Introduction
Papillary thyroid carcinoma (PTC) is the most common
type of thyroid cancer, accounting for 75–85% of all primary
thyroid carcinomas (1). The
classical and follicular variants are the most common types of PTC,
and they differ markedly regarding diagnosis, prognosis, treatment,
recurrence, molecular alterations, genetic alterations and
molecular biomarkers (2), such as
with the preoperative diagnosis of the follicular variant of PTC
being more challenging compared with that of classical PTC
(3).
Several markers for the diagnosis or prognosis of
PTC have been reported, including thyroglobulin for
well-differentiated PTC (4,5), ATP5E for early diagnosis (6), RET/PTC, RAS and B-type Raf kinase
(BRAF) mutations for diagnosis or prognosis (7). However, the mechanisms of action of
those markers require further study and validation. Therefore,
there is a clinical need for more reliable, sensitive and specific
markers for PTC.
Most human transcripts are composed of non-coding
RNA, and accumulating evidence supports the important regulatory
role of non-coding RNAs, such as microRNAs (miRNAs) (8) and long non-coding RNAs (lncRNAs)
(9), in several physiological and
pathophysiological processes. Recently, circular RNAs (circRNAs)
have attracted attention in the field of RNA research (10,11).
CircRNAs are a novel class of RNAs that have a closed loop
structure and are abundantly present in the eukaryotic
transcriptome (12,13). The majority of circRNAs are composed
of exon sequences, which are conserved across different species,
and have expression specificity for different tissues and
developmental stages (10). They
are primarily considered to act as sponges that bind competitively
with miRNAs to regulate the expression of target genes (10,14,15),
and dysregulated expression of circRNAs has been found in several
types of human cancer (16–19), such as gastric cancer (20, 21),
hepatocellular carcinoma (HCC) (17), colorectal cancer (18) and PTC (22–26).
However, the detailed function and molecular mechanism of circRNAs
in tumour diagnosis, prognosis and treatment have yet to be fully
elucidated.
Therefore, in the present study, the circRNA profile
in PTC tissues was screened with high-throughput RNA sequencing
(RNA-Seq) and the dysregulated circRNAs were validated with reverse
transcription-quantitative PCR (RT-qPCR) analysis. Subsequently,
the associations between circRNA expression and clinicopathological
characteristics were analysed, and the potential roles of circRNAs
were predicted with bioinformatics analysis, with the aim of
determining their functions in the tumourigenesis, progression and
diagnosis of PTC.
Materials and methods
Patient information
A total of 50 patients who underwent surgical
treatment with a final diagnosis of PTC at Peking Union Medical
College Hospital (Beijing, China) between January 2017 and December
2018 were enrolled in this study, and the clinicopathological
characteristics of the patients are listed in Tables I and III. The recruited patients did not
receive any adjunctive treatment prior to surgery, such as
radiotherapy, chemotherapy or targeted therapy. Clinical
information, including patient age, sex, multifocality, subtype
(classical/follicular variant PTC), Ki67, vascular endothelial
growth factor (VEGF), pT, pN, metastasis, BRAF mutation, capsular
invasion and thyroiditis, was collected from the clinical records
and stratified as characteristics according to the 8th edition of
the American Joint Committee on Cancer (AJCC) Cancer Staging Manual
(27).
 | Table I.Clinicopathologic characteristics of
the 5 patients for circRNA sequencing. |
Table I.
Clinicopathologic characteristics of
the 5 patients for circRNA sequencing.
| Patient no. | Age (years) | Sex | TNM | Subtype | Capsular
invasion |
Multicentricity |
|---|
| 1 | 46 | Male | T3N0M0 |
Classical+follicular variant | Yes | Yes |
| 2 | 78 | Male | T3N0M0 | Follicular
variant | Yes | Yes |
| 3 | 38 | Female | T1N1M0 | Classical | Yes | No |
| 4 | 36 | Male | T1N0M0 | Classical | Yes | No |
| 5 | 44 | Male | T1N1M0 | Classical | Yes | No |
 | Table III.Association between the 8 circRNA
expression levels and clinicopathological characteristics of the 45
PTC patients. |
Table III.
Association between the 8 circRNA
expression levels and clinicopathological characteristics of the 45
PTC patients.
|
|
|
chr5:161330882-161336769− |
chr12:129699809-129700698− |
chr9:22046750-22097364+ |
chr20:17456347-17465553+ |
chr7:116699070-116700284+ |
chr8:18765448-18804898− |
chr7:22308338-22318037− |
chr5:38523418-38530666− |
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Clinico-pathologic
parameters | No. of cases
(%) | Mean ± SD | P-value | Mean ± SD | P-value | Mean ± SD | P-value | Mean ± SD | P-value | Mean ± SD | P-value | Mean ± SD | P-value | Mean ± SD | P-value | Mean ± SD | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
<45 | 28 | 1.21E-02± | 0.797 | 2.26E-03± | 0.574 | 4.34E-04± | 0.386 | 1.52E-04± | 0.708 | 2.39E-02± | 0.761 | 2.13E-02± | 0.374 | 8.81E-03± | 0.963 | 8.12E-04± | 0.673 |
|
| (62.2) | 0.014 |
| 0.006 |
| 0.001 |
| 0.000 |
| 0.039 |
| 0.018 |
| 0.011 |
| 0.001 |
|
|
≥45 | 17 | 1.25E-02± |
| 2.12E-03± |
| 4.45E-04± |
| 1.54E-04± |
| 2.32E-02± |
| 2.21E-02± |
| 8.51E-03± |
| 8.01E-04± |
|
|
| (37.8) | 0.014 |
| 0.005 |
| 0.001 |
| 0.000 |
| 0.038 |
| 0.018 |
| 0.011 |
| 0.001 |
|
| Sex |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Female | 33 | 1.29E-02± | 0.209 | 2.06E-03± | 0.259 | 4.47E-04± | 0.472 | 1.57E-04± | 0.590 | 2.37E-02± | 0.505 | 2.26E-02± | 0.228 | 8.61E-03± | 0.878 | 7.99E-04± | 0.342 |
|
| (73.3) | 0.014 |
| 0.005 |
| 0.001 |
| 0.000 |
| 0.039 |
| 0.019 |
| 0.011 |
| 0.001 |
|
|
Male | 12 | 1.12E-02± |
| 2.26E-03± |
| 4.33E-04± |
| 1.56E-04± |
| 2.47E-02± |
| 2.08E-02± |
| 9.05E-03± |
| 8.61E-04± |
|
|
| (26.7) | 0.014 |
| 0.006 |
| 0.001 |
| 0.000 |
| 0.040 |
| 0.018 |
| 0.011 |
| 0.001 |
|
| Multifocal |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| No | 31 | 1.19E-02± | 0.641 | 2.16E-03± | 0.148 | 4.24E-04± | 0.477 | 1.47E-04± | 0.961 | 2.32E-02± | 0.492 | 2.13E-02± | 0.941 | 8.52E-03± | 0.961 | 8.15E-04± | 0.902 |
|
| (68.9) | 0.013 |
| 0.005 |
| 0.001 |
| 0.000 |
| 0.039 |
| 0.018 |
| 0.011 |
| 0.001 |
|
|
Yes | 14 | 1.29E-02± |
| 2.06E-03± |
| 4.47E-04± |
| 1.57E-04± |
| 2.37E-02± |
| 2.26E-02± |
| 8.61E-03± |
| 7.99E-04± |
|
|
| (31.1) | 0.014 |
| 0.005 |
| 0.001 |
| 0.000 |
| 0.039 |
| 0.019 |
| 0.011 |
| 0.001 |
|
| Ki67 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
<3 | 26 | 1.21E-02± | 0.516 | 2.26E-03± | 0.725 | 4.34E-04± | 0.725 | 1.52E-04± | 0.116 | 2.39E-02± | 0.892 | 2.13E-02± | 0.705 | 8.81E-03± | 0.850 | 8.12E-04± | 0.110 |
|
| (57.8) | 0.014 |
| 0.006 |
| 0.001 |
| 0.000 |
| 0.039 |
| 0.018 |
| 0.011 |
| 0.001 |
|
| ≥3 | 15 | 1.29E-02± |
| 2.06E-03± |
| 4.47E-04± |
| 1.57E-04± |
| 2.37E-02± |
| 2.26E-02± |
| 8.61E-03± |
| 7.99E-04± |
|
|
| (33.3) | 0.014 |
| 0.005 |
| 0.001 |
| 0.000 |
| 0.039 |
| 0.019 |
| 0.011 |
| 0.001 |
|
| VEGF(+) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 41 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (91.1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| No | 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| pT-stage |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| x | 1 (2.2) | 2.24E-03 | 0.375 | 2.16E-03 | 0.590 | 4.53E-03 | 0.098 | 1.57E-04 | 0.381 | 2.35E-02 | 0.319 | 2.25E-02 | 0.754 | 8.66E-03 | 0.237 | 7.81E-04 | 0.037a |
| 1 | 29 | 1.25E-02± |
| 2.12E-03± |
| 4.45E-04± |
| 1.54E-04± |
| 2.32E-02± |
| 2.21E-02± |
| 8.51E-03± |
| 8.01E-04± |
|
|
| (64.4) | 0.014 |
| 0.005 |
| 0.001 |
| 0.000 |
| 0.038 |
| 0.018 |
| 0.011 |
| 0.001 |
|
| 2 | 4 | 1.22E-02± |
| 2.21E-03± |
| 4.32E-04± |
| 1.50E-04± |
| 2.36E-02± |
| 2.16E-02± |
| 8.67E-03± |
| 7.94E-04± |
|
|
| (8.9) | 0.013 |
| 0.006 |
| 0.001 |
| 0.000 |
| 0.039 |
| 0.018 |
| 0.011 |
| 0.001 |
|
| 3 | 11 | 1.23E-02± |
| 2.16E-03± |
| 4.27E-04± |
| 1.53E-04± |
| 2.41E-02± |
| 2.15E-02± |
| 8.76E-03± |
| 8.32E-04± |
|
|
| (24.4) | 0.014 |
| 0.005 |
| 0.001 |
| 0.000 |
| 0.040 |
| 0.018 |
| 0.011 |
| 0.001 |
|
| pN stage |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0/x | 34 | 1.19E-02± | 0.291 | 2.16E-03± | 0.561 | 4.24E-04± | 0.428 | 1.47E-04± | 0.113 | 2.32E-02± | 0.398 | 2.13E-02± | 0.712 | 8.52E-03± | 0.653 | 8.15E-04± | 0.002a |
|
| (75.6) | 0.013 |
| 0.005 |
| 0.001 |
| 0.000 |
| 0.039 |
| 0.018 |
| 0.011 |
| 0.001 |
|
| 1 | 11 | 1.27E-02± |
| 2.16E-03± |
| 4.53E-04± |
| 1.57E-04± |
| 2.35E-02± |
| 2.25E-02± |
| 8.66E-03± |
| 7.81E-04± |
|
|
| (24.4) | 0.014 |
| 0.005 |
| 0.001 |
| 0.000 |
| 0.038 |
| 0.018 |
| 0.011 |
| 0.001 |
|
| BRAF mutation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 36 | 1.27E-02± | 0.015a | 2.16E-03± | 0.865 | 4.53E-04± | 0.041a | 1.57E-04± | 0.955 | 2.35E-02± | 0.496 | 2.25E-02± | 0.036a | 8.66E-03± | 0.670 | 7.81E-04± | 0.444 |
|
| (80.0) | 0.014 |
| 0.005 |
| 0.001 |
| 0.000 |
| 0.038 |
| 0.018 |
| 0.011 |
| 0.001 |
|
| No | 9 | 9.71E-03± |
| 1.95E-03± |
| 3.96E-04± |
| 1.38E-04± |
| 2.14E-02± |
| 1.75E-02± |
| 8.59E-03± |
| 7.24E-04± |
|
|
| (20.0) | 0.012 |
| 0.006 |
| 0.001 |
| 0.000 |
| 0.037 |
| 0.016 |
| 0.011 |
| 0.001 |
|
| Capsular
invasion |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 34 | 1.25E-02± | 0.792 | 2.12E-03± | 0.025a | 4.45E-04± | 0.509 | 1.54E-04± | 0.937 | 2.32E-02± | 0.771 | 2.21E-02± | 0.561 | 8.51E-03± | 0.979 | 8.01E-04± | 0.544 |
|
| (75.6) | 0.014 |
| 0.005 |
| 0.001 |
| 0.000 |
| 0.038 |
| 0.018 |
| 0.011 |
| 0.001 |
|
| No | 11 | 1.19E-02± |
| 2.25E-03± |
| 4.33E-04± |
| 1.50E-04± |
| 2.18E-02± |
| 2.13E-02± |
| 8.38E-03± |
| 8.68E-04± |
|
|
| (24.4) | 0.014 |
| 0.006 |
| 0.001 |
| 0.000 |
| 0.037 |
| 0.018 |
| 0.011 |
| 0.001 |
|
| With
thyroiditis |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 9 | 1.27E-02± | 0.610 | 2.16E-03± | 0.061 | 4.53E-04± | 0.222 | 1.57E-04± | 0.364 | 2.35E-02± | 0.570 | 2.25E-02± | 0.212 | 8.66E-03± | 0.395 | 7.81E-04± | 0.281 |
|
| (20.0) | 0.014 |
| 0.005 |
| 0.001 |
| 0.000 |
| 0.038 |
| 0.018 |
| 0.011 |
| 0.001 |
|
| No | 36 | 1.06E-02± |
| 1.89E-03± |
| 4.07E-04± |
| 1.59E-04± |
| 2.18E-02± |
| 2.03E-02± |
| 8.39E-03± |
| 7.89E-04± |
|
|
| (80.0) | 0.013 |
| 0.006 |
| 0.001 |
| 0.000 |
| 0.039 |
| 0.019 |
| 0.011 |
| 0.001 |
|
A total of 50 paired specimens (tumour and normal
tissues) were obtained from the same patients during surgery.
Following gross examination, the tissues were resected and washed
with PBS (pH 7.2). One part of each specimen was immediately
snap-frozen using liquid nitrogen within 30 min and cryopreserved
in liquid nitrogen (−196°C) until RNA sequencing and gene
expression analysis. The remaining part of the specimen was fixed
in formalin at room temperature for 48 h and embedded in paraffin,
and then 4-µm sections were cut from each block and stained with
haematoxylin and eosin (H&E). The morphology of H&E slides
were reviewed by 2 experienced pathologists to confirm the final
diagnosis (28).
The present study was approved by the Institutional
Review Board of Peking Union Medical College Hospital, and written
informed consent was obtained from all patients regarding the use
of their tissues for research purposes.
Sample preparation and circRNA
sequencing
A total amount of 5 µg RNA per sample was used as
input material for RNA sample preparation. First, ribosomal RNAs
were depleted using a Ribo-Zero™ rRNA Removal Kit (EPIcentre) to
obtain rRNA-depleted RNAs. The rRNA-depleted RNAs were further
treated with RNase R (EPIcentre) and were then subjected to TRIzol
extraction (Thermo Fisher Scientific, Inc.). Subsequently,
sequencing libraries were generated using the rRNA-depleted and
RNaseR-digested RNAs by NEBNext Ultra™ Directional RNA Library Prep
Kit for Illumina® (New England Biolabs) following the
manufacturer's recommendations. Then, clustering of the index-coded
samples was performed on a cBot Cluster Generation System using
HiSeq PE Cluster Kit v4 cBot (Illumina, Inc.) according to the
manufacturer's instructions. After cluster generation, the library
preparations were sequenced on an Illumina Hiseq 2500 platform and
125-bp paired-end reads were generated.
Subsequently, the circRNAs were identified according
to the manual (10). Differential
expression in normal tissues and PTC was evaluated by using DESeq2
from Dr. Michael Love (version 1.6.3) (29). P-values were adjusted as q (Padj)
values by the Benjamini & Hochberg method (30); both q (Padj) <0.05 and
fold-change in expression of >2 was set as the threshold for
differential expression by default.
Total RNA extraction
Thyroid tissues were processed using the IKA
ULTRA-TURRAX T10 basic Disperser/Homogenizer (IKA Works, Inc.), and
then total RNA was extracted using TRIzol reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. The
total RNA concentrations and quality were determined using Nano
Drop One (Thermo Fisher Scientific, Inc.). The RNA integrity was
assessed through denaturing agarose gel electrophoresis.
RT-qPCR
The RT-qPCR experiments were conducted as previously
described (31,32). Total RNA was digested with RNaseR at
37°C for 30 min and reverse-transcribed to synthesise cDNA using
M-MLV reverse transcriptase (Promega Corporation; cat. no. M1701)
and random primers at 70°C for 10 min followed by a 60-min
incubation at 37°C. Then the cDNA was analysed by qPCR with Fast
SYBR® Green Master Mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.; cat. no. 4385612) and a 7500 Fast
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Amplification conditions were 95°C (20 sec), 40 cycles of
95°C (3 sec) and 60°C (30 sec) according to the manufacturer's
instructions. GAPDH was used as the internal control. The divergent
primers used for circRNA validation are listed in Table SI, the specificity of primers was
assessed in NCBI BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi), and the
backsplice junction of circRNA in the PCR product was verified with
Sanger sequencing. The relative expression level of each circRNA
with GAPDH was calculated using the 2−ΔΔCq method
(24,33).
Immunohistochemistry (IHC)
IHC staining was performed on 4-µm sections using
the DAKO Autostainer Link 48 (Dako; Agilent Technologies, Inc.).
The tissue epitopes were repaired using the automated water bath
heating process in Dako PT Link (Dako; Agilent Technologies, Inc.);
the sections were incubated in Tris-EDTA retrieval solution (10 mM
Tris, 1 mM EDTA; pH 9.0) at 98°C for 20 min. The sections were
subsequently incubated for 50 min at room temperature with the
primary anti-Ki67 (cat. no. MIB1; Dako; Agilent Technologies, Inc.)
and anti-VEGF (cat. no. VG1; Dako; Agilent Technologies, Inc.)
antibodies, diluted at 1:200 and 1:100, respectively, in Dako
Envision™ Flex antibody diluents, followed by anti-rabbit
immuno-peroxidase polymer (Envision FLEX/HRP) for 20 min at room
temperature according to the manufacturer's instructions, and
developed with freshly prepared 0.05% 3,3′-diaminobenzidine
tetrahydrochloride. Finally, the slides were counterstained with
hematoxylin, dehydrated, and mounted.
BRAFV600E mutation
detection
Genomic DNA was extracted from fresh-frozen tissues
using the TIAamp Genomic DNA Kit (Tiangen Biotech Co., Ltd.; cat.
no. DP304). Subsequently, the genomic DNA was used to detect the
V600E mutation in the BRAF oncogene through the AmoyDx
BRAFV600E Mutations Detection Kit (AmoyDx; cat.
no. ADx-BR04).
Statistical analysis
Statistical analyses were performed using SPSS 19.0
(IBM Corp.). The significance of the RT-qPCR validation between the
PTC and the normal tissue groups was assessed by the paired
Student's t-test (18,34,35).
The Mann-Whitney U test was used to analyse the associations
between the mean ± standard deviation of circRNA expression and the
clinicopathological characteristics of PTC, including age, sex,
multifocality, Ki67, pN stage, BRAF mutation status, capsular
invasion and thyroiditis, and the Kruskal-Wallis test followed with
post-hoc Dunn's multiple comparison test was used to analyse the
association between circRNA expression and pT-stage (35,36,37).
P<0.05 was considered to indicate statistically significant
differences (38). Receiver
operating characteristics (ROC) curves were established to evaluate
the diagnostic value of circRNAs for PTC. An area under the ROC
curve (AUC) of 0.5 was considered to represent a test with no
discriminating ability (i.e., no better than chance), while an AUC
of 1.0 was considered to represent a test with perfect
discrimination (39).
Results
circRNA expression profiles in PTC
tissues relative to adjacent tissues
The clinicopathological characteristics of the 5
patients are listed in Table I. The
majority were male (4 cases, 75%), 3 cases were classical PTCs, 1
case was follicular PTC and 1 case was mixed classical and
follicular PTC, with different tumour-node-metastasis (TNM) stages.
Two cases were multifocal and all displayed capsular invasion. The
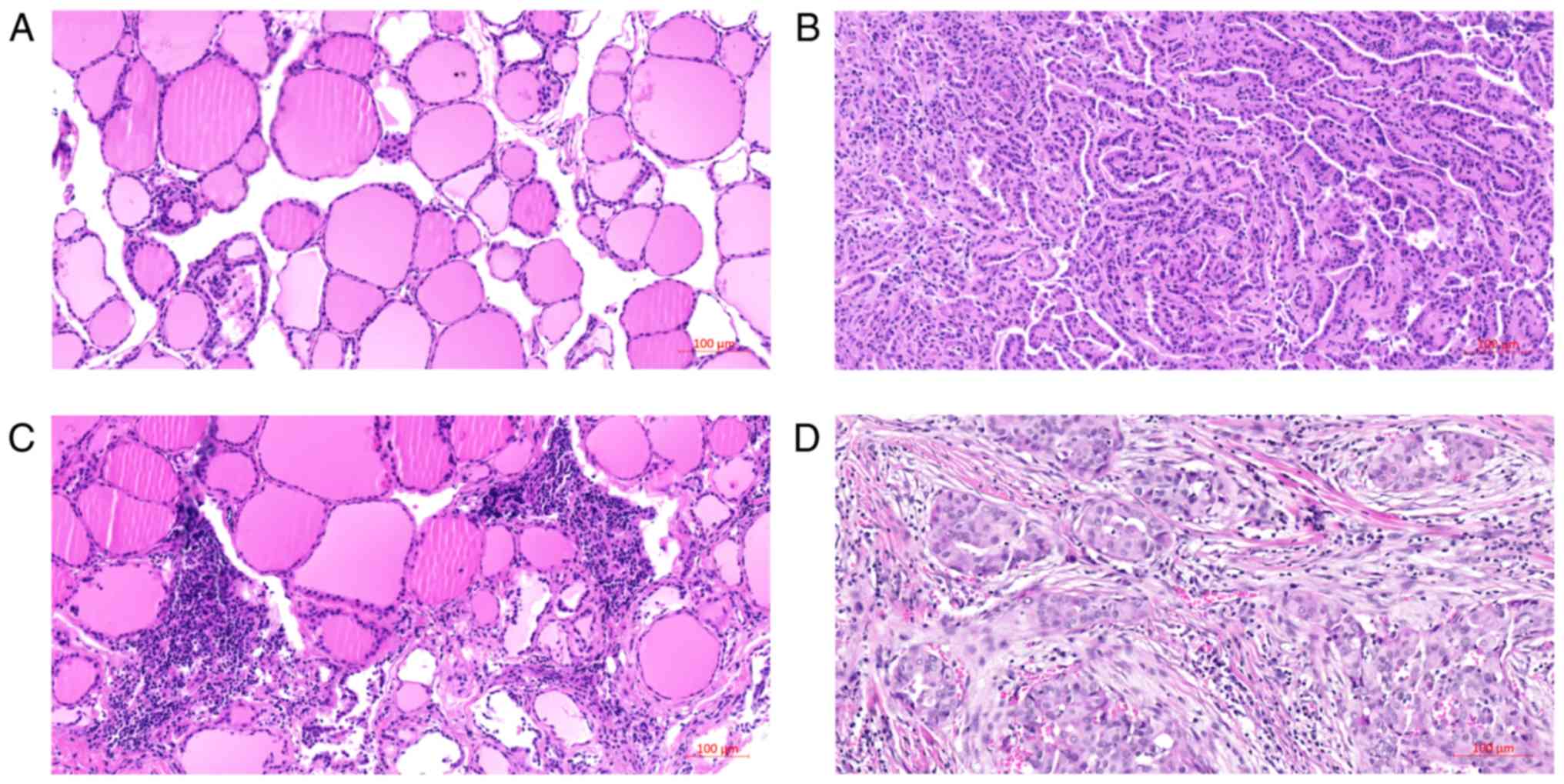
histomorphology of tissues are shown in Fig. 1. Among them, Fig. 1A and C shows the histomorphology of
normal tissues and with lymphocyte infiltration. Fig. 1B and represent the classical PTC and
follicular variant of PTC, respectively.
Subsequently, RNA-seq demonstrated that the circRNA
expression profile of the 5 paired normal and PTC tissue samples
exhibited significant differences. The hierarchical clustering
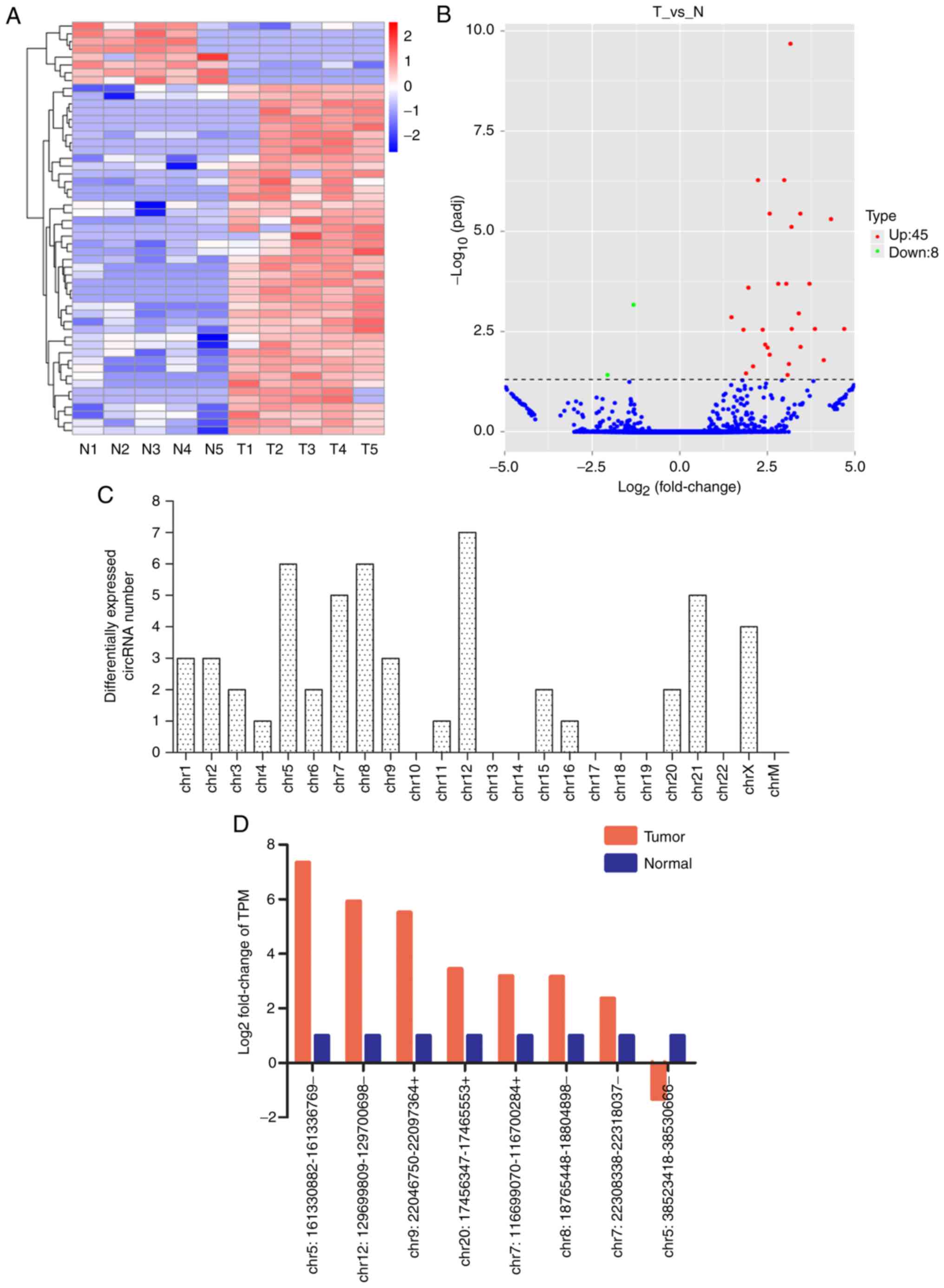
(Fig. 2A) and volcano plots
(Fig. 2B) revealed that there were
53 circRNAs differentially expressed in carcinoma tissues, with
both q (Padj) <0.05 and fold-change in expression of >2 set
as the threshold. Among those, 45 circRNAs were upregulated and 8
were downregulated in tumour tissues.
In the dysregulated circRNAs (Table SII), the Log2 (FC) of
abnormally highly expressed circRNAs ranged from 1.48 to 7.34, and
the Log2 (FC) of abnormally lowly expressed circRNAs
ranged from-1.31 to −5.593. In addition, the magnitude of fold
change was highest for chr5:161330882-161336769- in the upregulated
circRNAs (fold change, 162; P<0.01), and
chr20:20425608-20472956- for downregulated circRNAs (fold changes,
48; P=0.01). As presented in Fig.
2C, the transcription of the differentially regulated circRNAs
was found to be widely distributed among all chromosomes, except
chr10, chr13, chr14, chr17, chr18, chr19, chr22 and chrM. Moreover,
30 were identified as new circRNAs that have not been annotated in
the circBase or circ2 Traits database. Furthermore, multiple
circRNAs can be produced from a single gene and alternative
splicing events, so circRNAs can arise from exons, introns,
intergenic regions, UTRs, ncRNA loci and from locations antisense
(40,41). In our research, of the 53
dysregulated circRNAs (Table SII),
29 (54.7%) were from the antisense locus, 10 (18.9%) were formed
from exon-intron junction, 8 (15.1%) were from intron, 4 (7.5%)
were from exon, and the other 2 were from intergenic regions,
respectively.
Validation of circRNA expression
A total of 8 differentially expressed circRNAs were
selected according to transcripts per million reads (TPM), fold
change, q (Padj) value, junction read, features (arise from
exonic/intronic), distribution and length, and then were verified
using 45 pairs of tissues by RT-qPCR. The circRNA relative
expression level of GAPDH was calculated using the
2−ΔΔCq method. The results confirmed that the
upregulation of chr5: 161330882-161336769- (P<0.001),
chr12:129699809-129700698- (P<0.05), chr9:22046750-22097364+
(P<0.001), chr20:17456347-17465553+ (P<0.001),
chr7:116699070-116700284+ (P<0.01), chr8:18765448-18804898-
(P<0.001) and chr7:22308338-22318037- (P<0.001) and the
downregulation of chr5:38523418-38530666- (P<0.001) in carcinoma
(Fig. 3) were in accordance with
the RNA-seq data (Fig. 2D).
Furthermore, the mRNA expression of these 8 circRNAs
was accessed with ROC curve analysis (Fig. 4A), which revealed that all the AUCs
of circRNAs were >0.7 (P<0.001), and
chr5:161330882-161336769- with the highest AUC (0.878) could be
considered as a powerful marker for clinical PTC diagnosis
(Table II).
 | Table II.Area under the curve (AUC) of
RT-qPCR-validated circRNAs in the 45 cases of PTC. |
Table II.
Area under the curve (AUC) of
RT-qPCR-validated circRNAs in the 45 cases of PTC.
| circRNA | AUC | 95% CI | Sth. Error | P-value |
|---|
| chr5:
161330882-161336769- | 0.878 | 0.8068 to
0.9492 | 0.03632 | <0.0001 |
| chr12:
129699809-129700698- | 0.8099 | 0.7213 to
0.8984 | 0.04517 | <0.0001 |
| chr9:
22046750-22097364+ | 0.76 | 0.6610 to
0.8590 | 0.05049 | <0.0001 |
| chr20:
17456347-17465553+ | 0.759 | 0.6583 to
0.8597 | 0.05136 | <0.0001 |
| chr7:
116699070-116700284+ | 0.7763 | 0.6794 to
0.8732 | 0.04945 | <0.0001 |
| chr8:
18765448-18804898- | 0.8277 | 0.7387 to
0.9166 | 0.04538 | <0.0001 |
| chr7:
22308338-22318037- | 0.7017 | 0.5918 to
0.8116 | 0.05607 | 0.0009859 |
| chr5:
38523418-38530666- | 0.7101 | 0.6029 to
0.8174 | 0.05472 | 0.0006003 |
circRNA expression and clinical
characteristics
The clinical characteristics of 45 PTC patients are
summarized in Table III. The
median age of the patients at diagnosis was 45 years (range, 17–64
years), while the majority of the patients were female (n=33,
73.3%); 32 cases (71.1%) were classical PTC, 12 cases (26.7%) were
the follicular variant of PTC and only 1 case (2%) was a mixture of
the classical and follicular variant. Moreover, 31 cases (68.9%)
were unifocal, and 14 cases (31.1%) were multifocal. In regards to
pT stage, 29 cases (64.4%) had a diameter <2 cm or localized, 4
(8.9%) ranged between 2 and 4 cm, and 11 (24.4%) were >4 cm or
invading the soft tissues beyond the thyroid. Only one-quarter of
the patients had regional lymph node metastasis. None of the
patients had metastasis. The Ki67 index of 33.3% patients was very
high (≥3), and VEGF was positive for 91.1% patients.
BRAFV600E mutation, capsular invasion and thyroiditis
with diffuse lymphocytic infiltration were observed in 80, 75.6 and
20% of the cases, respectively.
Subsequently, we analysed the association between
the mean ± standard deviation of circRNA expression in the tumour
and the clinical characteristics in 45 PTC patients, including age,
sex, multifocality, Ki67, capsular invasion, VEGF,
BRAFV600E mutation, TNM stage and thyroiditis (Table III). Among the 8 dysregulated
circRNAs, chr5:161330882-161336769- (P=0.015),
chr9:22046750-22097364+ (hsa_circ_0008796, P=0.041) and
chr8:18765448-18804898- (hsa_circ_0002111, P=0.036) were associated
with the BRAFV600E mutation, and
chr12:129699809-129700698- was associated with capsular invasion
(P=0.025). Additionally, chr5:38523418-38530666- (hsa_circ_0072309)
was associated with pT (P=0.037) and pN (P=0.002) stage. This
association between circRNA expression and clinicopathological
characteristics indicated that those circRNAs may participate in
tumourigenesis and progression of PTC. The other three dysregulated
circRNAs exhibited no statistically significant association with
clinicopathological characteristics.
Furthermore, the association between the mRNA
expression of these 5 circRNAs and the clinical characteristics was
assessed with ROC curve analysis (Fig.
4B), revealing that the AUCs of all circRNAs were >0.7
(P<0.05), with chr5:38523418-38530666-(associated with pN stage)
having the highest AUC (0.8209, P<0.01; Table IV). This result was consistent with
the data mentioned above, and indicated that those 5 circRNAs
associated with BRAFV600E mutation, capsular invasion,
advanced pT stage and lymph node metastasis may be putative
biomarkers for the diagnosis and evaluation of the progression of
PTC.
 | Table IV.Area under the curve (AUC) of the
circRNA mRNA expression with the clinical characteristics of the 45
cases of PTC. |
Table IV.
Area under the curve (AUC) of the
circRNA mRNA expression with the clinical characteristics of the 45
cases of PTC.
| circRNA | Clinical
characteristics | AUC | 95% CI | Sth. Error | P-value |
|---|
|
chr5:161330882-161336769- | BRAF mutation | 0.7654 | 0.6142 to
0.9167 | 0.07714 | 0.01471 |
|
chr9:22046750-22097364+ | BRAF mutation | 0.7222 | 0.5562 to
0.8882 | 0.08468 | 0.0411 |
|
chr8:18765448-18804898- | BRAF mutation | 0.7284 | 0.5757 to
0.8811 | 0.0779 | 0.0358 |
|
chr12:129699809-129700698- | Capsular
invasion | 0.7273 | 0.5480 to
0.9066 | 0.09146 | 0.02482 |
|
chr5:38523418-38530666- | pN Stage | 0.8209 | 0.6905 to
0.9512 | 0.0665 | 0.001538 |
Gene ontology (GO) analysis and
pathway analysis of circRNA genes
GO enrichment analysis for the host genes of the
identified differentially expressed circRNAs was performed, and the
significantly enriched GO terms in the biological process, cellular
component and molecular function categories, e.g., carbohydrate
derivative catabolic process (GO: 1901136, P=0.00021557), membrane
(GO: 0016020, P=0.0012195) and GTPase regulator activity (GO:
0030695, P=0.00021632), are presented in Fig. 5A. A total of 28 host genes of the
differentially expressed circRNAs were associated with the membrane
GO term. The results of the Kyoto Encyclopaedia of Genes and
Genomes pathway analysis of the 20 highly related circRNA genes are
presented in Fig. 5B. Those circRNA
genes were mainly associated with important pathways (Rap1, VEGF
and Ras signalling pathways), tumourigenesis (transcriptional
misregulation and proteoglycans in cancer), cell signal
transduction (axon guidance, retrograde endocannabinoid signalling,
cytokine-cytokine receptor interaction), infection and immune
defence (bacterial invasion of epithelial cells, chemokine
signalling pathway). Furthermore, the Rap1 and Ras signalling
pathway contained more related circRNA genes, which suggested that
circRNAs may play a key role in PTC tumourigenesis and
progression.
Prediction for the circRNA-miRNA
network
circRNAs have been shown to contain miRNA-binding
sites and negatively regulate the inhibitory effects of miRNAs on
their target mRNAs, thereby essentially acting as miRNA sponges
(14). To evaluate the potential
functions of the identified differentially expressed circRNAs,
miRNAs that were potentially associated with those circRNAs were
investigated using the RegRNA2 platform and Circular RNA
Interactome (42,43). The first 5 predicted miRNAs for the
dysregulated circRNAs are listed in Table V. For example, circRNA
chr5:161330882-161336769- was predicted to harbour
hsa-miR-1273g-3p, hsa-miR-498, hsa-miR-1268a, has-miR-363-5p and
hsa-miR-566.
 | Table V.Predicted miRNAs for the top
upregulated or downregulated circRNAs in PTC. |
Table V.
Predicted miRNAs for the top
upregulated or downregulated circRNAs in PTC.
| circRNA | circBase ID | Predicted
miRNAs |
|---|
|
chr5:161330882-161336769- | / | miR-1273g-3p | miR-498 | miR-1268b | miR-363-5p | miR-566 |
|
chr12:129699809-129700698- | / | miR-4726-3p | miR-4793-3p | miR-766-3p | miR-1343 | miR-1226-5p |
|
chr9:22046750-22097364+ |
hsa_circ_0008796 | miR-490-5p | miR-224 | miR-1228 | miR-576-5p | miR-611 |
|
chr20:17456347-17465553+ | / | miR-939 | miR-576-3p | miR-3925-5p | miR-5189 | miR-4732-5p |
|
chr7:116699070-116700284+ |
hsa_circ_0082002 | miR-512-5p | miR-515-3p | miR-519e | miR-609 | miR-653 |
|
chr8:18765448-18804898- |
hsa_circ_0002111 | miR-647 | miR-532-3p | miR-526b | miR-1263 | miR-432 |
|
chr7:22308338-22318037- | / | miR-5095 | miR-5096 | miR-3159 | miR-4524a-3p | miR-5699 |
|
chr5:38523418-38530666- |
hsa_circ_0072309 | miR-1277 | miR-331-5p | miR-409-3p | miR-515-5p | miR-1276 |
To further explore which miRNAs may be
cancer-related, the starBase v2.0 (44) and miRPathDB v1.1 (45) platforms were used, and a network map
of circRNA-miRNA interactions was constructed to identify the
dysregulated circRNAs in PTC that potentially interacted with
cancer-related miRNAs. From the initial list of 40 potential miRNAs
identified by the RegRNA2 and circRNA Interactome analysis, several
cancer-related miRNAs were identified following a literature
research, including hsa-miR-498 (46), hsa-miR-766-3p (47,48)
and hsa-miR-661 (49,50), which are associated with cell
proliferation in several cancers. The results indicated that those
dysregulated circRNAs may act as miRNA sponges, leading to the
progression of PTC, but the specific underlying mechanism requires
further investigation.
Discussion
The present study profiled the circRNA expression in
papillary thyroid carcinoma (PTC) using RNA-seq, and a total of 45
upregulated and 8 downregulated circRNAs were identified in PTC
tumours. Among the 53 dysregulated circRNAs,
chr3:64594257-64596991-(hsa_circ_0066444) has been reported to be
significantly differentially expressed in hepatocellular carcinoma
(HCC) tissues by RNA-seq, but it exhibited no significant
difference on PCR verification (34). Additionally, chr8:18799294-18804898-
(hsa_circ_0004458) was reported to be upregulated in PTC tumours
(51) and cells (52), which was consistent with our
sequencing results. Another study verified that the silencing of
hsa_circ_0004458 inhibited PTC cell proliferation and promoted cell
cycle arrest and apoptosis in vitro (52), which may constitute the evidential
basis for further research.
Subsequently, the 8 dysregulated circRNAs were
screened according to high TPM, fold change, q (Padj) value,
junction reads and widespread distribution, and the results were
further validated with RT-qPCR. Among the 8 circRNAs,
chr5:161330882-161336769- had the highest area under the curve
(AUC) (0.878) and may be considered a powerful marker for clinical
diagnosis; in addition, chr8:18765448-18804898- (hsa_circ_0002111)
was found to be significantly upregulated in PTCs with microarray
profiling (51), which was
consistent with our results.
The associations between the circRNA expression
profile and clinical characteristics in PTC were then explored, and
it was observed that chr5:161330882-161336769-(P=0.015),
chr9:22046750-22097364+ (hsa_circ_0008796, P=0.041) and
chr8:18765448-18804898- (hsa_circ_0002111, P=0.036) were
significantly associated with the BRAFV600E mutation.
The BRAFV600E mutation is the most common genetic
aberration in PTC and has been found in up to 68% of PTCs in adults
with aggressive disease characteristics (53). Although it was reported that the
major driver mutation of follicular variant PTC and classical PTC
was RAS-related mutation and BRAFV600E mutation,
respectively (2), other studies
indicated that the follicular variant PTC also harbours the
BRAFV600E mutation (54,55).
In addition to our study, Ren et al reported that
hsa_circRNA_047771 is likely to be associated with the
BRAFV600E mutation (35). The results indicated that
BRAFV600E may be associated with multiple circRNAs, and
whether those circRNAs are involve in tumourigenesis should be the
focus of further investigation.
Furthermore, the host genes of
chr5:161330882-161336769-, chr9:22046750-22097364+
(hsa_circ_0008796) and chr8:18765448-18804898- (hsa_circ_0002111)
were γ-aminobutyric acid type A receptor subunit β2
(GABRB2), CDKN2B antisense RNA 1 (CDKN2B-AS1) and
pleckstrin and Sec7 domain containing 3 (PSD3),
respectively. Among those, GABRB2 was previously reported as
being overexpressed in PTC (56),
and the hypomethylation and overexpression of GABRB2 were
significantly altered in BRAFV600E-mutated PTC (57), further supporting that
chr5:161330882-161336769- and GABRB2 may play key roles in the
progression of PTC. However, there are yet no published data on
this association of CDKN2B-AS1, PSD3 and BRAFV600E
mutation.
In addition, the results of the present study also
demonstrated that chr12:129699809-129700698- was associated with
capsular invasion (P=0.025), which is considered a putative
biomarker for the progression of PTC. Furlan et al results
suggested that the presence of capsular invasion does not adversely
affect the biological behaviour of PTC or patient survival
(58), but another study indicated
that it is associated with a higher recurrence rate (59) and reduced survival time and quality
of life (60). Capsular invasion,
advanced pT stage and pathological positive lymph node metastasis
were independent predictors of multifocal PTC (61). Additionally, transmembrane protein
132D (TMEM132D), the host gene of
chr12:129699809-129700698-, was only reported to be associated with
anxiety phenotypes (62) and may
serve as a predictor of CD8+T-lymphocyte infiltration
and a favourable prognostic marker in early-stage ovarian cancer
(63). However, further study is
required to determine whether TMEM132D is associated with capsular
invasion and whether it may affect T-lymphocyte infiltration in
PTC.
Furthermore, the present study demonstrated that the
low expression of chr5:38523418-38530666- was associated with
advanced pT stage (P=0.037) and lymph node metastasis (P=0.002),
and may be considered as a putative biomarker for the progression
of PTC. According to the AJCC TNM staging system, T and N stages
are independent predictive factors of multifocal and bilateral PTC
(61,64). Ren et al reported that low
expression of hsa_circRNA_047771 was correlated with lymph node
metastasis and advanced TNM stage, whereas higher expression of
hsa_circRNA_007148 was correlated with lymph node metastasis
(35). In addition, LIF receptor
subunit α (LIFR), the host gene of chr5:38523418-38530666-, was
found to be a breast cancer metastasis suppressor through the
Hippo-YAP pathway, and is considered to have significant prognostic
power (65). However, the specific
function of chr5:38523418-38530666- in the progression and
metastasis of PTC requires further investigation.
In brief, the AUCs of the 8 dysregulated circRNAs
indicated that they were specific to PTC, and among them, the 5
circRNAs associated with BRAFV600E, capsular invasion,
advanced pT stage and lymph node metastasis, may be putative
biomarkers for the diagnosis and evaluation of progression of
PTC.
For circRNAs that were found to be associated with
the cellular RNA network and negatively regulating the inhibitory
effects of miRNAs on their target mRNAs, it was necessary to
analyse the downstream miRNAs and mRNA of circRNAs to gain insight
into the molecular mechanisms of action and the role of circRNAs in
tumourigenesis and cancer progression. Although the literature on
the circRNA-miRNA network in regard to other identified circRNAs is
limited, there was a considerable amount of literature on those
miRNAs. Therefore, in our results, miR-498 was predicted to be a
target miRNA of chr5:161330882-161336769-, and the expression of
miR-498 was also markedly downregulated in ovarian cancer cells and
tumours, acting as a new tumour suppressor by targeting the FOXO3
gene to inhibit cell proliferation in ovarian cancer (46). The present research demonstrated
that chr5:161330882-161336769- was significantly upregulated in
PTC, although further study is required to determine whether
miR-498 could be consequently downregulated in PTC as a result of
regulatory disinhibition, and the effects of dysregulation on
cancer-related target genes should be the focus of further
research.
In addition, miR-766-3p and miR-661, the predicted
miRNAs of chr12:129699809-129700698- and chr9:22046750-22097364+
(has_circ_0008796), may also be consequently dysregulated in PTC as
a result of regulatory disinhibition. For example, it was
previously demonstrated that miR-766-3p inhibits tumour progression
by targeting Wnt3a in HCC (47).
MiR-661 was shown to inhibit metastatic tumour antigen 1 in breast
cancer cells (50), and to inhibit
cell proliferation, migration and invasion by targeting hTERT in
glioma cells (49). Furthermore, as
the predicted miRNA of chr5:38523418-38530666- (hsa_circ_0072309),
miR-409-3p may promote epithelial-to-mesenchymal transition and
tumourigenesis via the RSU1 or STAG2 pathway in prostate cancer
(66,67). However, the specific function and
mechanism of action of circRNAs and miRNAs in PTC have not been
fully elucidated, and further studies should focus on the
circRNA/miRNA/mRNA axis in the regulation of PTC tumourigenesis, in
order to conclusively determine cancer-related target genes and
facilitate further biomarker identification for PTC.
In general, the function of the parental genes may
have some effect on the circRNA, but the effects on tumours are not
only associated with the parental genes, as circRNAs may also play
several important roles in biological processes, such as regulating
gene expression by acting as competitors of pre-mRNA splicing, as
decoys for microRNAs, as sponges for RNA-binding proteins, possibly
also as substrates for translation (68), and may regulate the transcription of
their parental genes (69). Ours is
a preliminary study of the expression profile of circRNAs in
thyroid cancer. In future studies, we would like to focus more on
the mechanisms of action of the dysregulated circRNAs and their
sponging miRNAs and potential mRNA targets, which may provide clues
to the molecular mechanisms of action of circRNAs in PTC.
Besides, there were certain limitations to the
present study, such as the small sample size, different
histological subtypes of PTC, and the fact that signaling pathways
were not studied. Thus, the results require verification using
larger sample sizes and more detailed investigation of the
differential expression of circRNAs between the two PTC subtypes
and their role in diagnosis, progression and evaluation of
prognosis.
In conclusion, the present study demonstrated that
several circRNAs are differentially expressed in PTC and were found
to be associated with the BRAFV600E mutation, capsular
invasion, advanced pT stage and lymph node metastasis, thereby
indicating that circRNAs may participate in tumourigenesis and
cancer progression. The detailed role of circRNAs as putative
biomarkers for the diagnostic and evaluation of progression of PTC
should be further explored and validated. Furthermore, those
differentially expressed circRNAs may participate in the cellular
RNA network with miRNAs and mRNAs, and further research should be
performed to gain a better insight into the molecular mechanisms of
circRNAs in PTC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Chinese
Academy of Medical Sciences (CAMS) Initiative for Innovative
Medicine (grant nos. 2017-I2M-1-001 and 2017-I2M-2-001); and The
National Key Research and Development program of China: the cluster
construction of human genetic resource Bio-bank in north China
(grant no. 2016YFC1201703).
Availability of data and materials
All data generated or analysed during the present
study are included in this published article.
Authors' contributions
DG carried out the study design and manuscript
preparation. FL conducted the manuscript preparation and data
analysis/interpretation. XZ carried out the experimental studies
and data analysis. BL carried out the manuscript editing and
revision. SZ conducted the experimental studies. AW performed the
statistical analysis. DC conducted the experimental studies. JS was
responsible for the specimen acquisition and diagnosis. BL was
responsible for the study conception and clinical studies.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Peking Union Medical College Hospital (Beijing,
China). Informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lam AK, Lo CY and Lam KS: Papillary
carcinoma of thyroid: A 30-yr clinicopathological review of the
histological variants. Endocr Pathol. 16:323–330. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abdullah MI, Junit SM, Ng KL, Jayapalan
JJ, Karikalan B and Hashim OH: Papillary thyroid cancer: Genetic
alterations and molecular biomarker investigations. Int J Med Sci.
16:450–460. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ng SC, Kuo SF, Hua CC, Huang BY, Chiang
KC, Chu YY, Hsueh C and Lin JD: Differentiation of the follicular
variant of papillary thyroid carcinoma from classic papillary
thyroid carcinoma: An ultrasound analysis and complement to
fine-needle aspiration cytology. J Ultrasound Med. 37:667–674.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin JD: Thyroglobulin and human thyroid
cancer. Clin Chim Acta. 388:15–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tuttle RM, Leboeuf R and Martorella AJ:
Papillary thyroid cancer: Monitoring and therapy. Endocrinol
Metabol Clin North Amer. 36:753–778. 2007. View Article : Google Scholar
|
|
6
|
Hurtado-Lopez LM, Fernandez-Ramirez F,
Martinez-Penafiel E, Carrillo Ruiz JD and Herrera Gonzalez NE:
Molecular analysis by gene expression of mitochondrial ATPase
subunits in papillary thyroid cancer: Is ATP5E transcript a
possible early tumor marker? Med Sci Monit. 21:1745–1751. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang KT and Lee CH: BRAF mutation in
papillary thyroid carcinoma: Pathogenic role and clinical
implications. J Chin Med Assoc. 73:113–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and Cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qu S, Yang X, Li X, Wang J, Gao Y, Shang
R, Sun W, Dou K and Li H: Circular RNA: A new star of noncoding
RNAs. Cancer Lett. 365:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo JU, Agarwal V, Guo H and Bartel DP:
Expanded identification and characterization of mammalian circular
RNAs. Genome Biol. 15:4092014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng Q, Bao C, Guo W, Li S, Chen J, Chen
B, Luo Y, Lyu D, Li Y, Shi G, et al: Circular RNA profiling reveals
an abundant circHIPK3 that regulates cell growth by sponging
multiple miRNAs. Nat Commun. 7:112152016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bachmayr-Heyda A, Reiner AT, Auer K,
Sukhbaatar N, Aust S, Bachleitner-Hofmann T, Mesteri I, Grunt TW,
Zeillinger R and Pils D: Correlation of circular RNA abundance with
proliferation-exemplified with colorectal and ovarian cancer,
idiopathic lung fibrosis, and normal human tissues. Sci Rep.
5:80572015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qin M, Liu G, Huo X, Tao X, Sun X, Ge Z,
Yang J, Fan J, Liu L and Qin W: Hsa_circ_0001649: A circular RNA
and potential novel biomarker for hepatocellular carcinoma. Cancer
Biomarkers. 16:161–169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie H, Ren X, Xin S, Lan X, Lu G, Lin Y,
Yang S, Zeng Z, Liao W, Ding YQ and Liang L: Emerging roles of
circRNA_001569 targeting miR-145 in the proliferation and invasion
of colorectal cancer. Oncotarget. 7:26680–26691. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X, Zhang Y, Huang L, Zhang J, Pan F,
Li B, Yan Y, Jia B, Liu H, Li S and Zheng W: Decreased expression
of hsa_circ_001988 in colorectal cancer and its clinical
significances. Int J Clin Exp Pathol. 8:16020–16025.
2015.PubMed/NCBI
|
|
20
|
Li P, Chen S, Chen H, Mo X, Li T, Shao Y,
Xiao B and Guo J: Using circular RNA as a novel type of biomarker
in the screening of gastric cancer. Clin Chim Acta. 444:132–136.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian M, Chen R, Li T and Xiao B: Reduced
expression of circRNA hsa_circ_0003159 in gastric cancer and its
clinical significance. J Clin Lab Anal. 32:e222812017. View Article : Google Scholar
|
|
22
|
Bi W, Huang J, Nie C, Liu B, He G, Han J,
Pang R, Ding Z, Xu J and Zhang J: CircRNA circRNA_102171 promotes
papillary thyroid cancer progression through modulating
CTNNBIP1-dependent activation of β-catenin pathway. J Exp Clin
Cancer Res. 37:2752018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wei H, Pan L, Tao D and Li R: Circular RNA
circZFR contributes to papillary thyroid cancer cell proliferation
and invasion by sponging miR-1261 and facilitating C8orf4
expression. Biochem Biophys Res Commun. 503:56–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lan X, Cao J, Xu J, Chen C, Zheng C, Wang
J, Zhu X, Zhu X and Ge M: Decreased expression of hsa_circ_0137287
predicts aggressive clinicopathologic characteristics in papillary
thyroid carcinoma. J Clin Lab Anal. 32:e225732018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang M, Chen B, Ru Z and Cong L: CircRNA
circ-ITCH suppresses papillary thyroid cancer progression through
miR-22-3p/CBL/β-catenin pathway. Biochem Biophys Res Commun.
504:283–288. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lan X, Xu J, Chen C, Zheng C, Wang J, Cao
J, Zhu X and Ge M: The landscape of circular RNA expression
profiles in papillary thyroid carcinoma based on RNA sequencing.
Cell Physiol Biochem. 47:1122–1132. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Doescher J, Veit JA and Hoffmann TK: The
8th edition of the AJCC Cancer Staging Manual. Updates in
otorhinolaryngology, head and neck surgery. HNO. 65:956–961.
2017.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
28
|
EI-Naggar AK, Chan JKC, Grandis JR, Takata
T and Slootweg PJ: WHO Classification of Head and Neck Tumours.
(Lyon). 2017.
|
|
29
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
D SJ: The positive false discovery rate: A
Bayesian interpretation and the q-value. Ann Statistics.
31:2013–2035. 2003. View Article : Google Scholar
|
|
31
|
Panda AC and Gorospe M: Detection and
analysis of circular RNAs by RT-PCR. Bio Protoc. 8(pii):
e27752018.PubMed/NCBI
|
|
32
|
Zhu M, Xu Y, Chen Y and Yan F: Circular
BANP, an upregulated circular RNA that modulates cell proliferation
in colorectal cancer. Biomed Pharmacother. 88:138–144. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sui W, Gan Q, Liu F, Chen H, Liu J and Dai
Y: The differentially expressed circular ribonucleic acids of
primary hepatic carcinoma following liver transplantation as new
diagnostic biomarkers for primary hepatic carcinoma. Tumour Biol.
40:10104283187669282018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shang X, Li G, Liu H, Li T, Liu J, Zhao Q
and Wang C: Comprehensive circular RNA profiling reveals that
hsa_circ_0005075, a new circular RNA biomarker, is involved in
hepatocellular crcinoma development. Medicine (Baltimore).
95:e38112016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ren H, Liu Z, Liu S, Zhou X, Wang H, Xu J,
Wang D and Yuan G: Profile and clinical implication of circular
RNAs in human papillary thyroid carcinoma. PeerJ. 6:e53632018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ouyang Q, Huang Q, Jiang Z, Zhao J, Shi GP
and Yang M: Using plasma circRNA_002453 as a novel biomarker in the
diagnosis of lupus nephritis. Mol Immunol. 101:531–538. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Peng Y, Song X, Zheng Y, Cheng H and Lai
W: circCOL3A1-859267 regulates type I collagen expression by
sponging miR-29c in human dermal fibroblasts. Eur J Dermatol.
28:613–620. 2018.PubMed/NCBI
|
|
38
|
Hu J, Li C, Liu C, Zhao S, Wang Y and Fu
Z: Expressions of miRNAs in papillary thyroid carcinoma and their
associations with the clinical characteristics of PTC. Cancer
Biomark. 18:87–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hoo ZH, Candlish J and Teare D: What is an
ROC curve? Emerg Med J. 34:357–359. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ebbesen KK, Hansen TB and Kjems J:
Insights into circular RNA biology. RNA Biol. 14:1035–1045. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dong Y, He D, Peng Z, Peng W, Shi W, Wang
J, Li B, Zhang C and Duan C: Circular RNAs in cancer: An emerging
key player. J Hematol Oncol. 10:22017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chang TH, Huang HY, Hsu BK, Weng SL, Horng
JT and Huang HD: An enhanced computational platform for
investigating the roles of regulatory RNA and for identifying
functional RNA motifs. BMC Bioinformatics. 14 (Suppl 2):S42013.
View Article : Google Scholar
|
|
43
|
Dudekula DB, Panda AC, Grammatikakis I, De
S, Abdelmohsen K and Gorospe M: CircInteractome: A web tool for
exploring circular RNAs and their interacting proteins and
microRNAs. RNA Biol. 13:34–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42:D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Backes C, Kehl T, Stöckel D, Fehlmann T,
Schneider L, Meese E, Lenhof HP and Keller A: miRPathDB: A new
dictionary on microRNAs and target pathways. Nucleic Acids Res.
45:D90–D96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu R, Liu F, Li L, Sun M and Chen K:
MiR-498 regulated FOXO3 expression and inhibited the proliferation
of human ovarian cancer cells. Biomed Pharmacother. 72:52–57. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
You Y, Que K, Zhou Y, Zhang Z, Zhao X,
Gong J and Liu Z: MicroRNA-766-3p inhibits tumour progression by
targeting Wnt3a in hepatocellular carcinoma. Mol Cells. 41:830–841.
2018.PubMed/NCBI
|
|
48
|
Chen C, Xue S, Zhang J, Chen W, Gong D and
Zheng J, Ma J, Xue W, Chen Y, Zhai W and Zheng J:
DNA-methylation-mediated repression of miR-766-3p promotes cell
proliferation via targeting SF2 expression in renal cell carcinoma.
Int J Cancer. 141:1867–1878. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li Z, Liu YH, Diao HY, Ma J and Yao YL:
MiR-661 inhibits glioma cell proliferation, migration and invasion
by targeting hTERT. Biochem Biophys Res Commun. 468:870–876. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Reddy SD, Pakala SB, Ohshiro K, Rayala SK
and Kumar R: MicroRNA-661, a c/EBPalpha target, inhibits metastatic
tumor antigen 1 and regulates its functions. Cancer Res.
69:5639–5642. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Peng N, Shi L, Zhang Q, Hu Y, Wang N and
Ye H: Microarray profiling of circular RNAs in human papillary
thyroid carcinoma. PLoS One. 12:e01702872017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jin X, Wang Z, Pang W, Zhou J, Liang Y,
Yang J, Yang L and Zhang Q: Upregulated hsa_circ_0004458
Contributes to Progression of Papillary Thyroid Carcinoma by
Inhibition of miR-885-5p and Activation of RAC1. Med Sci Monit.
24:5488–5500. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hardee S, Prasad ML, Hui P, Dinauer CA and
Morotti RA: Pathologic characteristics, natural history, and
prognostic implications of BRAF(V600E) mutation in pediatric
papillary thyroid carcinoma. Pediatr Dev Pathol. 20:206–212. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Walts AE, Mirocha JM and Bose S:
Follicular variant of papillary thyroid carcinoma (FVPTC):
Histological features, BRAF V600E mutation, and lymph node status.
J Cancer Res Clin Oncol. 141:1749–1756. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kim H, Kim BH, Kim YK, Kim JM, Oh SY, Kim
EH, Lee MJ, Kim JH, Jeon YK, Kim SS, et al: Prevalence of
BRAFV600E mutation in follicular variant of
papillary thyroid carcinoma and non-invasive follicular tumor with
papillary-like nuclear features (NIFTP) in a
BRAFV600E prevalent area. J Korean Med Sci.
33:e752018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jin Y, Jin W, Zheng Z, Chen E, Wang Q,
Wang Y, Wang O and Zhang X: GABRB2 plays an important role in the
lymph node metastasis of papillary thyroid cancer. Biochem Biophys
Res Commun. 492:323–230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Beltrami CM, Reis MBD, Barrosfilho MC,
Marchi FA, Kuasne H, Pinto CAL, Ambatipudi S, Herceg Z, Kowalski LP
and Rogatto SR: Integrated data analysis reveals potential drivers
and pathways disrupted by DNA methylation in papillary thyroid
carcinomas. Clin Epigenetics. 9:452017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Furlan JC, Bedard YC and Rosen IB:
Significance of tumor capsular invasion in well-differentiated
thyroid carcinomas. Am Surg. 73:484–491. 2007.PubMed/NCBI
|
|
59
|
Bolognamolina R, Gonzálezgonzález R,
Mosquedataylor A, Molinafrechero N, Damiánmatsumura P and
Dominguezmalagón H: Expression of syndecan-1 in papillary carcinoma
of the thyroid with extracapsular invasion. Arch Med Res. 41:33–37.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Meng X, Zhu P, Li N, Hu J, Wang S, Pang S
and Wang J: Expression of BMP-4 in papillary thyroid carcinoma and
its correlation with tumor invasion and progression. Pathol Res
Pract. 213:359–363. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Genpeng L, Jianyong L, Jiaying Y, Ke J,
Zhihui L, Rixiang G, Lihan Z and Jingqiang Z: Independent
predictors and lymph node metastasis characteristics of multifocal
papillary thyroid cancer. Medicine (Baltimore). 97:e96192018.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Erhardt A, Czibere L, Roeske D, Lucae S,
Unschuld PG, Ripke S, Specht M, Kohli MA, Kloiber S, Ising M, et
al: TMEM132D, a new candidate for anxiety phenotypes: Evidence from
human and mouse studies. Mol Psychiatr. 16:647–663. 2011.
View Article : Google Scholar
|
|
63
|
Karapetsas A, Giannakakis A, Dangaj D,
Lanitis E, Kynigopoulos S, Lambropoulou M, Tanyi JL, Galanis A,
Kakolyris S and Trypsianis G: Overexpression of GPC6 and TMEM132D
in early stage ovarian cancer correlates with CD8+ T-lymphocyte
infiltration and increased patient survival. Biomed Res Int.
2015:7124382015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Baek HJ, Kim DW and Ryu JH: Association
between TNM staging system and histopathological features in
patients with papillary thyroid carcinoma. Endocrine. 48:589–594.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chen D, Sun Y, Wei Y, Zhang P, Rezaeian
AH, Teruya-Feldstein J, Gupta S, Liang H, Lin HK, Hung MC and Ma L:
LIFR is a breast cancer metastasis suppressor upstream of the
Hippo-YAP pathway and a prognostic marker. Nat Med. 18:1511–1517.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Josson S, Gururajan M, Hu P, Shao C, Chu
GY, Zhau HE, Liu C, Lao K, Lu CL, Lu YT, et al: miR-409-3p/-5p
promotes tumorigenesis, epithelial-to-mesenchymal transition, and
bone metastasis of human prostate cancer. Clin Cancer Res.
20:4636–4646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Josson S, Gururajan M, Sung SY, Hu P, Shao
C, Zhau HE, Liu C, Lichterman J, Duan P, Li Q, et al: Stromal
fibroblast-derived miR-409 promotes epithelial-to-mesenchymal
transition and prostate tumorigenesis. Oncogene. 34:2690–2699.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yu CY and Kuo HC: The emerging roles and
functions of circular RNAs and their generation. J Biomed Sci.
26:292019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Li X, Yang L and Chen LL: The biogenesis,
functions, and challenges of circular RNAs. Mol Cell. 71:428–442.
2018. View Article : Google Scholar : PubMed/NCBI
|