Introduction
The renin-angiotensin (Ang) system (RAS) is
important in the control of systemic blood pressure (SBP). It is
also involved in the pathogenesis of hypertensive renal injury,
which is one of the leading causes of chronic kidney disease
worldwide (1,2). The analysis of the epidemiological
data from dialysis registries has shown that the incidence of
end-stage renal disease due to hypertensive nephrosclerosis is
progressively increasing in China and a number of other countries.
Therefore, novel preventive and therapeutic compounds for
hypertensive renal disease should be identified (3,4).
A stage I clinical trial on nicousamide, a potential
renal-protective compound, has been completed in China (5), while a stage II clinical trial is to
be conducted for the treatment of diabetic nephropathy. Nicousamide
is a novel coumarine-aspirin derivative. Recent studies have
suggested that nicousamide may reduce the progression of diabetic
nephropathy in streptozotocin-induced diabetic rats (6–8). The
inhibition of advanced glycation end product (AGE) formation and
phosphorylation by transforming growth factor-β (TGF-β) receptor II
may be associated with the mechanisms underlying the
nicousamide-mediated attenuation of diabetic nephropathy, as
suggested in previous findings (5).
By inhibiting the formation of AGEs, nicousamide blocks the effects
of AGEs, such as various intracellular events and increases the
activity of various growth factors including TGF-β1 and connective
tissue growth factor (CTGF) (9,10). By
inhibiting the phosphorylation of TGF-β receptor II, nicousamide
blocks the activity of TGFβ-Smad signal pathways, which are
actively involved in the final stage of kidney fibrosis (5).
Spontaneously hypertensive rats (SHRs) have been
widely used as a primary hypertension animal model, where the
hypertensive nephropathy is characterized by multiple renal
structural and functional alterations. Renal injury in SHRs
reportedly involves a complex pathological network including renin,
angiotensin (Ang) II, monocytes/macrophages, inflammatory cytokines
and oxidative stress (11,12). Generally, in SHRs of >6 months,
the kidney automatically progresses into severe renal injury
characterized by marked proteinuria, elevation of serum creatinine
(Scr) and blood urea nitrogen (BUN), reduced creatinine clearance
ratio (CCr), glomerulosclerosis, interstitial fibrosis and renal
vascular arteriosclerosis. These characteristics render SHRs a good
animal model of human hypertensive nephropathy (13).
Findings of recent studies have shown that the use
of RAS inhibitors, such as Ang-converting enzyme inhibitors (ACEs)
and Ang receptor blockers (ARBs), may effectively suppress the
progression of established renal disease (14,15).
These studies suggest the possibility of the early inhibition of
RAS as an effective strategy to prevent chronic kidney disease
development. In the present study, we aimed to use SHRs to
investigate whether or not nicousamide could alleviate chronic
kidney injury under hypertensive conditions.
Materials and methods
Ethical considerations
This study was approved by the Institutional Animal
Care Committee of the Peking Union Medical College (Beijing,
China), and was conducted in accordance with the US National
Institute of Health Guide for the Care and Use of Laboratory
Animals.
Animal treatment protocol
Male SHRs (age, 16 weeks; weight, 300–340 g) were
obtained from the Institute of Laboratory Animal Science, the
Chinese Academy of Medical Sciences (Beijing, China). They were
kept in a specific pathogen-free facility and provided with
adequate food and water ad libitum, under constant
temperature (22±2°C) conditions, with a 12-h (7:00 a.m.–7:00 p.m.)
dark-light cycle.
Rats were randomized into 5 groups (n=10): i) no
treatment control; ii) losartan [10 mg/kg, periocular (p.o.)]
treatment; iii) oral nicousamide at 15 mg/kg; iv) oral nicousamide
at 30 mg/kg and v) oral nicousamide at 45 mg/kg. Nicousamide with a
purity of 99% was synthesized by the Department of Medicinal
Chemistry, Institute of Materia Medica, the Chinese Academy of
Medical Sciences (Beijing, China). Losartan and nicousamide were
diluted in sodium carboxymethycellulose-Na (CMC-Na). The treatment
continued for up to 17 weeks, during which BP and heart rate (HR)
were measured every 4 weeks by tail-cuff plethysmography (BP-98A;
Softron, Tokyo, Japan) with prior training to minimize variability
in BP measurement.
Ten age-matched normotensive Wistar rats were also
raised under the same conditions as the normal controls.
Chemical analyses of blood and urine
At the end of the designated treatments, blood was
sampled through the eyes under anesthesia with diethyl ether. Urine
samples were continuously collected for 24 h from each animal after
placement in metabolic cage, the day prior to blood sample
collection. BUN and urinary albumin excretion (UAE) were measured
using the standard biochemical kits (Beijing BHKT Clinical Reagent
Co., Ltd., Beijing, China), respectively. CCr was calculated
according to the formula: CCr = urinary creatinine (mg/ml) × urine
volume (ml/kg)/creatinine in plasma (mg/ml).
Histopathological assessment
At the end of the 17-week treatment, the rats were
anaesthetized by ether and sacrificed. Kidneys and hearts were
removed and weighed. The weight ratio of the kidney or heart to the
body was calculated and termed kidney or heart index. Tissue blocks
of kidneys were fixed in 10% formalin solution, embedded in
paraffin and sectioned. The sections were stained with Periodic
Acid-Schiff (PAS) and haematoxylin and eosin (H&E) to evaluate
glomerulosclerosis. The percentage of sclerosis-positive glomeruli
was calculated. At least 30 glomeruli localized within a 1-mm depth
from the surface of the kidney cortex were analyzed in each
section. Interstitium-tubular injuries were classified into four
categories: interstitium infiltration, interstitium fibrosis,
tubular dilatation and tubule-interstitium protein casts. The
incidence in each group was determined semi-quantitatively.
Determination of Ang II level in
plasma
Ang II level in plasma was determined using the
Angiotensin II ELISA kit (Enzo Biochem Inc., New York, NY, USA),
according to the detailed procedure described in the manufacturer’s
manual.
Reverse transcription-polymerase chain
reaction (RT-PCR)
In order to further investigate the mechanism
underlying the effect of nicousamide on the RAS system, mRNA levels
of renin, ACE and chymase in kidney tissues were semi-quantitated
by RT-PCR.
Total RNA was extracted from the fresh kidney
tissues of animals using TRIzol reagent. For each RNA sample, 2 μg
RNA were reverse-transcribed into cDNA using oligo(dT) random
primers, with the SuperScript™ II RNase H Reverse Transcriptase.
PCR was performed with reaction mixtures containing 2.5 mM dNTP, 10
mM sense and antisense primers, and 5 U/ml TaqDNA polymerase in a
thermal cycler for 30 sec at 94°C, 30 sec at 58°C (β-actin), 58°C
(renin), 65°C (ACE), 59°C (chymase) and 1 min at 72°C, 35 cycles.
The final extension reaction was performed at 72°C for 5 min.
The PCR primers used in this study were: renin,
sense: 5′-GTGCAGCCGTCTCTAC-3′ and antisense: 5′-CCG
TGACCTCTCCAAAC-3′; ACE, sense: 5′-GCAAGGAGG CAGGCTATGAG-3′ and
antisense: 5′-CGGGTAAAACT GGAGGATG G-3′; chymase, sense:
5′-CTGAGAGGATGC TTCTTCCTG C-3′ and antisense: 5′-AGATCTTATTGATCCA
GGGCCG-3′; β-actin was used as an internal control, sense:
5′-GTGGGGCGCCCCAGGCACCA-3′ and antisense:
5′-CTTCCTTAATGTCACGCACGATTTC-3′. The PCR products were
electrophoresed on 1.5% agarose gels, visualized by ethidium
bromide staining, and photographed under ultraviolet (UV) light.
PCR was repeated three times and the average optical density was
analyzed using the ImageJ software.
Statistical analysis
Data were expressed as the mean ± standard deviation
(SD). Statistical comparisons were performed using the Student’s
t-test, with the exception of the histological analyses in
interstitium-tubular lesions, which were analyzed using the
Pearson’s Chi-square test. P<0.05 was considered to indicate a
statistically significant difference.
Results
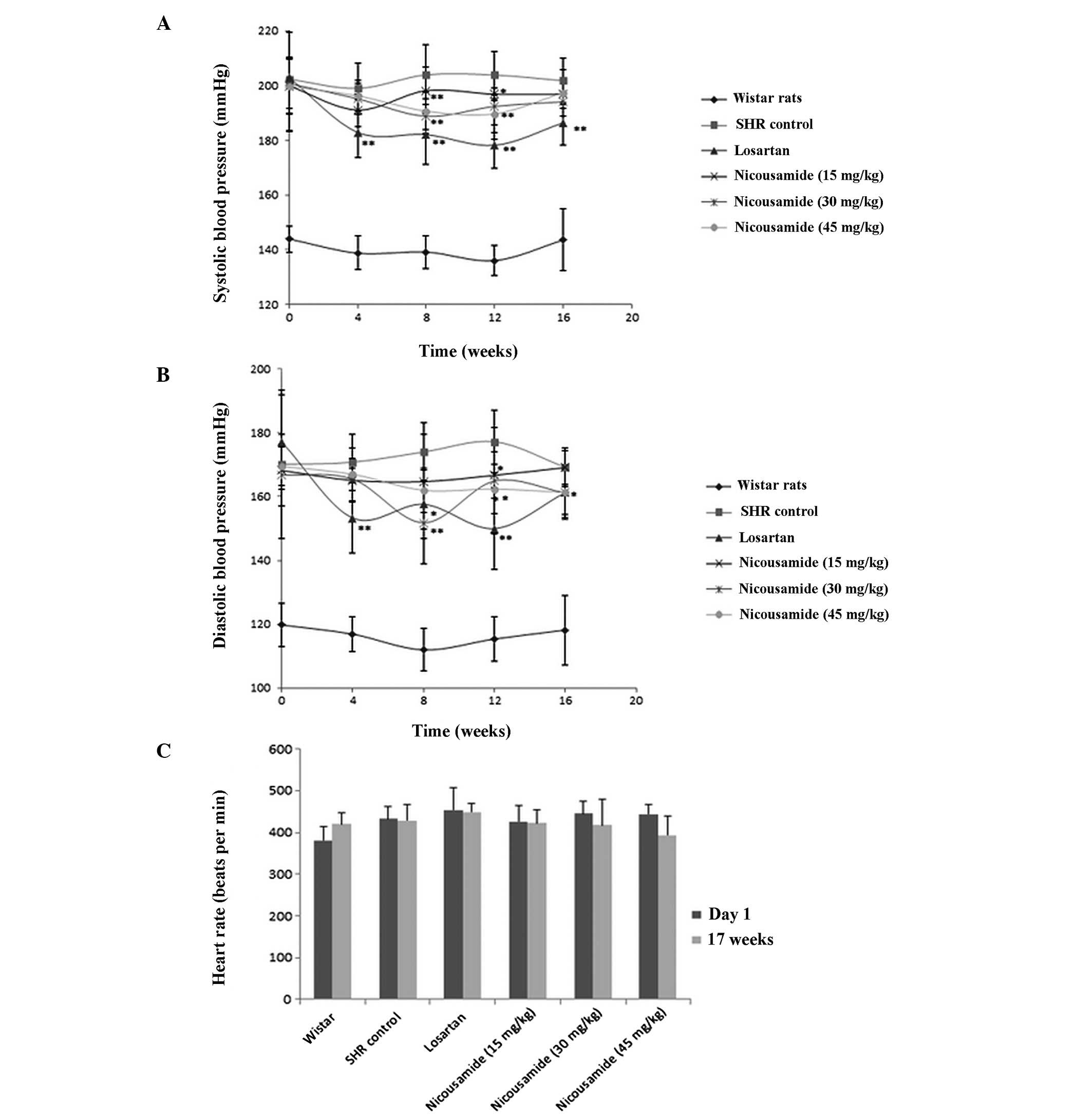
Effects of nicousamide on BP and HR
Prior to drug treatment, the average systolic
arterial pressure (SAP) of SHRs was 200 mmHg, 56 mmHg higher
compared to normotensive rats (P<0.01, Fig. 1A). The average diastolic pressure
(DAP) was 172 mmHg, 50 mmHg higher compared to normotensive rats
(P<0.01, Fig. 1B). The HR of
SHRs was also higher compared to normotensive rats (P<0.05)
(Fig. 1C).
Subsequent to drug treatment, BP was measured every
four weeks and the results are shown in Fig. 1A and B. Compared to untreated SHRs,
SHRs in the nicousamide treatment groups showed a decreased SAP and
DAP, especially at doses of 30 and 45 mg/kg (P<0.05 or
P<0.01), however, its BP-lowering effect was less effective
compared to losartan.
No statistically significant difference was observed
on HRs among the groups of experimental SHRs after the 17-week
treatment, although nicousamide (45 mg/kg) treatment decreased HR
by 8.24% compared to untreated SHRs, no statistically significant
difference was found (Fig. 1C).
Benefits of nicousamide on renal
function
The results of BUN, CCr and UAE measurements are
presented in Table I. Compared to
normotensive rats, untreated SHRs showed markedly higher levels in
these three parameters (P<0.01).
 | Table I.UAE, BUN, and CCr values after a
16-week nicousamide treatment in SHRs. |
Table I.
UAE, BUN, and CCr values after a
16-week nicousamide treatment in SHRs.
| Parameters | Normotensive
control | Control SHRs | Losartan (10
mg/kg) | Nicousamide (mg/kg)
|
|---|
| 15 | 30 | 45 |
|---|
| No. | 10 | 10 | 10 | 10 | 10 | 10 |
| UAE (mg/ml/day) | 21.47±6.91 | 24.76±8.23a | 19.11±5.86b | 20.08±10.04b | 25.32±6.46 | 24.58±4.50 |
| BUN (mg/dl) | 17.79±2.06 | 23.88±1.78a | 21.76±2.12b | 20.06±0.95b | 23.84±2.22 | 22.82±2.33 |
| CCr (ml/min/100 g
body weight) | 1.57±0.47 | 0.77±0.39a | 1.23±0.33b | 0.83±0.26 | 0.80±0.22 | 0.83±0.15 |
Treatment with nicousamide at 15 mg/kg decreased BUN
level by 16.01% (P<0.05) as compared to the untreated control
(P<0.05). Treatment of SHRs with nicousamide at 45 mg/kg
improved CCr values by 16.73% as compared to the control
(P<0.05). A modest improvement on UAE level was observed at a
15-mg/kg dose of nicousamide treatment.
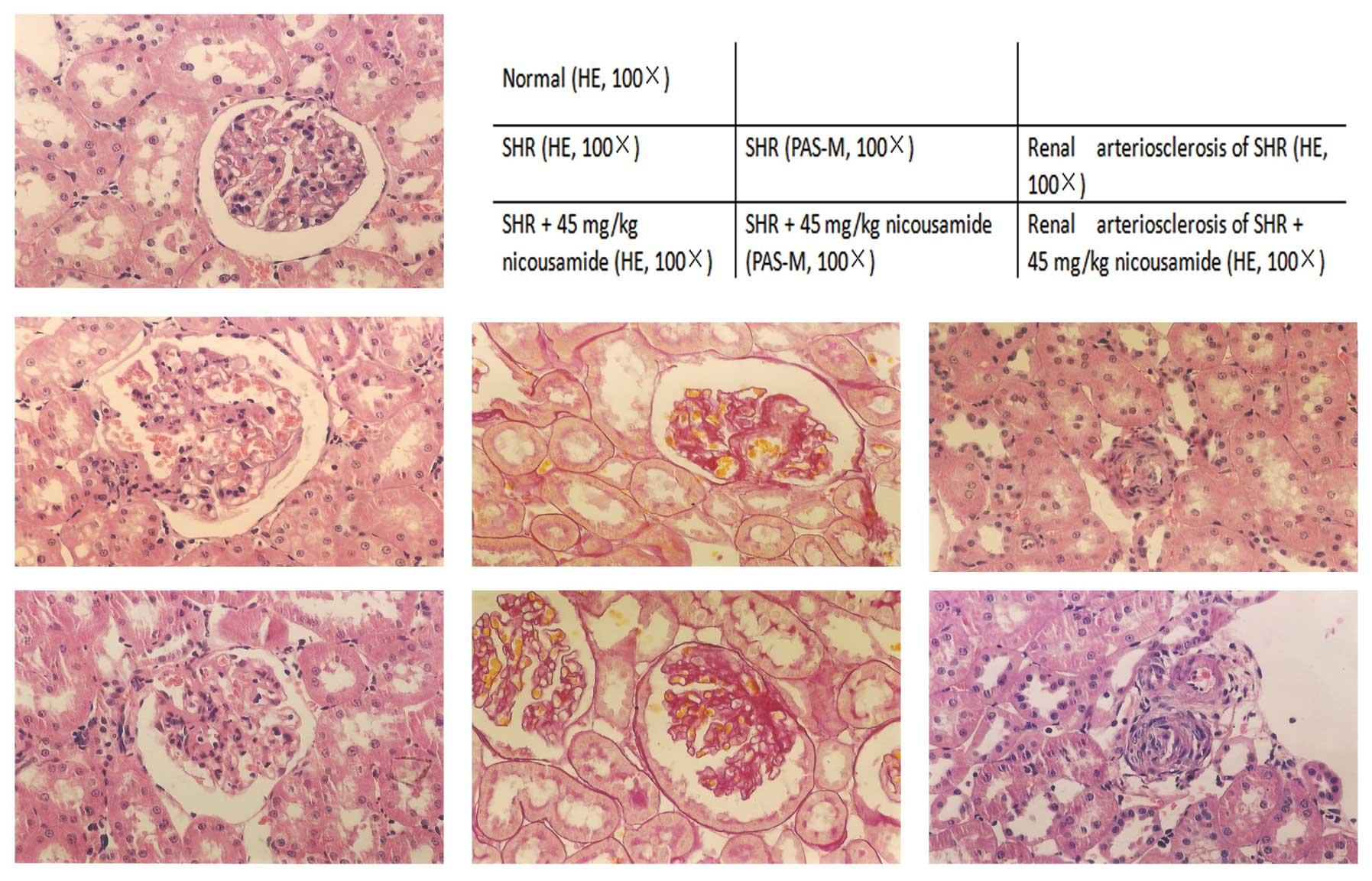
Benefits of nicousamide on kidney
histopathology
Histopathological findings in SHRs in various
treatment groups and in normotensive rats are shown in Fig. 2 and Table II. In the absence of drug
treatments, SHRs exhibited higher indices in kidney and heart
indices (P<0.01), glomerulosclerosis and apparent histological
abnormalities in the interstitium-tubular areas including
interstitium infiltration of inflammatory cells, fibrosis, tubular
dilatation and protein casts, as compared to normotensive rats.
 | Table II.Kidney histopathological
characteristics of the rats at the17th week. |
Table II.
Kidney histopathological
characteristics of the rats at the17th week.
| Parameters | Normotensive
control | Control SHRs | Losartan (10
mg/kg) | Nicousamide (mg/kg)
|
|---|
| 15 | 30 | 45 |
|---|
| No. | 10 | 10 | 10 | 10 | 10 | 10 |
| Kidney index (mg/kg
BW) | 0.574±0.05 | 0.687±0.04a | 0.679±0.03 | 0.674±0.05 | 0.670±0.07 | 0.672±0.02 |
| Heart index (mg/kg
BW) | 0.255±0.023 | 0.388±0.019a | 0.355±0.016 | 0.369±0.012 | 0.358±0.026 | 0.371±0.018 |
| Glomerulosclerosis
(%) | 3.67±4.29 | 12.34±4.45a | 6.68±5.00b | 7.51±2.52b | 7.32±4.39b | 4.66±3.59b |
| Incidence of
interstitium infiltration | 10 (0) | 10 (4)a | 10 (5) | 10 (4) | 10 (4) | 10 (3) |
| Incidence of
interstitium fibrosis | 10 (0) | 10 (2)a | 10 (2) | 10 (1) | 10 (3) | 10 (0)b |
| Incidence of tubular
dilatation | 10 (0) | 10 (4)a | 10 (1) | 10 (1)b | 10 (2) | 10 (1)b |
| Incidence of
tubule-interstitium protein casts | 10 (2) | 10 (4)a | 10 (1)b | 10 (2) | 10 (3) | 10 (1)b |
Treatment with either nicousamide or losartan for 17
weeks did not markedly improve the kidney or heart index, although
a modest amelioration was observed (P>0.05). By contrast,
nicousamide treatment resulted in significant alleviation of
glomerulosclerosis (P<0.05). When used at 15 and 30 mg/kg,
amelioration of glomeruloscerosis was evident (P<0.05), while
when used at 45 mg/kg, the level of glomerulosclerosis decreased
from 12.34 to 4.66% (P<0.05). At the three doses, nicousamide
markedly reduced interstitium infiltration of inflammatory cells,
fibrosis, tubular dilatation and protein casts in SHRs (P<0.05).
In the present study, nicousamide showed a stronger effect compared
to losartan on histopathological improvements.
Nicousamide decreased Ang II
concentrations of plasma in SHRs
As shown in Fig. 3,
the Ang II level in the nicousamide-treated groups was reduced in a
dose-dependent manner compared to the untreated controls. At the
dose of 45 mg/kg, nicousamide reduced the plasma Ang II by 27.2%
(P<0.05), while losartan markedly increased plasma Ang II level
(P<0.01).
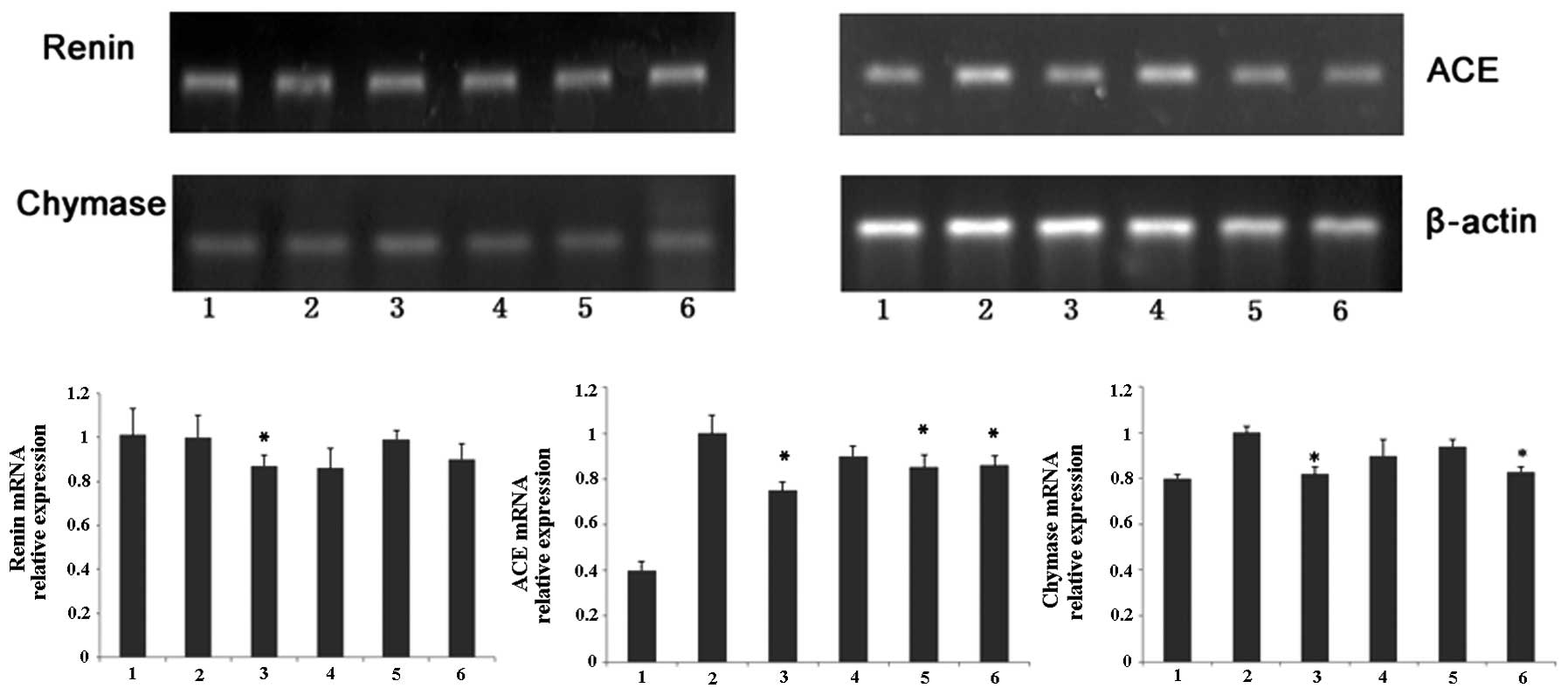
Effects of nicousamide on renin, ACE and
chymase mRNA levels
As shown in Fig. 4,
SHRs demonstrated higher mRNA levels of chymase and ACE in kidney
tissues compared to normotensive rats. Using optical density
analysis, we found that nicousamide and losartan could slightly but
markedly decrease ACE and chymase mRNA levels in SHR kidney
tissues, especially at 45 mg/kg (P<0.05). Although nicousamide
also showed a certain inhibitory effect on renin mRNA expression in
kidney tissues, no statistical significance was observed
(P>0.05).
Discussion
Nicousamide is a class 1.1 drug protected by
domestic and international patents. A phase II clinical trial for
diabetic nephropathy is currently being conducted in the Peking
Union Hospital (Beijing, China). In addition to its potential in
treating diabetic nephropathy, in the present study we aimed to use
SHRs as model animals to investigate whether or not it also has a
renal-protective effect on hypertensive nephropathy in rats.
In the present study, 16-week-old SHRs were used. At
the end of the 17-week treatment, the rats progressed into the
stage of chronic renal dysfunction with low creatinine clearance,
high UAE and BUN. Nicousamide markedly attenuated renal dysfunction
and improved the three renal function para meters, as demonstrated
by the biochemical analysis. Compared to losartan, nicousamide was
less effective in improving the biochemical parameters of renal
function.
Notably, histopathological analysis showed that
nicousamide markedly alleviated injuries in the glomeruli and
proximal tubules. The incidence of glomerulosclerosis in the
nicousamide-treated SHRs was markedly lower compared to that of the
control SHRs, and its beneficial effect was even better compared to
that of losartan. The impairment in tubulointerstitial fibrosis and
other tubular-interstitium injuries (interstitium infiltration,
interstitium fiborosis, tubular dilatation and tubule-interstitium
protein casts) were also markedly reduced after nicousamide
treatment (P<0.05). These observations suggest a therapeutic
potential of nicousamide for hypertensive nephropathy.
Regarding the underlying mechanisms involved in the
renal-protective effect on hypertensive nephropathy, we
hypothesized that the BP-lowering action constituted one of the
most important reasons. Our results have shown that nicousamide
markedly decreased SAP and DAP in SHRs during the long-term
treatment. Obviously, anti-hypertension is helpful for nicousamide
to retard the progression of hypertensive nephropathy.
To investigate the nicousamide effect on the
regulation of the BP-lowering action, mRNA levels of certain
important components in the RAS system, such as renin, ACE and
chymase in the animal kidneys were evaluated using RT-PCR. Renin,
produced by collecting duct cells, circulates in the bloodstream
and hydrolyzes Ang to Ang I (16,17).
In the present study, compared to the control model SHRs, only the
losartan-treated group demonstrated a significant decrease in the
renin level, and although nicousamide treatment also showed a
certain reduction in renin mRNA expression, no statistical
significance was found.
Chymase is a serine protease, released by mucosal
mast cells upon challenge with parasites and parasite antigens
promoting an inflammatory response (18). Chymase is also known to convert Ang
I to Ang II and is, thus, crucial in hypertension and
atherosclerosis (19). In the
present study, losartan and nicousamide (45 mg/kg) treatment
markedly downregulated chymase mRNA expression in the kidneys of
SHRs. Thus, it was hypothesized that by inhibiting chymase
expression, nicousamide might reduce the concentration of Ang II
and finally reduce BP.
ACE, which is present in vascular and tubular
epithelium, catalyzes the conversion of decapeptide Ang I to active
Ang II (20), potentially inducing
strong vascular contraction. In the present study, subsequent to
nicousamide treatment (30 or 45 mg/kg), the mRNA level of ACE in
animal kidneys was slightly but markedly reduced and similar
results were also observed in the losartan-treated group. By
reducing the ACE expression, nicousamide may decrease the
concentration of Ang II in plasma and eventually reduce BP.
Ang II levels in the plasma were assessed in order
to confirm our hypothesis. The results, according to which the Ang
II level in plasma was reduced in a dose-dependent manner compared
to the model group, confirmed our hypothesis. Consequently,
decreasing Ang II is crucial in anti-hypertension for
nicousamide.
In conclusion, nicousamide may moderately reduce BP,
thus having a beneficial effect on individuals with hypertensive
nephropathy. This BP-lowering effect may be achieved through the
reduction of the plasma Ang II and the decrease of ACE and chymase
expression.
Acknowledgements
The authors appreciate the support
provided by the Key Project of the National Eleventh-Five Year
Research Program of China.
References
|
1.
|
Azegami T, Sasamura H, Hayashi K and Itoh
H: Vaccination against the angiotensin type 1 receptor for the
prevention of L-NAME-induced nephropathy. Hypertens Res.
35:492–499. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Hill GS: Hypertensive nephrosclerosis.
Curr Opin Nephrol Hypertens. 17:266–270. 2008. View Article : Google Scholar
|
|
3.
|
Chen Y, Lipkowitz MS, Salem RM, et al:
Progression of chronic kidney disease: adrenergic genetic influence
on glomerular filtration rate decline in hypertensive
nephrosclerosis. Am J Nephrol. 32:23–30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Wang G, Lai FM, Kwan BC, et al: Expression
of ACE and ACE2 in patients with hypertensive nephrosclerosis.
Kidney Blood Press Res. 34:141–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Zhang H, Jin J, Zhou W, et al:
Nicousamide, a potent inhibitor of phosphorylation by TGF-β
receptor II. Acta Pharmaceutica Sinica. 1:160–165. 2011.
|
|
6.
|
Li H, Zheng X, Wang H, Zhang Y, Xin H and
Chen X: XLF-III-43, a novel coumarin-aspirin compound, prevents
diabetic nephropathy in rats via inhibiting advanced glycation end
products. Eur J Pharmacol. 627:340–347. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Li H, Zhang Y, Wang H, Zheng X and Chen X:
Nicousamide blocks the effects of advanced glycation end products
on renal cells. Eur J Pharmacol. 674:455–459. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Sheng L, Chen H and Li Y: A HPLC method
for determination of nicousamide in dog plasma and its application
to pharmaco-kinetic studies. J Chromatogr B Analyt Technol Biomed
Life Sci. 854:99–103. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Jakus V and Rietbrock N: Advanced
glycation end-products and the progress of diabetic vascular
complications. Physiol Res. 53:131–142. 2004.PubMed/NCBI
|
|
10.
|
Wendt TM, Tanji N, Guo J, et al: RAGE
drives the development of glomerulosclerosis and implicates
podocyte activation in the pathogenesis of diabetic nephropathy. Am
J Pathol. 162:1123–1137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Tian D, Ling S, Chen G, et al:
Hypertensive nephropathy treatment by heart-protecting musk pill: a
study of anti-inflammatory therapy for target organ damage of
hypertension. Int J Gen Med. 4:131–139. 2011.PubMed/NCBI
|
|
12.
|
Sun L, Ke Y, Zhu CY, et al: Inflammatory
reaction versus endogenous peroxisome proliferator-activated
receptors expression, re-exploring secondary organ complications of
spontaneously hypertensive rats. Chin Med J (Engl). 121:2305–2311.
2008.
|
|
13.
|
Koshikawa S, Nishikimi T, Inaba C, Akimoto
K and Matsuoka H: Fasudil, a Rho-kinase inhibitor, reverses L-NAME
exacerbated severe nephrosclerosis in spontaneously hypertensive
rats. J Hypertens. 26:1837–1848. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Alfie J, Aparicio LS and Waisman GD:
Current strategies to achieve further cardiac and renal protection
through enhanced renin-angiotensin-aldosterone system inhibition.
Rev Recent Clin Trials. 6:134–146. 2011. View Article : Google Scholar
|
|
15.
|
Berl T: Review: renal protection by
inhibition of the renin-angiotensin-aldosterone system. J Renin
Angiotensin Aldosterone Syst. 10:1–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Susic D, Frohlich ED, Kobori H, Shao W,
Seth D and Navar LG: Salt-induced renal injury in SHRs is mediated
by AT1 receptor activation. J Hypertens. 29:716–723. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Varagic J, Ahmad S, Brosnihan KB, et al:
Salt-induced renal injury in spontaneously hypertensive rats:
effects of nebivolol. Am J Nephrol. 32:557–566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Ehara T and Shigematsu H: Mast cells in
the kidney. Nephrology (Carlton). 8:130–138. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Jones C: Matrix degradation in renal
disease. Nephrology (Carlton). 2:13–23. 1996. View Article : Google Scholar
|
|
20.
|
Siragy HM: Angiotensin II
compartmentalization within the kidney: effects of salt diet and
blood pressure alterations. Curr Opin Nephrol Hypertens. 15:50–53.
2006. View Article : Google Scholar : PubMed/NCBI
|