, apoptosis, cell cycle regulation and
genotoxic stress (57). However,
GADD45 genes are epigenetically inactivated in different
types of cancer cells (1). In TNBC,
there is a strong association between a low level of the
GADD45A gene and the lack of the three hormone receptors,
ER, PR and Her2/neu (58). Moreover,
in BC, the hypermethylated GADD45A gene is regulated by two
major tumor suppressor proteins: p53 and BRCA1 (59). Furthermore, the upregulation of
GADD45A may be involved in apoptosis by activating the JNK
and/or p38 MAPK signaling pathways (60,61).
In both MM-231 and MM-468 cell lines, GOSS
upregulated BNIP3 expression. However, the increase in gene
expression in MM-231 cells was ~2-fold greater than that in its
counterpart cell line, MM-468 (Figs.
6 and 7 and Table I). The death-inducing mitochondrial
protein BNIP3 is a member of the proapoptotic Bcl-2 family
of proteins that promotes both apoptosis and autophagy.
BNIP3 contains a C-terminal transmembrane (TM) domain as
well as a sequence resembling a BH3 domain, and both are essential
for apoptosis induction (62,63). Moreover, BNIP3 mediates novel
necrosis-like mechanisms by opening the mitochondrial permeability
transition (PT) pore independent of caspase activation, cytochrome
c release, Apaf-1, or the nuclear translocation of
apoptosis-inducing factor (64).
Similar to our finding, in BC, the induction of BNIP3 was
found to induce apoptosis via (FAS) inhibition, which leads to the
suppression of cell proliferation (63,65).
Various stimuli can trigger apoptosis, one of which
is the signaling protein TNF superfamily (66). In the present study, TNF, also
known as the TNF-α gene (67),
was the most upregulated in MM-468 cells. The compound GOSS
markedly increased the expression of TNF by 159-fold
(Fig. 7 and Table I). The fact that TNF is the
most potent inducer of apoptosis in the TNF superfamily
(68) can explain the higher response
of MM-468 cells to the apoptotic effect induced by the compound.
Furthermore, multifunctional TNF can trigger both cell
proliferation and cell death. In particular, the upregulation of
NF-κB-related genes increases cell viability and proliferation.
However, the activation of different caspases leads to apoptosis
induction (68,69). Contrary to healthy cells, the
multifunctional proinflammatory gene TNF-α is detected in
many cancers, including ovarian, renal and breast (70–72), and
has been found to increase cell survival and proliferation through
NF-κB activation (73). Additionally,
the TNF superfamily also plays a crucial role in regulating
tissue homeostasis by activating the extrinsic apoptotic pathway
(74) and triggering various
signaling pathways, including the activation of caspases, impacting
proliferation and inducing apoptosis (75).
In MM-231 cells, a 12-fold upregulation in
TNF receptor superfamily 9 (TNFRSF9 also known as
CD137, 4–1BB, or ILA) was found (Fig.
5 and Table I). TNFRSF9 is
a costimulatory receptor that is involved in apoptosis.
TNFRSF9 is expressed by activated monocytes and lymphocytes
(76), while its ligand is expressed
by monocytes and B cells (3). In
MM-231 cells, the most important ability is for CD137 to synergize
the apoptotic effect of suberoylanilide hydroxamic acid (SAHA), a
histone deacetylase (HDAC) inhibitor with anticancer properties
(77).
GOSS markedly and similarly decreased the expression
of BIRC5 in both TNBC cell lines, as shown in Figs. 5 and 6
and Table I. BIRC5, or the
survivin gene is the smallest member of the IAP family (78). In the human genome, the gene encoding
BIRC5 is among one of the highly specific tumor genes
(79) that prevent apoptosis by
inhibiting the activity of different caspases (80), leading to aggressive tumor behavior
and poor clinical outcomes (81).
Compared to normal tissues, an increase in BIRC5 expression
has been demonstrated in different types of cancers, including
pancreatic (82), lung (83), colon and ovarian (84), esophageal and skin (85), colorectal and lymphoma (86) and prostate (87). In particular, in TNBC cells, the
highly expressed BIRC5 is considered a marker in the early
diagnosis of BC and was found to correlate with the resistance to
radiation and chemotherapy (79,81,84).
Additionally, two more apoptosis-related genes, DAPK1 and
TP73, were significantly downregulated in GOSS-treated
MM-231 and MM-468 cells, respectively (Figs. 6 and 7
and Table I).
The proapoptotic death-associated protein kinase 1
(DAPK1) is characterized by its C-terminal death domain and
plays an essential role in cell survival, proliferation and death
(88). Compared to normal cells,
lower expression of DAPK1 mRNA has been observed in
different cancer cell lines; however, in the presence of
TNF-α, the gene can decrease cell growth (89). The present study indicated repression
of DAPK1 gene expression in treated MM-231 cells. In
addition, TNF-α-stimulated apoptosis was inhibited by
overexpression of DAPK1 (89).
Taken together, our results suggest that DAPK1
downregulation may be a promising target in cancer therapy,
particularly in MM-231 TNBC cells.
Surprisingly, the compound GOSS downregulated the
gene TP73 in MM-468 cells. Tumor protein p73 (TP73)
is a member of the TP53 (p53) tumor-suppressor gene family. This
family is characterized by a proapoptotic role (90) and is involved in cell cycle regulation
and apoptosis (91). In TNBC MM-231
cells (92,93), upregulated TP73 can replace
(92) or activate P53 gene
transcription, induce apoptosis and considerably affect tumor
progression (94). On a molecular
basis, gene polymorphisms can modify their specific functions
(95). Certainly, there is an
association between the TP73 polymorphism (G4C14-A4T14) and
cancer risk (94). Although it was
not found in BC, in sympathetic neurons, the p73 isoform
lacking the transactivation domain has been found to act as a
neuronal antiapoptotic protein and counteract the proapoptotic
function of p53 (90).
The data obtained in this study provide evidence
that the apoptotic effect of GOSS is related to either the
upregulated proapoptotic genes and TNF-α or repression of the
inhibitor of apoptosis genes or both. However, previous studies
have shown the ability of GOSS to induce apoptotic effects by DNA
fragmentation (96), DNA synthesis
inhibition (30), and arresting
S-phase without affecting RNA and protein synthesis (97). The molecular mechanism of GOSS-induced
apoptosis was previously determined to be associated with the
interaction between the compound and the antiapoptotic proteins
Bcl-2 and Bcl-xl in the mitochondria (98). In other words, the BH3 mimetic GOSS
ectopically expresses Bcl-2 and Bcl-xL (25), inhibiting the Bcl-2/Mcl-1 pathway
(33) and the heterodimerization of
Bcl-xL/Bcl-2 by releasing proapoptotic proteins (apoptosis-inducing
factor, AIF) (37). GOSS can also
induce apoptosis by releasing cytochrome c and activating
the proapoptotic protein Bak or Bax/Bak via conformational changes
in overexpressed Bcl-2 (99,100). Moreover, inhibiting the
phosphorylation of ERK1/2 and AKT, stimulating p38 and JNK1/2
protein phosphorylation, and inhibiting ErbB2 protein expression
have been found to be mechanisms of GOSS-induced apoptosis
(35).
In conclusion, the present study elucidated the
genes involved in the apoptotic molecular mechanism of the
polyphenol compound GOSS in two different TNBC cell models, MM-231
and MM-468. This study demonstrated that GOSS was more potent in
MM-468 cells in inducing apoptosis and delaying colony formation.
However, the impact of the compound on cell viability and
proliferation was almost the same, and the data obtained did not
show a considerable difference in cell response. In parallel, GOSS
upregulated two proapoptotic genes (GADD45A and
BNIP3) and attenuated the expression of BIRC5 in both
MM-231 and MM-468 cells. Additionally, GOSS increased the
expression of TNFRSF9 and repressed DAPK1 in MM-231
cells, while an increase in TNF gene expression (159-fold)
and repression in TP73 were detected in MM-468 cells. The
results obtained emphasize the importance of the polyphenol
gossypol as a compound that can induce cancer cell apoptosis
through extrinsic apoptosis-related genes. This compound may be
targeted for TNBC treatment, particularly in African-American
patients.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Institute of Minority Health and Health Disparity through grants
G12 MD007582 and P20 MD006738.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
Conceptualization of the study was achieved by SSM
and KFAS. The research methodology was designed by SSM, HA, CC,
NOZ, PM and KFAS. Formal analysis of the data was conducted by SSM
and KFAS. Funding acquisition was accomplished by KFAS. Project
administration was carried out by KFAS, and study resources were
obtained by SSM and KFAS. Software analysis of data and figures was
conducted by SSM and KFAS, and supervision of the research was
conducted by KFAS. Writing of the original draft was undertaken by
SSM, and writing, review and editing of the manuscript were carried
out by SSM, NOZ, PM, HA, CC and KFAS. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Al-Romaih K, Sadikovic B, Yoshimoto M,
Wang Y, Zielenska M and Squire JA: Decitabine-induced demethylation
of 5′ CpG island in GADD45A leads to apoptosis in osteosarcoma
cells. Neoplasia. 10:471–480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Albain KS, Unger JM, Crowley JJ, Coltman
CA Jr and Hershman DL: Racial disparities in cancer survival among
randomized clinical trials patients of the Southwest oncology
group. J Natl Cancer Inst. 101:984–992. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alderson MR, Smith CA, Tough TW,
Davis-Smith T, Armitage RJ, Falk B, Roux E, Baker E, Sutherland GR
and Din WS: Molecular and biological characterization of human
4-1BB and its ligand. Eur J Immunol. 24:2219–2227. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schulze-Osthoff K, Ferrari D, Los M,
Wesselborg S and Peter ME: Apoptosis signaling by death receptors.
Eur J Biochem. 254:439–459. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mishra AP, Salehi B, Sharifi-Rad M,
Pezzani R, Kobarfard F, Sharifi-Rad J and Nigam M: Programmed cell
death, from a cancer perspective: An overview. Mol Diagn Ther.
22:281–295. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wali JA, Masters SL and Thomas HE: Linking
metabolic abnormalities to apoptotic pathways in Beta cells in type
2 diabetes. Cells. 2:266–283. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miyashita T, Krajewski S, Krajewska M,
Wang HG, Lin HK, Liebermann DA, Hoffman B and Reed JC: Tumor
suppressor p53 is a regulator of bcl-2 and bax gene expression in
vitro and in vivo. Oncogene. 9:1799–1805. 1994.PubMed/NCBI
|
|
8
|
Kang MH and Reynolds CP: Bcl-2 inhibitors:
Targeting mitochondrial apoptotic pathways in cancer therapy. Clin
Cancer Res. 15:1126–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fulda S: Targeting inhibitor of apoptosis
proteins (IAPs) for cancer therapy. Anticancer Agents Med Chem.
8:533–539. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mannhold R, Fulda S and Carosati E: IAP
antagonists: Promising candidates for cancer therapy. Drug Discov
Today. 15:210–219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
DeSantis CE, Fedewa SA, Goding Sauer A,
Kramer JL, Smith RA and Jemal A: Breast cancer statistics, 2015:
Convergence of incidence rates between black and white women. CA
Cancer J Clin. 66:31–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Anders CK and Carey LA: Biology,
metastatic patterns, and treatment of patients with triple-negative
breast cancer. Clin Breast Cancer. 9 (Suppl 2):S73–S81. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beaumont T and Leadbeater M: Treatment and
care of patients with metastatic breast cancer. Nurs Stand.
25:49–56. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fernández Y, Cueva J, Palomo AG, Ramos M,
de Juan A, Calvo L, García-Mata J, García-Teijido P, Peláez I and
García-Estévez L: Novel therapeutic approaches to the treatment of
metastatic breast cancer. Cancer Treat Rev. 36:33–42. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu P, Kumar IS, Brown S, Kannappan V,
Tawari PE, Tang JZ, Jiang W, Armesilla AL, Darling JL and Wang W:
Disulfiram targets cancer stem-like cells and reverses resistance
and cross-resistance in acquired paclitaxel-resistant
triple-negative breast cancer cells. Br J Cancer. 109:1876–1885.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Craig DW, O'Shaughnessy JA, Kiefer JA,
Aldrich J, Sinari S, Moses TM, Wong S, Dinh J, Christoforides A,
Blum JL, et al: Genome and transcriptome sequencing in prospective
metastatic triple-negative breast cancer uncovers therapeutic
vulnerabilities. Mol Cancer Ther. 12:104–116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao H, Sethumadhavan K and Bland JM:
Isolation of cottonseed extracts that affect human cancer cell
growth. Sci Rep. 8:104582018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He Z, Zhang H and Olk DC: Chemical
composition of defatted cottonseed and soy meal products. PLoS One.
10:e01299332015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sharifi-Rad M, Fokou PVT, Sharopov F,
Martorell M, Ademiluyi AO, Rajkovic J, Salehi B, Martins N, Iriti M
and Sharifi-Rad J: Antiulcer agents: From plant extracts to
phytochemicals in healing promotion. Molecules. 23(pii): E17512018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin TS, Schinazi RF, Zhu J, Birks E,
Carbone R, Si Y, Wu K, Huang L and Prusoff WH: Anti-HIV-1 activity
and cellular pharmacology of various analogs of gossypol. Biochem
Pharmacol. 46:251–255. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Janero DR and Burghardt B: Protection of
rat myocardial phospholipid against peroxidative injury through
superoxide-(xanthine oxidase)-dependent, iron-promoted fenton
chemistry by the male contraceptive gossypol. Biochem Pharmacol.
37:3335–3342. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu S, Kulp SK, Sugimoto Y, Jiang J, Chang
HL, Dowd MK, Wan P and Lin YC: The (−)-enantiomer of gossypol
possesses higher anticancer potency than racemic gossypol in human
breast cancer. Anticancer Res. 22:33–38. 2002.PubMed/NCBI
|
|
23
|
Moon DO, Kim MO, Lee JD and Kim GY:
Gossypol suppresses NF-kappaB activity and NF-kappaB-related gene
expression in human leukemia U937 cells. Cancer Lett. 264:192–200.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Voss V, Senft C, Lang V, Ronellenfitsch
MW, Steinbach JP, Seifert V and Kögel D: The pan-Bcl-2 inhibitor
(−)-gossypol triggers autophagic cell death in malignant glioma.
Mol Cancer Res. 8:1002–1016. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang M, Liu H, Guo R, Ling Y, Wu X, Li B,
Roller PP, Wang S and Yang D: Molecular mechanism of
gossypol-induced cell growth inhibition and cell death of HT-29
human colon carcinoma cells. Biochem Pharmacol. 66:93–103. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pang X, Wu Y, Wu Y, Lu B, Chen J, Wang J,
Yi Z, Qu W and Liu M: (−)-Gossypol suppresses the growth of human
prostate cancer xenografts via modulating VEGF signaling-mediated
angiogenesis. Mol Cancer Ther. 10:795–805. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Flack MR, Pyle RG, Mullen NM, Lorenzo B,
Wu YW, Knazek RA, Nisula BC and Reidenberg MM: Oral gossypol in the
treatment of metastatic adrenal cancer. J Clin Endocrinol Metab.
76:1019–1024. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moon DO, Choi YH, Moon SK, Kim WJ and Kim
GY: Gossypol decreases tumor necrosis factor-α-induced
intercellular adhesion molecule-1 expression via suppression of
NF-κB activity. Food Chem Toxicol. 49:999–1005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gilbert NE, O'Reilly JE, Chang CJ, Lin YC
and Brueggemeier RW: Antiproliferative activity of gossypol and
gossypolone on human breast cancer cells. Life Sci. 57:61–67. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu YF, Chang CJ, Brueggemeier RW and Lin
YC: Gossypol inhibits basal and estrogen-stimulated DNA synthesis
in human breast carcinoma cells. Life Sci. 53:Pl433–Pl438. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ye W, Chang HL, Wang LS, Huang YW, Shu S,
Sugimoto Y, Dowd MK, Wan PJ and Lin YC: Induction of apoptosis by
(−)-gossypol-enriched cottonseed oil in human breast cancer cells.
Int J Mol Med. 26:113–119. 2010.PubMed/NCBI
|
|
32
|
Lin J, Wu Y, Yang D and Zhao Y: Induction
of apoptosis and antitumor effects of a small molecule inhibitor of
Bcl-2 and Bcl-xl, gossypol acetate, in multiple myeloma in
vitro and in vivo. Oncol Rep. 30:731–738. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sadahira K, Sagawa M, Nakazato T, Uchida
H, Ikeda Y, Okamoto S, Nakajima H and Kizaki M: Gossypol induces
apoptosis in multiple myeloma cells by inhibition of interleukin-6
signaling and Bcl-2/Mcl-1 pathway. Int J Oncol. 45:2278–2286. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baoleri X, Dong C, Zhou Y, Zhang Z, Lu X,
Xie P and Li Y: Combination of L-gossypol and low-concentration
doxorubicin induces apoptosis in human synovial sarcoma cells. Mol
Med Rep. 12:5924–5932. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Benvenuto M, Mattera R, Masuelli L,
Taffera G, Andracchio O, Tresoldi I, Lido P, Giganti MG, Godos J,
Modesti A and Bei R: (±)-Gossypol induces apoptosis and autophagy
in head and neck carcinoma cell lines and inhibits the growth of
transplanted salivary gland cancer cells in BALB/c mice. Int J Food
Sci Nutr. 68:298–312. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao GX, Xu LH, Pan H, Lin QR, Huang MY,
Cai JY, Ouyang DY and He XH: The BH3-mimetic gossypol and
noncytotoxic doses of valproic acid induce apoptosis by suppressing
cyclin-A2/Akt/FOXO3a signaling. Oncotarget. 6:38952–38966. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang M, Liu H, Tian Z, Griffith BN, Ji M
and Li QQ: Gossypol induces apoptosis in human PC-3 prostate cancer
cells by modulating caspase-dependent and caspase-independent cell
death pathways. Life Sci. 80:767–774. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Volate SR, Kawasaki BT, Hurt EM, Milner
JA, Kim YS, White J and Farrar WL: Gossypol induces apoptosis by
activating p53 in prostate cancer cells and prostate
tumor-initiating cells. Mol Cancer Ther. 9:461–470. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu MD, Li LY, Li PH, You T, Wang FH, Sun
WJ and Zheng ZQ: Gossypol induces cell death by activating
apoptosis and autophagy in HT-29 cells. Mol Med Rep. 16:2128–2132.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jin L, Chen Y, Mu X, Lian Q, Deng H and Ge
R: Phosphoproteomic analysis of gossypol-induced apoptosis in
ovarian cancer cell line, HOC1a. Biomed Res Int. 2014:1234822014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang J, Jin L, Li X and Deng H, Chen Y,
Lian Q, Ge R and Deng H: Gossypol induces apoptosis in ovarian
cancer cells through oxidative stress. Mol Biosyst. 9:1489–1497.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xin J, Zhan YH, Xia LM, Zhu HW, Nie YZ,
Liang JM and Tian J: ApoG2 as the most potent gossypol derivatives
inhibits cell growth and induces apoptosis on gastric cancer cells.
Biomed Pharmacother. 67:88–95. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cheng W, Zhao YQ, Li YM and Yang DJ:
Effects of gossypol acetate on apoptosis in primary cultured cells
from patients with lymphoid leukemia and its synergy with
dexamethasone. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 20:229–234.
2012.(In Chinese). PubMed/NCBI
|
|
44
|
Balakrishnan K, Wierda WG, Keating MJ and
Gandhi V: Gossypol, a BH3 mimetic, induces apoptosis in chronic
lymphocytic leukemia cells. Blood. 112:1971–1980. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tang J, Wang Z, Chen L, Huang G and Hu X:
Gossypol acetate induced apoptosis of pituitary tumor cells by
targeting the BCL-2 via the upregulated microRNA miR-15a. Int J
Clin Exp Med. 8:9079–9085. 2015.PubMed/NCBI
|
|
46
|
Karaca B, Atmaca H, Uzunoglu S, Karabulut
B, Sanli UA and Uslu R: Enhancement of taxane-induced cytotoxicity
and apoptosis by gossypol in human breast cancer cell line MCF-7. J
BUON. 14:479–485. 2009.PubMed/NCBI
|
|
47
|
Yoshida R, Niki M, Jyotaki M, Sanematsu K,
Shigemura N and Ninomiya Y: Modulation of sweet responses of taste
receptor cells. Semin Cell Dev Biol. 24:226–231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tate CR, Rhodes LV, Segar HC, Driver JL,
Pounder FN, Burow ME and Collins-Burow BM: Targeting
triple-negative breast cancer cells with the histone deacetylase
inhibitor panobinostat. Breast Cancer Res. 14:R792012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Messeha SS, Zarmouh NO, Mendonca P,
Alwagdani H, Kolta MG and Soliman KFA: The inhibitory effects of
plumbagin on the NF-κB pathway and CCL2 release in racially
different triple-negative breast cancer cells. PLoS One.
13:e02011162018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Citalingam K, Abas F, Lajis NH, Othman I
and Naidu R: Anti-proliferative effect and induction of apoptosis
in androgen-independent human prostate cancer cells by
1,5-bis(2-hydroxyphenyl)-1,4-pentadiene-3-one. Molecules.
20:3406–3430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chougule MB, Patel AR, Jackson T and Singh
M: Antitumor activity of noscapine in combination with doxorubicin
in triple negative breast cancer. PLoS One. 6:e177332011.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Plati J, Bucur O and Khosravi-Far R:
Dysregulation of apoptotic signaling in cancer: Molecular
mechanisms and therapeutic opportunities. J Cell Biochem.
104:1124–1149. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kretschmer C, Sterner-Kock A, Siedentopf
F, Schoenegg W, Schlag PM and Kemmner W: Identification of early
molecular markers for breast cancer. Mol Cancer. 10:152011.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ma XJ, Salunga R, Tuggle JT, Gaudet J,
Enright E, McQuary P, Payette T, Pistone M, Stecker K, Zhang BM, et
al: Gene expression profiles of human breast cancer progression.
Proc Natl Acad Sci USA. 100:5974–5979. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Van Poznak C, Seidman AD, Reidenberg MM,
Moasser MM, Sklarin N, Van Zee K, Borgen P, Gollub M, Bacotti D,
Yao TJ, et al: Oral gossypol in the treatment of patients with
refractory metastatic breast cancer: A phase I/II clinical trial.
Breast Cancer Res Treat. 66:239–248. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhan Q: Gadd45a, a p53- and
BRCA1-regulated stress protein, in cellular response to DNA damage.
Mutat Res. 569:133–143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tront JS, Willis A, Huang Y, Hoffman B and
Liebermann DA: Gadd45a levels in human breast cancer are hormone
receptor dependent. J Transl Med. 11:1312013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Desjardins S, Ouellette G, Labrie Y,
Simard J; INHERIT BRCAs, ; Durocher F: Analysis of GADD45A sequence
variations in French Canadian families with high risk of breast
cancer. J Hum Genet. 53:490–498. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Takekawa M and Saito H: A family of
stress-inducible GADD45-like proteins mediate activation of the
stress-responsive MTK1/MEKK4 MAPKKK. Cell. 95:521–530. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Harkin DP, Bean JM, Miklos D, Song YH,
Truong VB, Englert C, Christians FC, Ellisen LW, Maheswaran S,
Oliner JD and Haber DA: Induction of GADD45 and JNK/SAPK-dependent
apoptosis following inducible expression of BRCA1. Cell.
97:575–586. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yasuda M, Theodorakis P, Subramanian T and
Chinnadurai G: Adenovirus E1B-19K/BCL-2 interacting protein BNIP3
contains a BH3 domain and a mitochondrial targeting sequence. J
Biol Chem. 273:12415–12421. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bandyopadhyay S, Zhan R, Wang Y, Pai SK,
Hirota S, Hosobe S, Takano Y, Saito K, Furuta E, Iiizumi M, et al:
Mechanism of apoptosis induced by the inhibition of fatty acid
synthase in breast cancer cells. Cancer Res. 66:5934–5940. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Vande Velde C, Cizeau J, Dubik D, Alimonti
J, Brown T, Israels S, Hakem R and Greenberg AH: BNIP3 and genetic
control of necrosis-like cell death through the mitochondrial
permeability transition pore. Mol Cell Biol. 20:5454–5468. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Khan A, Aljarbou AN, Aldebasi YH, Faisal
SM and Khan MA: Resveratrol suppresses the proliferation of breast
cancer cells by inhibiting fatty acid synthase signaling pathway.
Cancer Epidemiol. 38:765–772. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Silva JC, Ferreira-Strixino J, Fontana LC,
Paula LM, Raniero L, Martin AA and Canevari RA:
Apoptosis-associated genes related to photodynamic therapy in
breast carcinomas. Lasers Med Sci. 29:1429–1436. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ruddle NH: Tumor necrosis factor
(TNF-alpha) and lymphotoxin (TNF-beta). Curr Opin Immunol.
4:327–332. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Rath PC and Aggarwal BB: TNF-induced
signaling in apoptosis. J Clin Immunol. 19:350–364. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Robbs BK, Lucena PI and Viola JP: The
transcription factor NFAT1 induces apoptosis through cooperation
with Ras/Raf/MEK/ERK pathway and upregulation of TNF-α expression.
Biochim Biophys Acta. 1833:2016–2028. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Balkwill F: TNF-alpha in promotion and
progression of cancer. Cancer Metastasis Rev. 25:409–416. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Szlosarek PW and Balkwill FR: Tumour
necrosis factor alpha: A potential target for the therapy of solid
tumours. Lancet Oncol. 4:565–573. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Balkwill F: Tumor necrosis factor or tumor
promoting factor? Cytokine Growth Factor Rev. 13:135–141. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Luo JL, Maeda S, Hsu LC, Yagita H and
Karin M: Inhibition of NF-kappaB in cancer cells converts
inflammation-induced tumor growth mediated by TNFalpha to
TRAIL-mediated tumor regression. Cancer Cell. 6:297–305. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hahne M, Kataoka T, Schröter M, Hofmann K,
Irmler M, Bodmer JL, Schneider P, Bornand T, Holler N, French LE,
et al: APRIL, a new ligand of the tumor necrosis factor family,
stimulates tumor cell growth. J Exp Med. 188:1185–1190. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Schwarz H, Valbracht J, Tuckwell J, von
Kempis J and Lotz M: ILA, the human 4-1BB homologue, is inducible
in lymphoid and other cell lineages. Blood. 85:1043–1052.
1995.PubMed/NCBI
|
|
77
|
Bellarosa D, Bressan A, Bigioni M, Parlani
M, Maggi CA and Binaschi M: SAHA/Vorinostat induces the expression
of the CD137 receptor/ligand system and enhances apoptosis mediated
by soluble CD137 receptor in a human breast cancer cell line. Int J
Oncol. 41:1486–1494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hingorani P, Dickman P, Garcia-Filion P,
White-Collins A, Kolb EA and Azorsa DO: BIRC5 expression is a poor
prognostic marker in ewing sarcoma. Pediatr Blood Cancer. 60:35–40.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Jha K, Shukla M and Pandey M: Survivin
expression and targeting in breast cancer. Surg Oncol. 21:125–131.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC,
Hwang JI, Chung CW, Jung YK and Oh BH: An anti-apoptotic protein
human survivin is a direct inhibitor of caspase-3 and −7.
Biochemistry. 40:1117–1123. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ghaffari K, Hashemi M, Ebrahimi E and
Shirkoohi R: BIRC5 genomic copy number variation in early-onset
breast cancer. Iran Biomed J. 20:241–245. 2016.PubMed/NCBI
|
|
82
|
Mahlamäki EH, Bärlund M, Tanner M,
Gorunova L, Höglund M, Karhu R and Kallioniemi A: Frequent
amplification of 8q24, 11q, 17q, and 20q-specific genes in
pancreatic cancer. Genes Chromosomes Cancer. 35:353–358. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Baykara O, Bakir B, Buyru N, Kaynak K and
Dalay N: Amplification of chromosome 8 genes in lung cancer. J
Cancer. 6:270–275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Gunaldi M, Isiksacan N, Kocoglu H,
Okuturlar Y, Gunaldi O, Topcu TO and Karabulut M: The value of
serum survivin level in early diagnosis of cancer. J Cancer Res
Ther. 14:570–573. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kato J, Kuwabara Y, Mitani M, Shinoda N,
Sato A, Toyama T, Mitsui A, Nishiwaki T, Moriyama S, Kudo J and
Fujii Y: Expression of survivin in esophageal cancer: Correlation
with the prognosis and response to chemotherapy. Int J Cancer.
95:92–95. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Coumar MS, Tsai FY, Kanwar JR, Sarvagalla
S and Cheung CH: Treat cancers by targeting survivin: Just a dream
or future reality? Cancer Treat Rev. 39:802–811. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Gu J, Ren L, Wang X, Qu C and Zhang Y:
Expression of livin, survivin, and caspase-3 in prostatic cancer
and their clinical significance. Int J Clin Exp Pathol.
8:14034–14039. 2015.PubMed/NCBI
|
|
88
|
Wu B, Yao H, Wang S and Xu R: DAPK1
modulates a curcumin-induced G2/M arrest and apoptosis by
regulating STAT3, NF-κB, and caspase-3 activation. Biochem Biophys
Res Commun. 434:75–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yoo HJ, Byun HJ, Kim BR, Lee KH, Park SY
and Rho SB: DAPk1 inhibits NF-κB activation through TNF-α and
INF-γ-induced apoptosis. Cell Signal. 24:1471–1477. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Pozniak CD, Radinovic S, Yang A, McKeon F,
Kaplan DR and Miller FD: An anti-apoptotic role for the p53 family
member, p73, during developmental neuron death. Science.
289:304–306. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Melino G, De Laurenzi V and Vousden KH:
p73: Friend or foe in tumorigenesis. Nat Rev Cancer. 2:605–615.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
92
|
Huang L, Li A, Liao G, Yang F, Yang J,
Chen X and Jiang X: Curcumol triggers apoptosis of p53 mutant
triple-negative human breast cancer MDA-MB 231 cells via activation
of p73 and PUMA. Oncol Lett. 14:1080–1088. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Yamamoto T, Oda K, Kubota T, Miyazaki K,
Takenouti Y, Nimura Y, Hamaguchi M and Matsuda S: Expression of p73
gene, cell proliferation and apoptosis in breast cancer:
Immunohistochemical and clinicopathological study. Oncol Rep.
9:729–735. 2002.PubMed/NCBI
|
|
94
|
Yu XJ, Fang F and Xie J: Relationship
between TP73 polymorphism (G4C14-A4T14) and cancer risk: A
meta-analysis based on literatures. Gene. 484:42–46. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Jung JH, Chae YS, Moon JH, Kang BW, Kim
JG, Sohn SK, Park JY, Lee MH and Park HY: TNF superfamily gene
polymorphism as prognostic factor in early breast cancer. J Cancer
Res Clin Oncol. 136:685–694. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Jarvis WD, Turner AJ, Povirk LF, Traylor
RS and Grant S: Induction of apoptotic DNA fragmentation and cell
death in HL-60 human promyelocytic leukemia cells by
pharmacological inhibitors of protein kinase C. Cancer Res.
54:1707–1714. 1994.PubMed/NCBI
|
|
97
|
Wang Y and Rao PN: Effect of gossypol on
DNA synthesis and cell cycle progression of mammalian cells in
vitro. Cancer Res. 44:35–38. 1984.PubMed/NCBI
|
|
98
|
Kitada S, Leone M, Sareth S, Zhai D, Reed
JC and Pellecchia M: Discovery, characterization, and
structure-activity relationships studies of proapoptotic
polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J Med
Chem. 46:4259–4264. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Oliver CL, Miranda MB, Shangary S, Land S,
Wang S and Johnson DE: (−)-Gossypol acts directly on the
mitochondria to overcome Bcl-2- and Bcl-X(L)-mediated apoptosis
resistance. Mol Cancer Ther. 4:23–31. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Lei X, Chen Y, Du G, Yu W, Wang X, Qu H,
Xia B, He H, Mao J, Zong W, et al: Gossypol induces
Bax/Bak-independent activation of apoptosis and cytochrome c
release via a conformational change in Bcl-2. FASEB J.
20:2147–2149. 2006. View Article : Google Scholar : PubMed/NCBI
|