Please visit "Information for authors" for each of our journals:
Online Submission
Spandidos Publications journals utilize an online submission and tracking system designed to provide a faster, more efficient service to authors.
Submission guidelines



- The principal aim of Spandidos Publications is to publish promptly original works of high quality in English.
- Manuscripts will be considered on the understanding that they report original work, or are review articles summarizing and interpreting progress in a thematic area and are not under consideration for publication by another journal.
- Before submitting a manuscript, authors are invited to ensure that it adheres to the guidelines outlined below.
- The corresponding author is responsible for the submission on behalf of all authors.
- All manuscript files and correspondence submitted by the authors will be reviewed by appropriate referees selected by the Editorial Office.
- All manuscripts will be examined to detect inappropriate use of previously published material without attribution. Spandidos Publications uses iThenticate to screen submitted manuscripts against previously published studies and other relevant sources. Authors are also encouraged to use iThenticate to screen their manuscript prior to submission.
- All submitted figures submitted may be subject to checks using the MOTUIN image authenticity detector.
- Manuscripts should be written in clear, concise English and should contain all essential data in order to make the presentation clear and the results of the study replicable.
- Authors wish to improve the language grammar, spelling and style of their manuscript to ensure its meaning is clear to the readers can make use of the English Language Editing service offered by Spandidos Publications.
- During the editorial process, Spandidos Publications reserves the right to improve the grammar and style of all manuscripts.
Prior to submitting your manuscript, please ensure that it has been prepared according to the guidelines below.
1. Submission method
Manuscripts may only be submitted through our online submission system, which is accessible via our
website. Authors must create a user account, log in and follow the on-screen directions.
2. Cover letter
All submissions should be accompanied by a cover letter that briefly summarizes the important points of the submitted work, including a brief description of the study. In the cover letter, authors should also confirm that the submitted manuscript reports the results of an original study presenting novel work, that it has not been previously submitted to or accepted by any other journal, that is has been approved by all author and that ethics approval and written informed consent have been obtained, where applicable. Any competing interests must also be disclosed to the Editor in the cover letter.
3. Format of articles and reviews
3.1 General style
- Times New Roman. Font size 12. Spacing 1.5. Alignment Justified.
- Use a single tab on the first line of each new paragraph.
- Do not use page breaks or multiple returns between sections (one section should directly follow the previous one on the page).
- Do not insert page numbers or line numbers.
- Sub-titles and general headings should be presented in lower case letters (not capitals).
- Authors may use either British English or American English spellings throughout your manuscript, but not both.
3.2 Manuscripts
All manuscripts must contain a title page that includes all of the following information:
- The title of the manuscript in sentence case. This should not contain any abbreviation unless they are commonly used. Gene symbols may be used.
- Full names and full postal addresses, but not including street names, of all authors and ORCID if desired.
- Affiliations of the authors indicated by numbers (not symbols).
- Equal contribution, if applicable, indicated by asterisk.
- Name, full postal address, including street number and name, and e-mail address of the corresponding author(s).
- Abbreviations, if relevant.
- Key words (5 – 10).
- Running title preceded by the first author’s name (maximum 100 characters with spaces, including the author’s name). For example: PEARSON et al: REGULATION OF HER2 EXPRESSION BY NASCENT GROWTH FACTORS.
Footnotes should not be used.
Original research articles must contain all of the following sections:
- Abstract. This section is usually 150‐300 words and does not contain any references. It should be continuous, and not contain any subsections (such as ‘Objectives’, ‘Methods’, ‘Results’ or ‘Conclusions’). Except for gene symbols, any terms that appear only once in this section must not be abbreviated. Terms used more than once must be abbreviated on first use, and the abbreviation must then be used for the rest of Abstract. Any term abbreviated in the Abstract must be abbreviated again in the main body of the text.
- Introduction. All information provided in this section must be duly referenced.
- Materials and methods. This section should include sufficient technical information to allow the experiments to be repeated and the results to be reproduced by other researchers. Any experiment described in the Results section and presented in the Figures/Tables must be mentioned in Materials and methods. For each experiment, all steps (e.g., DNA and protein extraction, quantification, cloning, PCR and microscopy) need to be mentioned, along with the instruments the analyses were performed on and the reagents and methods used (e.g., BCA method for protein quantification, ΔΔCq method for qPCR). Any relevant citations must also be added.
- Minimum required information: For specific details on our standards of reporting for individual techniques, please click here. For PCRs, the name of the polymerase/polymerase kit used, the sequence of the primers in 5’-3’ orientation and the thermocycling conditions are required. Please carefully review your text to ensure that the type of PCR (i.e. quantitative or semi-quantitative) is clear. If the PCR is performed using cDNA synthesized from RNA samples by reverse transcription (RT), all steps and reagents should be described, and the method should be referred to as RT-PCR or, if quantitative, as RT-qPCR. For relative quantification, ΔΔCt should be referred to as ΔΔCq. When using the ΔΔCq method, a citation must be provided. (such as Livak and Schmittgen: Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCq method. Methods 25: 402-408, 2001). Manufacturer/supplier details need to be provided for all reagents used (including chemicals), instruments (e.g. thermocyclers, microscopes) and software, together with the corresponding catalogue number/model/version. For antibodies, the full name, catalogue number, dilution and supplier (and conjugate, such as HRP, if applicable) must be provided. Any antigen retrieval, fixing or blocking steps should be described. The temperature and duration of any incubation steps must be stated. For centrifugation steps, the centrifugal force units in x g rather than revolutions per minute (rpm) should be given.
- Commercial kits: For steps performed with commercial kits, the full name of the kit and its supplier must be provided, along with a catalogue number. It should be clearly stated whether the protocol of the manufacturer was followed or if any modifications were made to the standard protocol.
- Bioinformatics: As with any other methods, bioinformatics methods should contain sufficient information to allow the reader to i) access the exact same public datasets you have used for your analysis, ii) to replicate your experiments and iii) to reproduce your results. As with any other methods, bioinformatics methods should contain sufficient information to allow the reader to i) access the exact same public datasets you have used for your analysis, ii) to replicate your experiments and iii) to reproduce your results. We require the name of all software used, along with a reference or URL. The use of software should be described with regards to the parameters analysed and the applied thresholds. Please explicitly state the output (for example, ‘differentially expressed genes were defined as…’) and the parameters analysed (for example, FDR < XXX, log2 fold change > XXX or adjusted P-value < XXX). Any datasets downloaded from public databases need to be clarified in the text, together with the corresponding accession number of the dataset and a URL for the database. If Figures/Tables contain data from a public database, please cite the source in the figure legend explicitly.
- Flow cytometry experiments: Authors are encouraged to adhere to MIFlowCyt guidelines. Axis labels should include the name of the analyte used and the fluorochrome (for example, annexin-V-FITC, rather than generic flow cytometer labels, such as FL-1H). The scaling (log/lin) should be clearly displayed. If most events are “piled up” on the plot axes, the scale of the plots must be adjusted. If statistical analysis is provided, please clarify if it is the fluorescence intensity of the gated population (mean, median, geometric mean) or the proportion of cells within a specific gate that is examined.
- Sources of materials: The sources of all materials used and relevant ethical framework for all experiments should be clearly identified (ethics approval and/or written informed consent). For tissue samples, please explain how and where these were collected, handled and stored. For bacterial strains or cells, provide the name and supplier. For studies on involving humans, the following information is required: number of subjects, time and place of sample collection, age range, sex ratio, inclusion and exclusion criteria, criteria used for diagnosis, description of any control subjects. Please note that the term ‘normal’ should be avoided for controls. Instead, the precise health status needs to be described, such as ‘healthy’, or ‘individuals with no recorded tumor complication’.
- Statistical analysis: When statistical analyses have been performed, the following information should be provided in Materials and methods: the name, version and supplier of the statistical software used, the name of the statistical tests used, the number of experimental repeats and the P-value threshold considered to indicate statistical significance. It should be clear which statistical test was used to generate every P-value. Significance indicators (symbol such as *, ** or ***) should be used in all graphs and tables and described in the figure or table legends. The groups being compared must be clearly stated (e.g. *P<0.05, vs. healthy control). The word ‘significant’ must only be used if the result is statistically significant, and the relevant P-value should be clearly indicated. All graphs must contain error bars where applicable, and the author should clearly state whether this designates the standard deviation or a standard error. If your study involves performing statistical analysis of datasets containing 3 or more groups, please note that the use of Student's t-test, Mann-Whitney’s U-test or similar two-sample tests is not considered appropriate, as the familywise error rate is raised to unacceptably high levels when these tests are used to perform multiple comparisons. These data should be analyzed using tests that are adequate for multiple groups, such as analysis of variance (ANOVA) followed by a post hoc test (e.g. Tukey, Bonferroni, Dunnett, etc.) for parametric data, or Kruskal-Wallis test followed by Dunn's test for non-parametric data.
- Figure legends: The legends should include a title, followed by a brief description of what is shown in the figure. The Figure legends are not expected to contain information already described in Materials and methods, except for image-specific information. For example, for microscopy, the legend should state the type of image shown (e.g., immunofluorescence) and the original magnification and/or representative length of the scale bars. The x- and y-axes of the graphs labelled in the figure, and explained in the legend if necessary. If significance indicators (symbol such as *, ** or ***) are used in the figures, then these must be described in the figure legends. The groups being compared must be clearly stated (e.g. *P<0.05, vs. healthy control).
- Cell lines: For all manuscripts involving the use of cell lines, it is strongly advised to include the following information in the Materials and methods section of the manuscript: i) Whether mycoplasma testing has been carried out for the cell lines used; ii) Whether the cell lines used have been authenticated; if so, the methods used should be stated; and iii) the name of the supplier (or if not obtained commercially, the name, title and affiliation of the individual who provided the cells) and, if available, catalogue number of all cell lines used the study. The method used to maintain the cell lines in culture should be specified, according to international guidelines on good cell culture practice. For more information, please refer to the following links: (fundamental techniques, mycoplasma contamination, passage number, etc.). Furthermore, information regarding misidentified or cross-contaminated cell lines must be provided and cross-checked from the International Cell Line Authentication Committee and ExPASy Cellosaurus databases in order to exclude any possible contamination with other cell lines or misidentification. If any of the cell lines used in the study has been previously reported to be contaminated with another line and/or misidentified, the Editor may request an STR profile at any stage of the editorial process.
- Results. This section must contain a detailed description of all data presented in the form of a figure or a table. All figures and tables must be cited in the Results. The word ‘significant’ must only be used if the result is statistically significant, and the relevant P-value should be clearly indicated.
- Discussion. This should include a brief summary of the findings and discuss the present study in light of previous findings. Authors may wish to compare their own findings with those of previous studies or suggest further research on the topic and mention any limitations of the present study. This section may also be concluded by restating the main findings and their significance to the field.
- Acknowledgements
- Funding
- Availability of data and materials
- Authors' contributions
- Ethics approval and consent to participate
- Patient consent for publication
- Competing interests
- Authors' information (optional)
- Use of artificial intelligence tools (to be included only when AI tools are used)
- References
- Figure legends
- Tables
For Review articles:
- Abstract. This section is usually 150‐300 words and does not contain any references. It should be continuous, and not contain any subsections (such as ‘Objectives’, ‘Methods’, ‘Results’ or ‘Conclusions’). Except for gene symbols, any terms that appear only once in this section must not be abbreviated. Terms used more than once must be abbreviated on first use, and the abbreviation must then be used for the rest of Abstract. Any term abbreviated in the Abstract must be abbreviated again in the main body of the text.
- Reviews may have different sections and sub-headings according to the subject matter.
- The main headings of the review should be summarized as a numbered Contents section immediately following the Abstract.
- Acknowledgements
- Funding
- Availability of data and materials
- Authors' contributions
- Ethics approval and consent to participate
- Patient consent for publication
- Competing interests
- Authors' information (optional)
- Use of artificial intelligence tools (to be included only when AI tools are used)
- References
- Figure legends
- Tables
3. Format of articles and reviews
3.3 Figures
Submission of figures to Spandidos Publications implies that the images or parts thereof have not been published elsewhere (unless mentioned and/or cited in the text and permission has been obtained and provided to us).
Images showing any patient or patient’s medical scans or other data should not contain information, that might identify them, either directly or indirectly, unless written permission is obtained from the patient allowing use of the specific image. For more information, please refer to our editorial policies regarding patient consent for publication.
Figures should be prepared in a way that ensures to ensure that the version submitted to us is an honest and accurate representation of the original observation(s) made by the authors and minimizes any possible misinterpretation of what was done experimentally. Cosmetic adjustments are only acceptable if they help show the experimental results in a clearer or more intuitive manner.
The Editors reserve the right to examine the submitted images for unacceptable manipulation using forensic tools or other available means at any point during the editorial process. This might delay progress of your manuscript and/or lead to further investigations and action to preserve the integrity of the scientific record, such as not accepting or revoking a manuscript. The Editors may also request any or all original unmanipulated source files for further review and may contact the authors’ institution for assistance with enquiries. Our guidance builds on that described by Rossner and Yamada (1).
- If brightness, contrast or colour balance is altered, the change should apply to the entire image shown and not a selected part. For images from gels or filters, ensure that details are not lost from bright areas or obscured in dark areas.
- For gels and blots, features (such as DNA or protein bands) should never be selectively enhanced, obscured, removed or added. If the individual lanes show bands from a sample or groups of samples analysed separately (or from different exposures), then the grouping should be made obvious using black or white lines and explained in the figure legend.
- For tiled images, the boundaries between individual parts should also be made obvious.
- If any changes are made, the figure legends should clearly describe why this was done and how. For example, “Figure 99. Light microscopy of a frozen section of a lesion stained with toluidine blue. Original magnification x100. Uneven illumination was corrected using a control image as described by Marty GD (2)”.
(1) Rossner M and Yamada KM: What's in a picture? The temptation of image manipulation. J Cell Biol 166: 11-15, 2004 (http://jcb.rupress.org/content/166/1/11).
(2) Marty GD: Blank-field correction for achieving a uniform white background in brightfield digital photomicrographs. BioTechniques 42:716-720, 2007 (https://www.ncbi.nlm.nih.gov/pubmed/17612294).
3.3.1 File format
- Acceptable
- TIFF without layers and preferably using Lempel–Ziv–Welch (LZW) compression as it does not reduce image quality.
- JPEG (only if originally saved at the highest quality).
- Unacceptable
- Images imported or copy pasted into Word or PowerPoint
- BMP, GIF, PCT, PNG or low quality JPEG files originally saved at low quality.
3.3.2 Color mode
- Acceptable
- Color figures: Use RGB as this will offer the best reproduction of your data in the final PDF version of your article on screen. CMYK mode is also acceptable. Fluorescence images must be submitted for publication in color.
- Black and white figures and line art: grey scale mode or RGB mode.
- Combination figures with colour images and line art: RGB mode.
- PLEASE NOTE
- Color figures are welcome but must be submitted only if reproduction in color is intended (a charge will apply).
- There is a charge of Euro 390 per each published page containing color.
- Changing color figures to black and white following evaluation is NOT possible.
3.3.3 Image size
- Image size is measured in centimeters or inches
- Create your figures at the size (width) at which they will be printed
- 8.00 cm (3.15 in) wide for a single-column figure
- 17.00 cm (6.70 in) maximum for a double-column (full page width) figure
- Maximum height 20.00 cm (7.87 in)
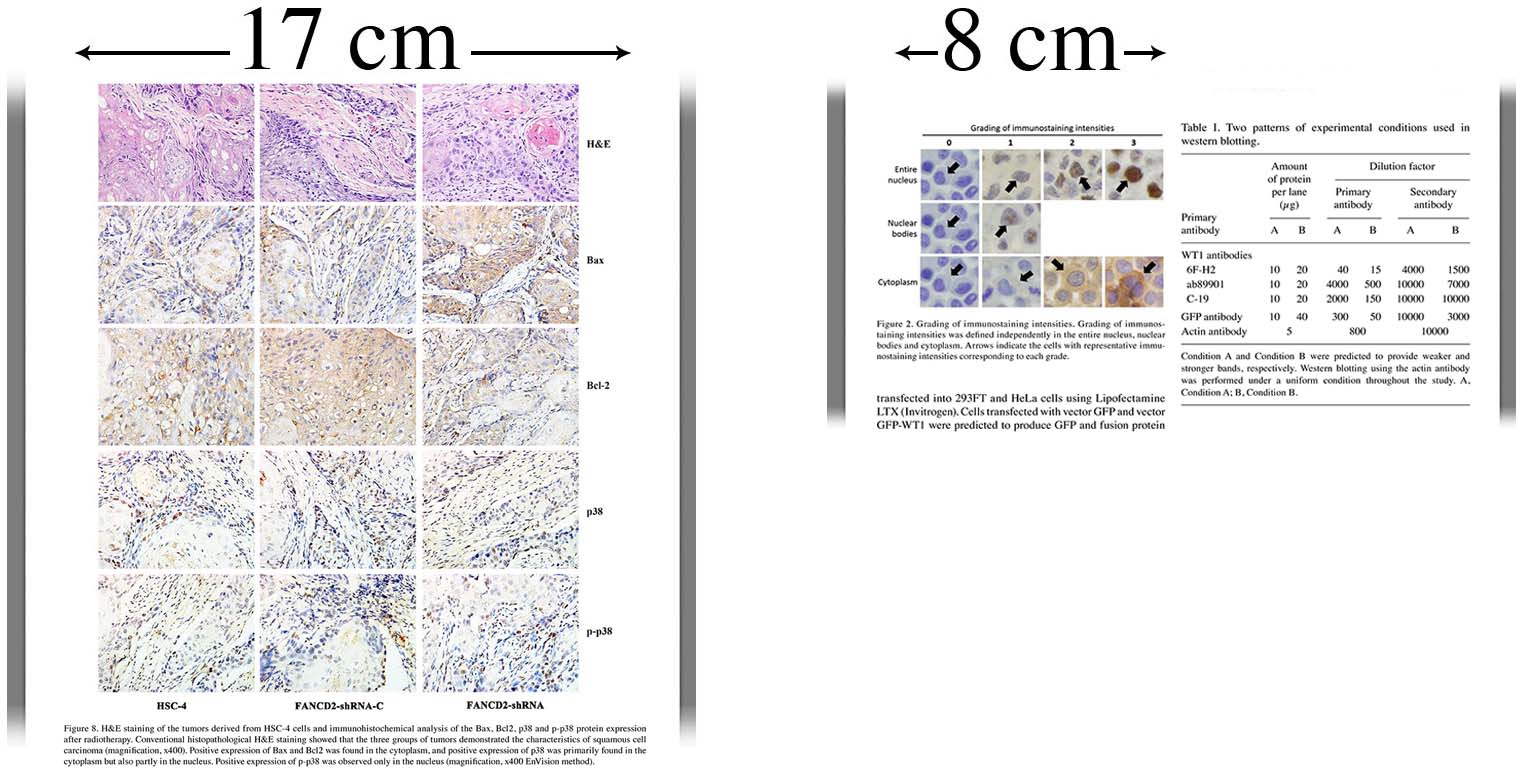
- Empty white space surrounding a figure should NOT be included when calculating image size. Images should therefore be cropped (cut) as close to the outside edges of the figure as possible.

- If a figure is too wide or contains too much information to be fit within 17 cm while keeping details clearly visible, figures must be divided into several clearly labeled separate parts.
3.3.4 Image resolution
- Image resolution in this context is simply a measure of the number of pixels per inch (also called dots per inch, dpi) defining the image and does not relate to the quality of an image in terms of focus, contrast and legibility.
- Images must be clear, of good contrast and legible at the size they are to appear in the journal.
- Images should be AT LEAST 300 dpi, at the size at which they will be printed (8 or 17 cm wide).
- Insufficient image size and/or resolution (dpi) will result in poor quality (blurred) printed figures if they are upscaled.
3.3.5 Exporting/capturing/saving figures
Figures may be produced by scanning, digital photography, or exporting from scientific software or a program such as PowerPoint.
- Scanning
- use a good quality scanner set to scan in RGB for color images or grey scale for line art or to scan gel images, at a resolution of at least 300 dpi and with the output file type set preferably to TIFF, or JPG highest quality (lowest compression).
- Digital photographs
- Set simple cameras to a ‘fine’ or ‘extra fine’ setting to help ensure that images have sufficient pixels.
- Exporting
- When exporting from scientific graphing software, choose settings to ensure the highest possible final size and resolution with lines of sufficient thickness to be seen at final printed size.
- When exporting from PowerPoint, DO NOT choose ‘Save as TIFF’ from the Save as dialogue box as this will NOT result in an image of sufficiently high resolution. Instead, save the individual slide image as a PDF (from the Print dialogue box), THEN open the PDF with image editing software, such as Photoshop or GIMP, and when prompted specify 300 dpi resolution. Finally, save the resulting image as a TIFF (with LZW compression).
- Note: figures initially scanned, photographed or exported at an insufficient size and resolution cannot be improved by upscaling, i.e., artificially increasing the resolution of a low-quality figure. Using image-editing software to keep the figure size the same while raising the dpi will NOT improve its quality.
3.3.6 File size
- If saved according to our guidelines, files will rarely exceed 10 MB.
- To reduce the file size of images:
- Ensure figures are the exact width and height they should be for publication (not smaller), make sure the figures are saved at no more than 300 dpi.
- Ensure that layers in the image have been flattened.
- Save black and white figures as grey scale.
- Ensure that TIFF are saved with LZW compression.
- Consider saving files as highest quality JPEGs. These may be smaller files than TIFF with LZW compression, but will lose some detail.
- Try using a compression or stuffing utility, such as WinZip or StuffIt.
3.3.7 Figure labels
- Font size
- Labels must be sized in proportion to the image, sharp and clearly legible.
- When figures are prepared at the correct size (8 or 17 cm at 300 dpi) the font size for labels should be 8‐10 points.
- If the figure is saved at a size larger than that needed for printing, the font size of labels must also be larger to maintain the correct proportions.
- If labels cannot fit on an 8‐cm‐wide page unless the font size is smaller than 8 points, the figure must be prepared as a double column figure (14‐17 cm wide). If labels cannot fit on the 17‐cm‐wide page unless the font size is smaller than 8 points, the figure must be split into several parts.
- Font style and appearance
- Labels must be saved using standard fonts (Times New Roman, Times, Arial, Helvetica or Symbol font).
- The labels should be of the same font and size in all figures. Also, the numbering should be of the same font and size in all figures.
- Labels should be evenly spaced and aligned, easy to see (including exponential numbers around figure axes), and NOT faded, broken, or distorted by JPG compression artifact. Do NOT use light grey color lines or labels.
- There must be strong contrast between labels and their background (e.g., labels placed over shaded bar graphs should be in a color that stands out against the shading, NOT blend in with it). Whenever possible, labels should be placed in black font on a white background. Consider using a black label with a white stroke applied to create contrast.
- Letters of labels must NOT be overlapping, condensed, expanded, have unnecessary gaps between them or be otherwise irregularly spaced, and must NOT be stretched (distorted) horizontally or vertically.
- Labels must NOT overlap or be concealed by other parts of the image, or be cropped (cut off) by the edge of the figure.
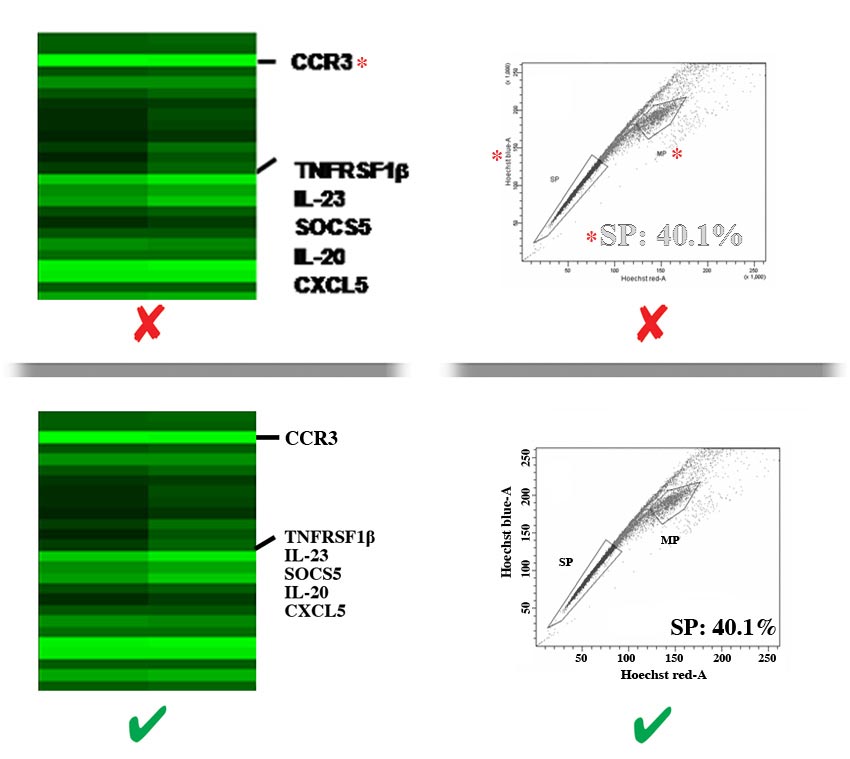
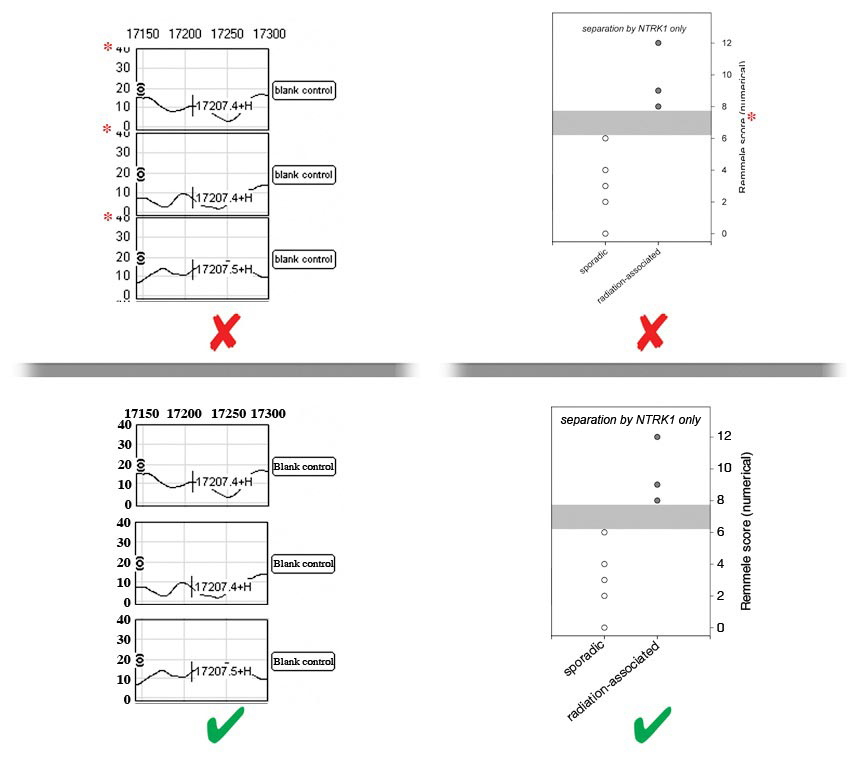
- Label styles and language
- Labels must be prepared according to our in‐house style, be phrased in accordance to the manuscript, and free of spelling and other language errors.
- The first letter of each phrase, NOT each word, must be capitalized [e.g., ‘Overall survival (months)’ not ‘Overall survival (months)’ and not ‘overall survival (months)]’.
- Always use a leading zero (0) before decimal points: 0.5 NOT .5.
- Decimal points must use a full stop/period (.) NOT a comma (,).
- A space must be inserted before measurement units: 132 bp NOT 132bp, 5 mm NOT 5mm, 1 h NOT 1h.
- Measurements must be written as:
- second(s): sec
- minute(s): min
- hour(s): h
- day(s): day(s)
- week(s): week(s)
- month(s): month(s)
- micro: μ, μ (available in Times and Helvetica) NOT u
- liter(s): l NOT L
- kilo Dalton: kDa NOT kD, Da, bp, kb
- 5 units BUT 5 U/ml
- Greek letters must be inserted using the correct Greek symbol (using Times, Helvetica or Symbol font), NOT written in full, i.e., alpha: α; beta: β, ß, (available in Times and Helvetica); and gamma: γ, etc.
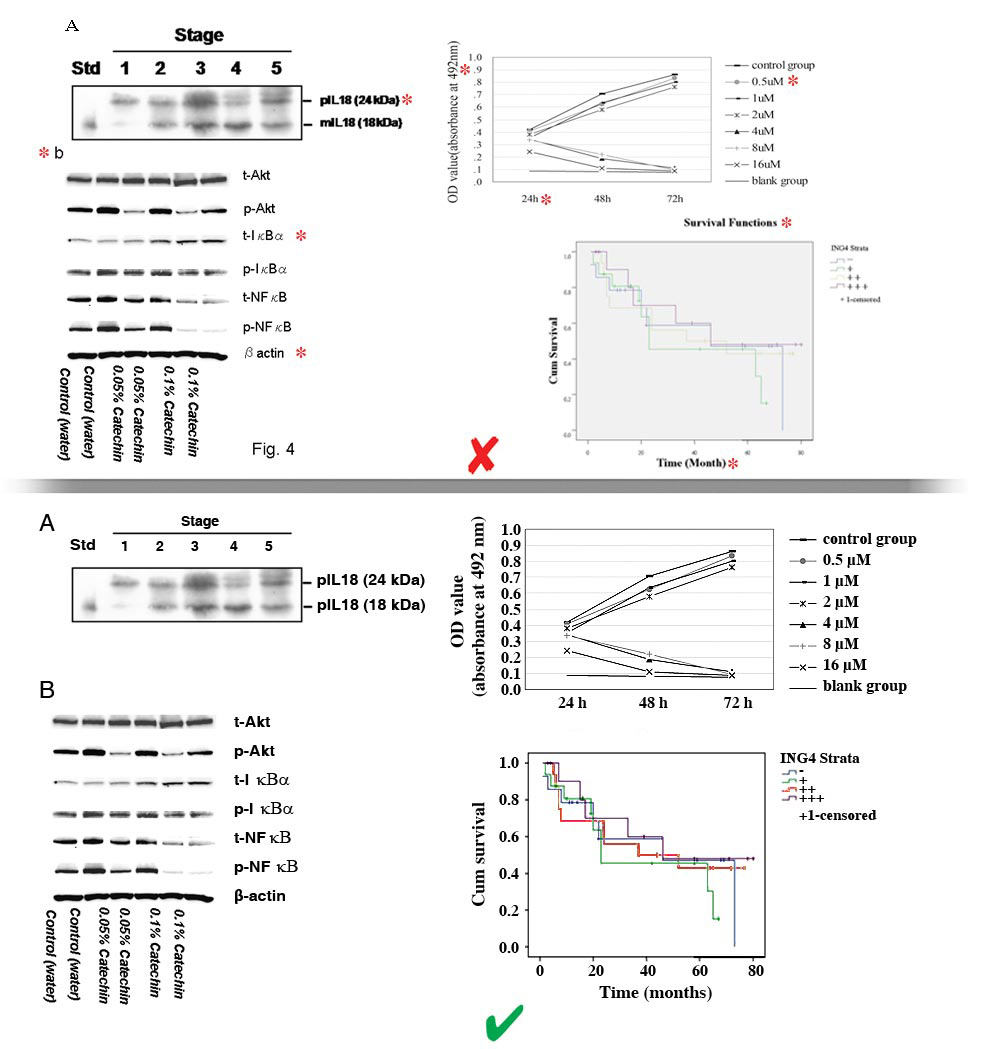
- Figures may be divided into separate sections. Each section may be saved as a separate file (clearly indicated in file name) or included together in one file (with parts clearly labeled).
- Separate parts of a figure should be labeled using just A, B, C, NOT 1A, 1B, 1C.
- Figure sections may be divided and subdivided as follows:
- A, B, C
- A a,b,c; B a,b,c; C a,b,c
- A a-1, a-2, b-1, b-2; B, a-1, a-2, b-1, b-2
- The number of the figure must NOT be included in the image, especially if placed on the overlapping part of the image. Instead, the file itself should be named using the figure number.
- A, B, Cs must be placed to the top left of each section of the figure, NOT overlapping the image.
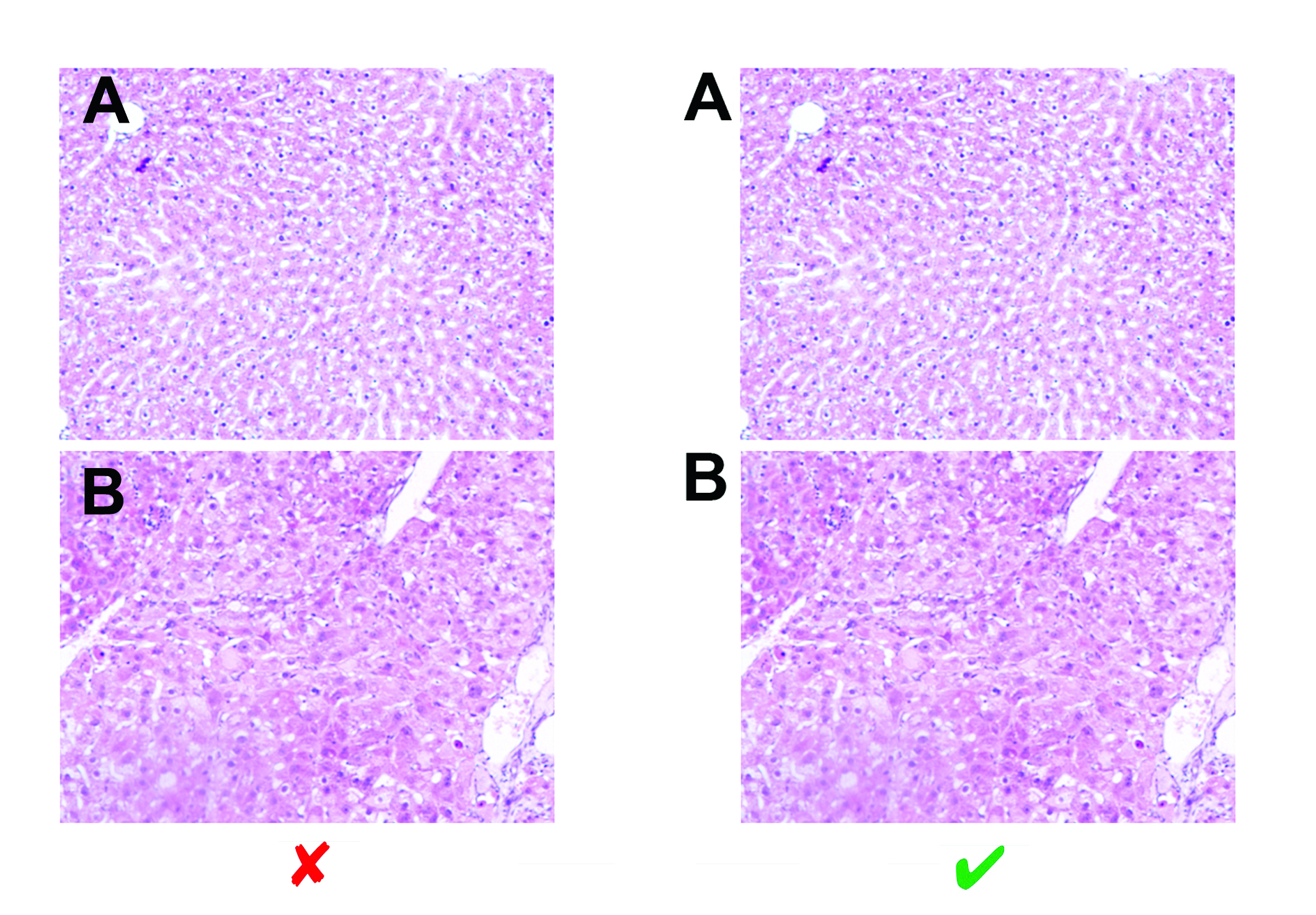
3.3.8 Figure appearance
- Figure backgrounds must be white. Grey backgrounds (or backgrounds of any other color) are NOT acceptable.
- White space surrounding figures should be cropped so that the image is as close to the edges of the page as possible.
- Figures and specific sections of figures should NOT be surrounded by borders (frames).
- Figures should NOT be stretched out of proportion (distorted) horizontally or vertically.
- Yellow must NOT be used for lines in diagrams. Any darker color may be used instead.
- Line art should be dark, and lines and labeling thick enough to be clearly visible, even at small sizes.
- Charts, graphs and diagrams should NOT use more than 5 shades of grey. Patterns are acceptable.
- In charts, graphs and diagrams, unnecessary colors should be avoided (e.g., color that does not impart any additional information and is used for slight emphasis only, or color that can be replaced by shades of grey, patterns or shapes).
3.3.9 Copyright
- If a figure or table has been published previously (even if you were the author of the manuscript), copyright permission for re-use of the figure or table will often be required.
- You must acknowledge the original source and submit written permission from the copyright holder to reproduce the material where necessary.
- As an author of your manuscript, you are responsible for obtaining permissions to use material owned by others.
3.4 Figure legends
- Figure legends should be listed one after the other, as part of the text document and separate from the figure files.
- Figure legends must begin with a brief title for the whole figure and continue with a short description of each panel or part.
- All symbols (eg. asterisks, hashtags) used to indicate significant differences in the figures must be defined accordingly in the figure legend.
- All error bars must be defined in the figure legend.
- Legends should not contain any details of the methods.
3.5 Tables
- Each table should be submitted on a separate Word file.
- Times New Roman. Font size 12. Spacing 1.5.
- Tables must be labelled using Roman numerals, (Table I, Table II, etc.)
- Include a short title immediately before the table.
- Any abbreviations or symbols should be defined immediately below the table.
3.6 Supplementary material
Supplementary data and other materials can now be submitted to all of our journals to support and enhance research manuscripts. The material should be directly relevant to your paper and can include information in the form of audio, video, tables and figures. Supplementary data should be submitted together with the original manuscript, as these data will undergo the peer review process as well.
Acceptable file types are: JPG, JPEG, EPS, TIFF, TIF, DOC, DOCX, ODT, ODF and for video/audio: MKV, MOV, AVI, MPG, MPEG, MP4.
Please note that supplementary materials should be referenced in the text as: 'Fig. S1', 'Table SI, Table SII e.t.c', 'Data S1' or 'Appendix S1'. Supplementary video/audio clips should be called “Supplementary_Data1.mp4"
3.7 Nomenclature and abbreviations
3.8 Chemical and biomolecular characterization
Manuscripts submitted to Spandidos Publications must have sufficient information to substantiate the identity and purity of novel compounds. This must be supported by a statement, which validates the origin, identity and purity of these compounds, irrespective of their supplier or synthetic methodology.
3.8.1. Identity of novel compounds
The identity of compounds containing carbon-hydrogen bonds with or without a metal should be validated using spectroscopy.1H-NMR and proton-decoupled 13C-NMR must be supplemented for novel compounds. Additional NMR information can be reported(31P-NMR, 19F-NMR) if necessary. Mass spectrometry results should be supplemented to confirm identification of novel compounds. Additional spectroscopic information (UV or IR) can be used to support the presence of specific functional groups. Melting-point data are required for crystalline compounds. The characterization of chiral compounds can be supported by rotational data. References can be provided, instead of comprehensive methods for known compounds unless the authors followed a modification of the published methods.
3.8.2. Combinatorial compound libraries
Authors studying the use of combinatorial libraries must provide standard characterization results that correspond to a wide range of library components.
3.8.3. Biomolecular identity
If direct structural analysis of novel biopolymeric compounds (e.g. oligosaccharides, peptides, nucleic acids) by NMR spectroscopy is not possible, the identification of these compounds should be performed by sequence and mass spec characterization.
3.8.4. Biological constructs
Sequencing or functional data should be provided to support the identification of biological constructs (e.g. plasmids or recombinant proteins) upon request.
3.8.5. Sample purity
Data supporting sample purity are required for novel compounds. For compounds containing carbon-hydrogen bonds with or without a metal atom, purity can be confirmed by1H-NMR or 13C-NMR, whereas elemental analysis is preferred for small-molecular weight molecules. Chromatography-based or electrophoretic methods can also be used to assess purity of polymers or small-molecular weight compounds.
3.8.6. Spectroscopic information
Comprehensive spectroscopic information for novel compounds should be supplemented in the Materials and methods section. Figures containing spectra must be made available to the Editor upon request. The authors should explain how specific, unambiguous NMR assignments were made in the Materials and methods section.
3.8.7. Crystallographic data for small molecules
Authors presenting novel structural data of small-molecular weight compounds from crystallography-based methods must be able to provide a standard crystallographic information file (.cif); structure factors for each structure; and a structural figure with probability ellipsoids upon request. The associated parameters and structural output should be checked using International Union of Crystallography checkCIF. The results of the crystallographic analysis should be deposited to a relevant public database and the deposition number must be referenced in the manuscript.
3.8.8. Macromolecular structural data
Manuscripts reporting new structures must include a summary in a table format of the statistical analysis of structural and refinement parameters, and the different programs used in the analysis should be mentioned and referenced. To assess the structural information, a stereo image of a portion of the electron density map (for crystallography papers), of the superimposed lowest energy structures (>10; for NMR papers), or of the entire structure (as a backbone trace) in case a new overall fold is presented, must be provided upon request. For cryo-EM structures, a characteristic micrograph indicating sole particles must be supplemented at submission. Protein structures should be deposited in the Protein Data Bank PDB and the deposition number must be referenced in the manuscript.

3.8.9. Chemical structures
Structures of compounds should be prepared using a drawing program, such as ChemDraw. Please ensure to use the following settings (ACS Style sheet in ChemDraw):
- Chain angle: 120º
- Space between bonds: 18% of width
- Fixed length: 14.4 pt (0.508 cm, 0.2 in.)
- Bold width: 2.0 pt (0.071 cm, 0.0278 in.)
- Line width: 0.6 pt (0.021 cm, 0.0084 in.)
- Margin width: 1.6 pt (0.056 cm, 0.0222 in.)
- Hash spacing: 2.5 pt (0.088 cm, 0.0347 in.)
- Font: Arial/Helvetica
- Size: 10 pt
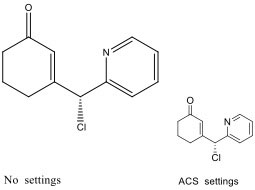
Figure 1: Representative molecule and lettering in ChemDraw default (left) and ACS (right) format.
3.9 References
- Spandidos Publications uses Edifix, to automatically link and correct bibliographic references.
- The first 9 authors are listed as they appear. When more than 10 authors are listed,Edifix will automatically include only the first 10 authors, followed by et al.
- Journal titles must be cited using the NLM Title Abbreviation (see U.S. National Library of Medicine Catalog online).
- In the reference list, references must be numbered consecutively in the order in which they appear in the main text.
- Do NOT use full stop after initials or abbreviations.
- In the text, references must be cited using numbers in parentheses e.g., (1-3) (1,2).
- Inclusive page numbers should be given.
- Personal communications should be avoided.
- Manuscripts in preparation or submitted (but not yet accepted) and abstracts, may be cited in the text, but should NOT be included in the list of references.
- Non-English references should not be included in the Reference list. The entire manuscript cited must be in English.
- Output Style files are available to download in Endnote format from Spandidos Publications or from Clarivate and also in CSL 1.0.1 format from here to work with software such as Mendeley, Papers and Zotero (www.zotero.org) .
- The following are examples of order and style, which should be strictly adhered to:
- Spandidos DA and Wilkie NM: Malignant transformation of early passage rodent cells by a single mutated human oncogene. Nature 310: 469-475, 1984.
- Spandidos A, Wang X, Wang H and Seed B: PrimerBank: A resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res 38: D792-D799, 2010.
- Barbu CG, Arsene AL, Florea S, Albu A, Sirbu A, Martin S, Nicolae AC, Burcea-Dragomiroiu GTA, Popa DE, Velescu BS, et al: Cardiovascular risk assessment in osteoporotic patients using osteoprotegerin as a reliable predictive biochemical marker. Mol Med Rep 16: 6059-6067, 2017.
- Hall A, Morris JDH, Price B, Lloyd A, Hancock JF, Gardener S, Houslay MD, Wakelam MJO and Marshall CJ: The function of the mammalian Ras proteins. In: Ras oncogenes. Spandidos DA (ed.) Plenum Publ. Corp., New York, pp99-104, 1989.
3.10 Declarations
The following sections must be added at the end of the manuscript, prior to the Reference list:
- Acknowledgements
- Funding
- Availability of data and materials
- Authors' contributions
- Ethics approval and consent to participate
- Patient consent for publication
- Competing interests
- Authors' information (optional)
- Use of artificial intelligence tools (to be included only when AI tools are used)
If any of the sections are not relevant to your manuscript, please include the heading and write 'Not applicable' for that section.
Acknowledgements
Any individuals who contributed to the study but do not meet the requirements to qualify for authorship may be listed in the ‘Acknowledgements’ section. For example, this may include a person who provided minimal technical assistance or writing advice, or the Chair/Head of department who provided general support. Where any other individual, such as a scientific or medical writer, assisted in preparing the contents of the manuscript, this should be explicitly acknowledged. Authors should obtain permission to acknowledge from all those mentioned in the Acknowledgements section. If there are no individuals to acknowledge, the authors should write "Not applicable" in this section. When the authors wish to thank a specific individual, their full name, title, affiliation and specific contribution must be stated. The acknowledgements section should not be used to thank companies (including language editing companies), patients, friends or family.
Funding
All sources of funding for the reported research should be declared in the ‘Funding’ section. If the funding body had any role in the design of the study or in the collection, analysis and interpretation of data, this should also be declared in this section. If there were no funding bodies supporting the study described in the manuscript, the ‘Funding’ section should state: “No funding was received”.
Availability of data and materials
For all manuscripts submitted to Spandidos Publications, the authors must make provisions for all materials described in the study, including all relevant raw data, to be freely available to any researchers who may wish to use them for non-commercial purposes, while preserving confidentiality and anonymity. If any conclusions made in the paper depend on a particular, dataset, then this dataset must be made available to the readers (unless it is already provided as part of the submitted article).
If public datasets are included in the study, the authors must include the following information in the ‘Availability of data and materials’ section of the article: the accession number of the dataset, the name of the repository in which it can be found, and a direct URL for the dataset. Authors who are unable to share their data must disclose this and provide a compelling explanation as to why the data are unavailable.
Availability of data and materials’ statements can take one of the following forms (or a combination of more than one if required for multiple datasets)::
- The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
- The datasets generated and/or analyzed during the current study are available in the [NAME] repository, [PERSISTENT WEB LINK TO DATASETS].
- All data generated or analyzed during this study are included in this published article.
- The datasets generated and/or analyzed during the current study are not publicly available due to [REASON WHY DATA ARE NOT PUBLIC] but are available from the corresponding author on reasonable request.
- Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
- The data that support the findings of this study are available from [THIRD PARTY NAME] but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of [THIRD PARTY NAME].
- Not applicable. If your manuscript does not contain any data, please state 'Not applicable' in this section.
For large sequencing or proteomic datasets
Authors are required to deposit their sequencing, X-ray crystallography and microarray datasets in public repositories, unless there is a compelling reason for them not to do so (such as protection of patient privacy, pending or approved patents, biosecurity reasons or any other legislation prohibiting public data sharing). Authors who are unable to share their data must disclose this at submission and provide an explanation in the manuscript as to why the data are unavailable. Publicly available datasets must be described in the ‘Availability of data and materials’ section of the manuscript, which must include the accession number and the name and URL of the repository in which the datasets are available. We leave the selection of the repository entirely at the authors' discretion, although the data must be freely available to readers. A list of recommended repositories is featured below; consulting the Registry of Research Data Repositories (http://www.re3data.org/) may also be useful in this regard.
Please note that a manuscript may be rejected if the Editor considers that the manuscript does not comply with our data sharing policies, and the authors have failed to provide a compelling reason to withhold their data and materials.
List of suggested repositories
Authors' contributions
The individual contributions of the authors of the manuscript should be specified in this section. The authors should be referred to using their initials, for example: "MA carried out the high-throughput sequencing experiments and performed the bioinformatics analysis. VA performed the histological examination of the kidney, and was a major contributor in writing the manuscript. MA and VA confirm the authenticity of all the raw data. All authors read and approved the final manuscript."
An 'author' is generally considered to be someone who has made substantive intellectual contributions to a study. According to the ICMJE guidelines, to qualify as an author, one should have i) substantially contributed to the conception and the design of the study, or in the acquisition, analysis and interpretation of the data; ii) been contributed to manuscript drafting or critical revisions on the intellectual content; iii) approved of the final manuscript version to be published; and iv) agreed to be accountable for all aspects of the work, so that any questions relating to research integrity or scientific accuracy in any part of the study are appropriately investigated and resolved. Each author listed in the manuscript should have participated sufficiently in the study to be publicly responsible for the content (or portions of the content). Authors do not normally qualify for authorship if their sole contribution is, for instance, the procurement of funding, acquisition of data, or general study supervision. Publication of a manuscript in Spandidos Publications journals requires at least two authors who can confirm the authenticity of all the raw data. These authors must be mentioned in the Authors’ contributions section of the declarations, using the following format: “MA and VA confirm the authenticity of all the raw data”.
Changes to authorship after manuscript acceptance are at the Editor’s discretion. If a manuscript requires a change in authorship, such as the addition or removal of an author, changes in the order of the authors, changes in affiliations or corresponding authors, the authors must must contact the Editor and provide reason to justify the change. In such cases, and if the changes are deemed appropriate, the Editor will request a letter of agreement, clearly stating the changes made, that has been signed by all authors (removed or added). Spandidos Publications will individually inform anyone who is added or removed from the author list.
Ethics approval and consent to participate
Studies involving humans
Ethics approval
Research performed on human subjects, materials or data must follow international and national regulations and be in agreement with the Declaration of Helsinki, or any other relevant set of ethical principles. Generally, relevant human material includes any material made inside the human body, including all cellular material (unless it has divided in culture), body fluids (such as blood, urine, feces, saliva, pus) and fetal tissue (including amniotic fluid, umbilical cord, placenta and membrane). Primary cells maintained in culture are also relevant until their first division.
All manuscripts reporting such research studies must include the name of the ethics committee that approved the work and the reference number where appropriate. If a study is exempt from requiring ethics approval, this should also be detailed in the ‘Ethics approval and consent to participate’ section of the manuscript (including the name of the ethics committee that can confirm the exemption and the reason for the exemption). Additional information and official documentation to support this may be requested by the Editor. Please note that manuscripts that do not adhere to our ethics policies may be rejected at any stage of the editorial process
Embryonic stem cell research
Embryonic stem cell research must be subject to review and monitoring by a specialized Embryonic Stem Cell Research Oversight Committee (ESCRO). This includes all scientific research on human development at any pre-implantation stage, human embryos or embryo-derived cells or involving the production of human gametes in vitro (tested/used by fertilization or for the creation of embryos). The overseeing committee must be duly qualified and able to evaluate such studies for their adherence to the guidelines of the International Society for Stem Cell Research (ISSCR). In particular, studies in Category 3 of these guidelines (heritable genome editing, transferring mtDNA-modified- not including MRT - embryos into a uterus, using gametes differentiated from human stem cells for reproduction, gestating human stem cell-based embryo models, human reproductive cloning, breeding human-animal chimeras where there may be human germ cells, transferring human-animal chimeric embryo(s) to a human or ape uterus, transferring human embryo(s), irrespective of origins, to an animal uterus) will not be considered for publication, as they are currently prohibited by the ISSCR due to their lack of safety and other scientific and ethical concerns (see link).
Retrospective ethics approval
For prospective studies, ethics approval must have been obtained prior to start of the study and cannot usually be sought retrospectively. In such cases, the manuscript may not be considered for peer review, and the decision on whether to proceed with the review will remain at the Editor's discretion.
If the study describes a new medical procedure or tool in patients, authors are required to justify their use, including a reason why the new procedure/tool was considered more appropriate than usual clinical practice for the purpose of patient treatment. Both ethics approval from a competent ethics committee and consent to participate in the study are required in such cases.
Clinical trials
In case of clinical trials, which are defined as "any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes", the trial registration number (TRN) and the date of the registration must be stated in the last line of the Abstract of the manuscript. Appropriate public registries are listed in theICMJE website and the WHO International Clinical Trials Registry Platform. If a clinical trial has not been registered prospectively, Spandidos Publications encourages retrospective registration to ensure that all results are fully available. In such cases, the last line of the Abstract of the manuscript should mention ‘retrospectively registered’, in addition to the TRN and date of registration.
Consent to participate
For all research involving human subjects or tissue, it must be stated that informed consent was obtained from all participants, for participation in the study or use of their tissue (or a parent/legal guardian in the case of children under 18 and patients otherwise considered minors under local legislation). Consent is also required for the procurement of biomaterials for stem cell research and translation, including gamete donors in IVF studies.
Studies involving vulnerable groups (i.e. individuals who are at higher risk of mistreatment or harm), with potential for coercion or exploitation (for example, prisoners or unconscious patients) or where consent may not have been fully informed (for example, due to a language barrier), will be considered at the Editor’s discretion under exceptional circumstances. Scientific research involving vulnerable groups can only be carried out if its aims and scope benefit those groups and meet their specific needs, and authors must be able to demonstrate this for their manuscript to considered for publication. For articles involving the use of human transplantation, authors must declare in the manuscript that no organs/tissues were obtained from prisoners. The institution(s) through which organs/tissues were obtained must be named.
Patient consent for publication
Patients have a right to anonymity and privacy, and authors have a legal and ethical responsibility to respect this right. Identifying information, including names, initials, date of birth or hospital numbers, images or statements should not be included in the manuscript unless the information is essential for scientific purposes and the patient (or parent or guardian) has provided written informed consent for the publication of this identifying information. It must be stated in the manuscript that the patient, or parent/guardian/next of kin (in case of minors or deceased patients) provided written informed consent for the publication of any data and/or accompanying images. The consent form may be requested by the Editor, in which case it will be treated confidentially by the editorial office. This applies to case reports or other studies in which case details, personal information or images are included and could allow an individual to be identified, either directly or indirectly. Authors should disclose to patients that personally identifiable material would be available via the Internet as well as in print after publication.
Under exceptional circumstances, the Editor will consider publication of clinical data without written consent if all identifying information is removed, if the risks and potential harm posed to the patient are far outweighed by the benefits of the study, if it is impossible to obtain permission or if it is considered that a reasonable individual would be unlikely to object to publication.
During clinical trials, informed consent for publication of clinical data should be sought from the participants at the point of recruitment to the trial. In cases where this is not possible, authors must demonstrate that the publication of the clinical data would not compromise anonymity and confidentiality, nor be in breach of any local data protection laws. The authors must determine whether the dataset might contain any patient identifiers, both direct and indirect. Before submission of the manuscript, the authors should also consult their local ethics committee or other appropriate regulatory body in order to confirm full patient anonymity. The manuscript must state whether informed consent was obtained for the publication of clinical data. If informed consent for publication was not obtained, authors must provide a compelling reason. The Editor may request a document from the relevant ethics committee, outlining the reason why the requirement for consent to publish was waived.
Please note that consent to participate and consent for publication are considered distinct at Spandidos Publications journals. Where applicable, the Editor may request evidence that both have been obtained. For example, in a study involving blood collection from a patient cohort, ‘consent to participate’ would be required, and the authors must be able to demonstrate that the participants consented to the use of their blood samples for the purpose of scientific research. By contrast, for a case report involving the publication of images, the authors must be able to demonstrate that both consent to participate and consent to publish was obtained, i.e. that the patient consented to the images being taken for the purpose of research and also consented to their publication.
The use of protected animals for the purpose of scientific research must follow internationally recognized guidelines on animal welfare, as well as local and national regulations, and be performed in accordance with the UK’s Animals (Scientific Procedures) Act 1986, and associated guidelines, the EU Directive 2010/63/EU for animal experiments, or the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. The name of the institutional ethics committee that approved the experiments undertaken, including the reference number where appropriate, should be stated in the manuscript. The ethics committee from which approval was obtained must be transparent in its functioning, independent of the researcher, the sponsor and any other unwarranted influence, and duly qualified.
All animal studies should also comply with the ARRIVE guidelines and the AVMA euthanasia guidelines 2020.
If a study is exempt from requiring ethics approval, this should also be stated in the ‘Ethics approval and consent to participate’ section of the manuscript (including the name of the ethics committee that can confirm the exemption and the reason for the exemption).
Animal welfare issues will be taken into account during peer review, and the Editor may reject a manuscript at any point during the editorial process if the research involves protocols that are inconsistent with commonly accepted norms of animal research. The Editor may request additional documentation, including approval forms and/or relevant citations from the literature if any of the experimental details described in the study are considered a deviation from common practice in animal research.
When rodents are used as in vivo cancer models, the tumor burden should not exceed the recommendations of the University of Pennsylvania Institutional Animal Care and Use Committee guidelines.
This animal welfare policy also applies to field studies, as well as other non-experimental research conducted on animals. If a study involves client-owned animals, the authors are also required to provide an informed consent from the client/owner of the animal. The research will also be expected to demonstrate adherence to the highest standards of veterinary care.
Competing interests
Authors, reviewers and editors must disclose any competing interests that may exist with respect to the publication of a study. Competing interests are at play if the interpretation and presentation of the data, the conclusions or any other information provided by the authors can be influenced (or may be perceived as such) by their personal or financial relationship with other individuals or organizations, such as reimbursement for salaries, equipment or supplies, or a personal belief that may influence their objectivity and affect their interpretation of the data and associated conclusions as a result. Examples include, but are not limited to financial relationships, such as competing patents, grants, funding, bursaries and employment history, as well as personal relationships and strong ethical or personal beliefs. Competing interest statements for public funding sources, including government agencies, charitable or academic institutions, need not be included, as these should be described in the ‘Funding’ section instead. For example, if a charitable foundation sponsored the study and a pharmaceutical company provided the drugs, the former would be mentioned in the ‘Funding’ section, whereas the latter would be acknowledged in the ‘Competing interests’ section.
All competing interests must be disclosed, as they may affect (or be perceived to affect) the integrity of the research study and the reliability of its scientific contents. Authors are expected to disclose any competing interests at the time of submission in their cover letter and in the manuscript at the time of submission, even if the authors consider that these have not influenced their work. In addition, competing interests (or lack thereof) should also be mentioned in the manuscript. If no conflict exists, this must also be stated clearly in the 'Competing interests’ section, using the following format: “The authors declare that they have no competing interests”, and all authors should confirm its accuracy. If there is a conflict, please state so in the 'Competing interests' section. Examples of conflict of interest statements include ‘Dr Jones is a part-time employee at the ABC company’, ‘Recombinant protein Z was kindly provided by The ABC Company, London, United Kingdom’. Authors may be asked to confirm, update or provide further details regarding such disclosure statements following acceptance of the manuscript. For further information, please refer to the following link:www.icmje.org/conflicts-of-interest.
Prior to peer review, the external reviewers are also required to disclose any competing interests. If any competing interest is identified regarding a particular study, the reviewers are asked to notify Spandidos Publications and should decline to review the article if appropriate. If any competing interest are disclosed and the reviewers proceed with their evaluation, the Editor will make the final decision regarding whether or not the comments made by the reviewers should be recognized or reinterpreted
Authors' information (optional)
This section is optional. The authors may wish to use this section to include additional information about any or all of the authors deemed relevant. Examples include details of the the authors' qualifications, roles they hold at external institutions or societies, or any other relevant background information. This section should not contain correspondence information, e-mail addresses, acknowledgements or competing interests. The authors should be referred to by their initials. For example: “MA is a member of the British Society for Immunology”.
Use of artificial intelligence tools
Authors should disclose the use of artificial intelligence (AI)-assisted tools such as chatGPT in the Declarations section (e.g. ‘Use of artificial intelligence tools - During the preparation of this work, AI tools were used to improve the readability and language of the manuscript or to generate images, and subsequently, the authors revised and edited the content produced by the AI tools as necessary, taking full responsibility for the ultimate content of the present manuscript’).
Please note that AI tools should only be employed for improving the language of the article, and NOT for generating scientific content or drawing scientific conclusions, neither for analysing or interpreting scientific data. Furthermore, authors are responsible for reviewing and editing the text/images generated by AI tools to ensure their accuracy and correctness. In addition, AI tools should not be listed as authors, since they are not capable of taking any responsibility or accountability for the content of the manuscript intended to be published in terms of its integrity, accuracy or originality.
3.11 Standards of reporting
Incomplete reporting of experimental details, study rationale, methodological design and research outcomes can greatly compromise the validity, accuracy and reproducibility of scientific results. Thus, it is important for authors to ensure that their manuscript adheres to certain standards of reporting. Spandidos Publications encourage authors to refer to the standards set out by the EQUATOR Network. A list of standard reporting checklists used in biomedical/biological research can also be found at FAIRsharing.org.
Commonly used checklists are described in the Standards of reporting section of our website .
Reporting sex and gender information
Sex and gender are important factors that may influence the outcome and interpretation of scientific research. Whereas sex commonly means ‘biological sex’ (i.e. male or female), gender refers to ‘the socially constructed roles, expectations, relationships, behaviours, relative power, and other traits that societies ascribe to women, men and people of diverse gender identities’ (1).
Authors are encouraged to follow the Sex and Gender Equity in Research (SAGER) guidelines (2). As with other types of study designs, both positive and negative results should be described for all studies reporting sex and/or gender-based analyses. This applies even in cases where the sex and/or gender-based differences were not anticipated at the time the study was first conceived. Specifically:
- If sex-related differences may be expected (or were found) in the results, the study should be designed and carried out in a way that to addresses them.
- If gender-related differences may be expected (or were found) in the results, the study should be designed and carried out in a way that to addresses them.
- The Materials and methods section should clearly explain how the study design addresses sex and/or gender-based differences.
If a study included only one sex and/or gender, the manuscript should clearly emphasize this (for example, in the manuscript title and in the Abstract). The authors may also wish to address or comment on the lack of sex- and/or gender-based analysis in the Discussion.
In addition, the terms ‘sex’ (biological) and ‘gender’ (social or cultural) should not be used interchangeably and/or incorrectly.
Day, S., Mason, R., Lagosky, S., & Rochon, P. A. (2016). Integrating and evaluating sex and gender in health research. Health Res Policy Syst, 14(1), 75.
Heidari, S., Babor, T.F., De Castro, P. et al. Sex and Gender Equity in Research: rationale for the SAGER guidelines and recommended use. Res Integr Peer Rev 1, 2 (2016).
4. Proofs
- Authors must check their manuscript carefully before submission, after responding to peer-review, and when answering queries raised at the proof stage. Any errors will be faithfully transferred into the final PDF and print versions.
5. Reprints
- A reprint order form will accompany notification of acceptance of a manuscript and should be completed and returned immediately. For further information, please email contact@spandidos-publications.com.
6. Article charges
World Academy of Sciences Journal, the International Journal of Functional Nutrition, the International Journal of Epigenetics and Medicine International are free to publish.
7. Open access
- All articles in the International Journal of Functional Nutrition are published online as "Open access" articles and can be freely accessed at the journal's website by all users. The articles are made immediately available as Open access.
- Articles are licensed under the Creative Commons Attribution 4.0 International License.
- Spandidos Publications’ publishing policies enable authors to comply fully with the public access requirements of the major worldwide funding agencies (visit here for more information). Authors must take the necessary actions to obtain this compliance, including self-archiving, utilization of Spandidos Publications manuscript deposition service and selection of Open access publication under the correct license.
- Spandidos Publications makes articles freely available 12 months after the publication.
- Authors wishing to purchase immediate Open access for International Journal of Oncology, Oncology Reports, International Journal of Molecular Medicine, Molecular Medicine Reports, Oncology Letters, Experimental and Therapeutic Medicine, Molecular and Clinical Oncology and Biomedical Reports must select option 2.
- Spandidos Publications will refund an article processing charge (APC) if an error on our part has resulted in a failure to publish an article under the Open access terms selected by the authors. This may include a failure to make an article openly available on the journal platform, or publication of an article under a different Creative Commons licence from that selected by the authors. If you become aware of an error in the Open access status or licensing of your article, please contact the appropriate journal immediately. Note that APCs will not be refunded in instances where articles are retracted or removed as a consequence of author error or misconduct. Neither we will be liable if delays in the processing of the article result from changes made to the authorship on the article, or in cases where delays result from editorial decisions.
8. Compliance with funding agencies
All manuscripts that are agency-funded (e.g. NIH, HHMI, Cancer Research UK, Wellcome Trust, the Chinese Academy of Sciences (CAS), the National Natural Science Foundation of China (NSFC), etc.) and paid for under the Open access οption will be deposited automatically by the publisher and become publicly available in PubMed Central and Europe PMC. Alternatively, agency-funded articles without Open access purchase will be deposited and made publicly available in PubMed Central and Europe PMC 6-12 months following publication, depending on the public access policy of the agency.
9. Funding body policies and agreements
- Spandidos Publications has obtained agreements and constructed policies to enable authors to comply with manuscript archiving requirements of the following funding bodies specified in conditions of researcher grant awards.
- Arthritis Research Campaign (UK)
- Austrian Science Fund (FWF)
- Biotechnology and Biological Sciences Research Council (BBSRC)
- British Heart Foundation (UK)
- Cancer Research (UK)
- Chief Scientist Office (UK)
- Chinese Academy of Sciences (CAS)
- Department of Health (UK)
- Dunhill Medical Trust
- Economic and Social Research Council (ESRC)
- Howard Hughes Medical Institute (US)
- Medical Research Council (UK)
- National Institutes of Health (US)
- National Natural Science Foundation of China (NSFC)
- Research Councils (UK)
- Telethon (Italy)
- Wellcome Trust (UK)
- This enables authors to follow their funding body's policies on archiving without breaching their publishing agreements with Spandidos Publications.
10. Author self-archiving
Authors are encouraged to submit the final publisher’s version PDF (Version of Record) to their institution’s repository immediately following publication, as well as to their funding body’s archive. A link to the published version on the Spandidos Publications website must be included with full citation details and acknowledgement of the journal as the original source. In addition, authors are allowed to archive their articles pre-print (i.e., pre-refereeing). Pre-print can be on author's personal website or pre-print server.
11. English language editing, manuscript formatting and figure preparation
If English is not your first language, you may decide to have your manuscript proofread or edited by an English speaker, prior to submission. A colleague or a professional service may do this. Clear and concise language enables editors and reviewers to concentrate on the scientific content of your manuscript. This facilitates the peer review process and ensures that your article will not be rejected based on English language alone. Spandidos Publications offers a number of Manuscript Services including an English Language Editing service, through which the use of English in your manuscript may be checked and refined. The editors of this service will focus on the spelling, grammar, clarity and style of your manuscript.
For more information on this and other services, please access: www.spandidos-publications.com/languageediting.
Please be aware that an author's use of this or any other service in no way guarantees that his or her submission will be accepted by a Spandidos journal or any other. Any arrangement an author enters into will be exclusively between the author and their chosen company. Using an editing service is neither a requirement for nor a guarantee of acceptance for publication. For further details, please refer to our disclaimer on our affiliated website.
1. Submission method
Manuscripts may only be submitted through our online submission system, which is accessible via our
website. Authors must create a user account, log in and follow the on-screen directions.
2. Cover letter
All submissions should be accompanied by a cover letter that briefly summarizes the important points of the submitted work, including a brief description of the study. In the cover letter, authors should also confirm that the submitted manuscript reports the results of an original study presenting novel work, that it has not been previously submitted to or accepted by any other journal, that is has been approved by all author and that ethics approval and written informed consent have been obtained, where applicable. Any competing interests must also be disclosed to the Editor in the cover letter.
3.8 Chemical and biomolecular characterization
Manuscripts submitted to Spandidos Publications must have sufficient information to substantiate the identity and purity of novel compounds. This must be supported by a statement, which validates the origin, identity and purity of these compounds, irrespective of their supplier or synthetic methodology.
3.8.1. Identity of novel compounds
The identity of compounds containing carbon-hydrogen bonds with or without a metal should be validated using spectroscopy.1H-NMR and proton-decoupled 13C-NMR must be supplemented for novel compounds. Additional NMR information can be reported(31P-NMR, 19F-NMR) if necessary. Mass spectrometry results should be supplemented to confirm identification of novel compounds. Additional spectroscopic information (UV or IR) can be used to support the presence of specific functional groups. Melting-point data are required for crystalline compounds. The characterization of chiral compounds can be supported by rotational data. References can be provided, instead of comprehensive methods for known compounds unless the authors followed a modification of the published methods.
3.8.2. Combinatorial compound libraries
Authors studying the use of combinatorial libraries must provide standard characterization results that correspond to a wide range of library components.
3.8.3. Biomolecular identity
If direct structural analysis of novel biopolymeric compounds (e.g. oligosaccharides, peptides, nucleic acids) by NMR spectroscopy is not possible, the identification of these compounds should be performed by sequence and mass spec characterization.
3.8.4. Biological constructs
Sequencing or functional data should be provided to support the identification of biological constructs (e.g. plasmids or recombinant proteins) upon request.
3.8.5. Sample purity
Data supporting sample purity are required for novel compounds. For compounds containing carbon-hydrogen bonds with or without a metal atom, purity can be confirmed by1H-NMR or 13C-NMR, whereas elemental analysis is preferred for small-molecular weight molecules. Chromatography-based or electrophoretic methods can also be used to assess purity of polymers or small-molecular weight compounds.
3.8.6. Spectroscopic information
Comprehensive spectroscopic information for novel compounds should be supplemented in the Materials and methods section. Figures containing spectra must be made available to the Editor upon request. The authors should explain how specific, unambiguous NMR assignments were made in the Materials and methods section.
3.8.7. Crystallographic data for small molecules
Authors presenting novel structural data of small-molecular weight compounds from crystallography-based methods must be able to provide a standard crystallographic information file (.cif); structure factors for each structure; and a structural figure with probability ellipsoids upon request. The associated parameters and structural output should be checked using International Union of Crystallography checkCIF. The results of the crystallographic analysis should be deposited to a relevant public database and the deposition number must be referenced in the manuscript.
3.8.8. Macromolecular structural data
Manuscripts reporting new structures must include a summary in a table format of the statistical analysis of structural and refinement parameters, and the different programs used in the analysis should be mentioned and referenced. To assess the structural information, a stereo image of a portion of the electron density map (for crystallography papers), of the superimposed lowest energy structures (>10; for NMR papers), or of the entire structure (as a backbone trace) in case a new overall fold is presented, must be provided upon request. For cryo-EM structures, a characteristic micrograph indicating sole particles must be supplemented at submission. Protein structures should be deposited in the Protein Data Bank PDB and the deposition number must be referenced in the manuscript.

3.8.9. Chemical structures
Structures of compounds should be prepared using a drawing program, such as ChemDraw. Please ensure to use the following settings (ACS Style sheet in ChemDraw):
- Chain angle: 120º
- Space between bonds: 18% of width
- Fixed length: 14.4 pt (0.508 cm, 0.2 in.)
- Bold width: 2.0 pt (0.071 cm, 0.0278 in.)
- Line width: 0.6 pt (0.021 cm, 0.0084 in.)
- Margin width: 1.6 pt (0.056 cm, 0.0222 in.)
- Hash spacing: 2.5 pt (0.088 cm, 0.0347 in.)
- Font: Arial/Helvetica
- Size: 10 pt

Figure 1: Representative molecule and lettering in ChemDraw default (left) and ACS (right) format.
3.10 Declarations
The following sections must be added at the end of the manuscript, prior to the Reference list:
- Acknowledgements
- Funding
- Availability of data and materials
- Authors' contributions
- Ethics approval and consent to participate
- Patient consent for publication
- Competing interests
- Authors' information (optional)
- Use of artificial intelligence tools (to be included only when AI tools are used)
If any of the sections are not relevant to your manuscript, please include the heading and write 'Not applicable' for that section.
Acknowledgements
Any individuals who contributed to the study but do not meet the requirements to qualify for authorship may be listed in the ‘Acknowledgements’ section. For example, this may include a person who provided minimal technical assistance or writing advice, or the Chair/Head of department who provided general support. Where any other individual, such as a scientific or medical writer, assisted in preparing the contents of the manuscript, this should be explicitly acknowledged. Authors should obtain permission to acknowledge from all those mentioned in the Acknowledgements section. If there are no individuals to acknowledge, the authors should write "Not applicable" in this section. When the authors wish to thank a specific individual, their full name, title, affiliation and specific contribution must be stated. The acknowledgements section should not be used to thank companies (including language editing companies), patients, friends or family.
Funding
All sources of funding for the reported research should be declared in the ‘Funding’ section. If the funding body had any role in the design of the study or in the collection, analysis and interpretation of data, this should also be declared in this section. If there were no funding bodies supporting the study described in the manuscript, the ‘Funding’ section should state: “No funding was received”.
Availability of data and materials
For all manuscripts submitted to Spandidos Publications, the authors must make provisions for all materials described in the study, including all relevant raw data, to be freely available to any researchers who may wish to use them for non-commercial purposes, while preserving confidentiality and anonymity. If any conclusions made in the paper depend on a particular, dataset, then this dataset must be made available to the readers (unless it is already provided as part of the submitted article).
If public datasets are included in the study, the authors must include the following information in the ‘Availability of data and materials’ section of the article: the accession number of the dataset, the name of the repository in which it can be found, and a direct URL for the dataset. Authors who are unable to share their data must disclose this and provide a compelling explanation as to why the data are unavailable.
Availability of data and materials’ statements can take one of the following forms (or a combination of more than one if required for multiple datasets)::
- The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
- The datasets generated and/or analyzed during the current study are available in the [NAME] repository, [PERSISTENT WEB LINK TO DATASETS].
- All data generated or analyzed during this study are included in this published article.
- The datasets generated and/or analyzed during the current study are not publicly available due to [REASON WHY DATA ARE NOT PUBLIC] but are available from the corresponding author on reasonable request.
- Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
- The data that support the findings of this study are available from [THIRD PARTY NAME] but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of [THIRD PARTY NAME].
- Not applicable. If your manuscript does not contain any data, please state 'Not applicable' in this section.
For large sequencing or proteomic datasets
Authors are required to deposit their sequencing, X-ray crystallography and microarray datasets in public repositories, unless there is a compelling reason for them not to do so (such as protection of patient privacy, pending or approved patents, biosecurity reasons or any other legislation prohibiting public data sharing). Authors who are unable to share their data must disclose this at submission and provide an explanation in the manuscript as to why the data are unavailable. Publicly available datasets must be described in the ‘Availability of data and materials’ section of the manuscript, which must include the accession number and the name and URL of the repository in which the datasets are available. We leave the selection of the repository entirely at the authors' discretion, although the data must be freely available to readers. A list of recommended repositories is featured below; consulting the Registry of Research Data Repositories (http://www.re3data.org/) may also be useful in this regard.
Please note that a manuscript may be rejected if the Editor considers that the manuscript does not comply with our data sharing policies, and the authors have failed to provide a compelling reason to withhold their data and materials.
List of suggested repositories
Authors' contributions
The individual contributions of the authors of the manuscript should be specified in this section. The authors should be referred to using their initials, for example: "MA carried out the high-throughput sequencing experiments and performed the bioinformatics analysis. VA performed the histological examination of the kidney, and was a major contributor in writing the manuscript. MA and VA confirm the authenticity of all the raw data. All authors read and approved the final manuscript."
An 'author' is generally considered to be someone who has made substantive intellectual contributions to a study. According to the ICMJE guidelines, to qualify as an author, one should have i) substantially contributed to the conception and the design of the study, or in the acquisition, analysis and interpretation of the data; ii) been contributed to manuscript drafting or critical revisions on the intellectual content; iii) approved of the final manuscript version to be published; and iv) agreed to be accountable for all aspects of the work, so that any questions relating to research integrity or scientific accuracy in any part of the study are appropriately investigated and resolved. Each author listed in the manuscript should have participated sufficiently in the study to be publicly responsible for the content (or portions of the content). Authors do not normally qualify for authorship if their sole contribution is, for instance, the procurement of funding, acquisition of data, or general study supervision. Publication of a manuscript in Spandidos Publications journals requires at least two authors who can confirm the authenticity of all the raw data. These authors must be mentioned in the Authors’ contributions section of the declarations, using the following format: “MA and VA confirm the authenticity of all the raw data”.
Changes to authorship after manuscript acceptance are at the Editor’s discretion. If a manuscript requires a change in authorship, such as the addition or removal of an author, changes in the order of the authors, changes in affiliations or corresponding authors, the authors must must contact the Editor and provide reason to justify the change. In such cases, and if the changes are deemed appropriate, the Editor will request a letter of agreement, clearly stating the changes made, that has been signed by all authors (removed or added). Spandidos Publications will individually inform anyone who is added or removed from the author list.
Ethics approval and consent to participate
Studies involving humans
Ethics approval
Research performed on human subjects, materials or data must follow international and national regulations and be in agreement with the Declaration of Helsinki, or any other relevant set of ethical principles. Generally, relevant human material includes any material made inside the human body, including all cellular material (unless it has divided in culture), body fluids (such as blood, urine, feces, saliva, pus) and fetal tissue (including amniotic fluid, umbilical cord, placenta and membrane). Primary cells maintained in culture are also relevant until their first division.
All manuscripts reporting such research studies must include the name of the ethics committee that approved the work and the reference number where appropriate. If a study is exempt from requiring ethics approval, this should also be detailed in the ‘Ethics approval and consent to participate’ section of the manuscript (including the name of the ethics committee that can confirm the exemption and the reason for the exemption). Additional information and official documentation to support this may be requested by the Editor. Please note that manuscripts that do not adhere to our ethics policies may be rejected at any stage of the editorial process
Embryonic stem cell research
Embryonic stem cell research must be subject to review and monitoring by a specialized Embryonic Stem Cell Research Oversight Committee (ESCRO). This includes all scientific research on human development at any pre-implantation stage, human embryos or embryo-derived cells or involving the production of human gametes in vitro (tested/used by fertilization or for the creation of embryos). The overseeing committee must be duly qualified and able to evaluate such studies for their adherence to the guidelines of the International Society for Stem Cell Research (ISSCR). In particular, studies in Category 3 of these guidelines (heritable genome editing, transferring mtDNA-modified- not including MRT - embryos into a uterus, using gametes differentiated from human stem cells for reproduction, gestating human stem cell-based embryo models, human reproductive cloning, breeding human-animal chimeras where there may be human germ cells, transferring human-animal chimeric embryo(s) to a human or ape uterus, transferring human embryo(s), irrespective of origins, to an animal uterus) will not be considered for publication, as they are currently prohibited by the ISSCR due to their lack of safety and other scientific and ethical concerns (see link).
Retrospective ethics approval
For prospective studies, ethics approval must have been obtained prior to start of the study and cannot usually be sought retrospectively. In such cases, the manuscript may not be considered for peer review, and the decision on whether to proceed with the review will remain at the Editor's discretion.
If the study describes a new medical procedure or tool in patients, authors are required to justify their use, including a reason why the new procedure/tool was considered more appropriate than usual clinical practice for the purpose of patient treatment. Both ethics approval from a competent ethics committee and consent to participate in the study are required in such cases.
Clinical trials
In case of clinical trials, which are defined as "any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes", the trial registration number (TRN) and the date of the registration must be stated in the last line of the Abstract of the manuscript. Appropriate public registries are listed in theICMJE website and the WHO International Clinical Trials Registry Platform. If a clinical trial has not been registered prospectively, Spandidos Publications encourages retrospective registration to ensure that all results are fully available. In such cases, the last line of the Abstract of the manuscript should mention ‘retrospectively registered’, in addition to the TRN and date of registration.
Consent to participate
For all research involving human subjects or tissue, it must be stated that informed consent was obtained from all participants, for participation in the study or use of their tissue (or a parent/legal guardian in the case of children under 18 and patients otherwise considered minors under local legislation). Consent is also required for the procurement of biomaterials for stem cell research and translation, including gamete donors in IVF studies.
Studies involving vulnerable groups (i.e. individuals who are at higher risk of mistreatment or harm), with potential for coercion or exploitation (for example, prisoners or unconscious patients) or where consent may not have been fully informed (for example, due to a language barrier), will be considered at the Editor’s discretion under exceptional circumstances. Scientific research involving vulnerable groups can only be carried out if its aims and scope benefit those groups and meet their specific needs, and authors must be able to demonstrate this for their manuscript to considered for publication. For articles involving the use of human transplantation, authors must declare in the manuscript that no organs/tissues were obtained from prisoners. The institution(s) through which organs/tissues were obtained must be named.
Patient consent for publication
Patients have a right to anonymity and privacy, and authors have a legal and ethical responsibility to respect this right. Identifying information, including names, initials, date of birth or hospital numbers, images or statements should not be included in the manuscript unless the information is essential for scientific purposes and the patient (or parent or guardian) has provided written informed consent for the publication of this identifying information. It must be stated in the manuscript that the patient, or parent/guardian/next of kin (in case of minors or deceased patients) provided written informed consent for the publication of any data and/or accompanying images. The consent form may be requested by the Editor, in which case it will be treated confidentially by the editorial office. This applies to case reports or other studies in which case details, personal information or images are included and could allow an individual to be identified, either directly or indirectly. Authors should disclose to patients that personally identifiable material would be available via the Internet as well as in print after publication.
Under exceptional circumstances, the Editor will consider publication of clinical data without written consent if all identifying information is removed, if the risks and potential harm posed to the patient are far outweighed by the benefits of the study, if it is impossible to obtain permission or if it is considered that a reasonable individual would be unlikely to object to publication.
During clinical trials, informed consent for publication of clinical data should be sought from the participants at the point of recruitment to the trial. In cases where this is not possible, authors must demonstrate that the publication of the clinical data would not compromise anonymity and confidentiality, nor be in breach of any local data protection laws. The authors must determine whether the dataset might contain any patient identifiers, both direct and indirect. Before submission of the manuscript, the authors should also consult their local ethics committee or other appropriate regulatory body in order to confirm full patient anonymity. The manuscript must state whether informed consent was obtained for the publication of clinical data. If informed consent for publication was not obtained, authors must provide a compelling reason. The Editor may request a document from the relevant ethics committee, outlining the reason why the requirement for consent to publish was waived.
Please note that consent to participate and consent for publication are considered distinct at Spandidos Publications journals. Where applicable, the Editor may request evidence that both have been obtained. For example, in a study involving blood collection from a patient cohort, ‘consent to participate’ would be required, and the authors must be able to demonstrate that the participants consented to the use of their blood samples for the purpose of scientific research. By contrast, for a case report involving the publication of images, the authors must be able to demonstrate that both consent to participate and consent to publish was obtained, i.e. that the patient consented to the images being taken for the purpose of research and also consented to their publication.
The use of protected animals for the purpose of scientific research must follow internationally recognized guidelines on animal welfare, as well as local and national regulations, and be performed in accordance with the UK’s Animals (Scientific Procedures) Act 1986, and associated guidelines, the EU Directive 2010/63/EU for animal experiments, or the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. The name of the institutional ethics committee that approved the experiments undertaken, including the reference number where appropriate, should be stated in the manuscript. The ethics committee from which approval was obtained must be transparent in its functioning, independent of the researcher, the sponsor and any other unwarranted influence, and duly qualified.
All animal studies should also comply with the ARRIVE guidelines and the AVMA euthanasia guidelines 2020.
If a study is exempt from requiring ethics approval, this should also be stated in the ‘Ethics approval and consent to participate’ section of the manuscript (including the name of the ethics committee that can confirm the exemption and the reason for the exemption).
Animal welfare issues will be taken into account during peer review, and the Editor may reject a manuscript at any point during the editorial process if the research involves protocols that are inconsistent with commonly accepted norms of animal research. The Editor may request additional documentation, including approval forms and/or relevant citations from the literature if any of the experimental details described in the study are considered a deviation from common practice in animal research.
When rodents are used as in vivo cancer models, the tumor burden should not exceed the recommendations of the University of Pennsylvania Institutional Animal Care and Use Committee guidelines.
This animal welfare policy also applies to field studies, as well as other non-experimental research conducted on animals. If a study involves client-owned animals, the authors are also required to provide an informed consent from the client/owner of the animal. The research will also be expected to demonstrate adherence to the highest standards of veterinary care.
Competing interests
Authors, reviewers and editors must disclose any competing interests that may exist with respect to the publication of a study. Competing interests are at play if the interpretation and presentation of the data, the conclusions or any other information provided by the authors can be influenced (or may be perceived as such) by their personal or financial relationship with other individuals or organizations, such as reimbursement for salaries, equipment or supplies, or a personal belief that may influence their objectivity and affect their interpretation of the data and associated conclusions as a result. Examples include, but are not limited to financial relationships, such as competing patents, grants, funding, bursaries and employment history, as well as personal relationships and strong ethical or personal beliefs. Competing interest statements for public funding sources, including government agencies, charitable or academic institutions, need not be included, as these should be described in the ‘Funding’ section instead. For example, if a charitable foundation sponsored the study and a pharmaceutical company provided the drugs, the former would be mentioned in the ‘Funding’ section, whereas the latter would be acknowledged in the ‘Competing interests’ section.
All competing interests must be disclosed, as they may affect (or be perceived to affect) the integrity of the research study and the reliability of its scientific contents. Authors are expected to disclose any competing interests at the time of submission in their cover letter and in the manuscript at the time of submission, even if the authors consider that these have not influenced their work. In addition, competing interests (or lack thereof) should also be mentioned in the manuscript. If no conflict exists, this must also be stated clearly in the 'Competing interests’ section, using the following format: “The authors declare that they have no competing interests”, and all authors should confirm its accuracy. If there is a conflict, please state so in the 'Competing interests' section. Examples of conflict of interest statements include ‘Dr Jones is a part-time employee at the ABC company’, ‘Recombinant protein Z was kindly provided by The ABC Company, London, United Kingdom’. Authors may be asked to confirm, update or provide further details regarding such disclosure statements following acceptance of the manuscript. For further information, please refer to the following link:www.icmje.org/conflicts-of-interest.
Prior to peer review, the external reviewers are also required to disclose any competing interests. If any competing interest is identified regarding a particular study, the reviewers are asked to notify Spandidos Publications and should decline to review the article if appropriate. If any competing interest are disclosed and the reviewers proceed with their evaluation, the Editor will make the final decision regarding whether or not the comments made by the reviewers should be recognized or reinterpreted
Authors' information (optional)
This section is optional. The authors may wish to use this section to include additional information about any or all of the authors deemed relevant. Examples include details of the the authors' qualifications, roles they hold at external institutions or societies, or any other relevant background information. This section should not contain correspondence information, e-mail addresses, acknowledgements or competing interests. The authors should be referred to by their initials. For example: “MA is a member of the British Society for Immunology”.
Use of artificial intelligence tools
Authors should disclose the use of artificial intelligence (AI)-assisted tools such as chatGPT in the Declarations section (e.g. ‘Use of artificial intelligence tools - During the preparation of this work, AI tools were used to improve the readability and language of the manuscript or to generate images, and subsequently, the authors revised and edited the content produced by the AI tools as necessary, taking full responsibility for the ultimate content of the present manuscript’).
Please note that AI tools should only be employed for improving the language of the article, and NOT for generating scientific content or drawing scientific conclusions, neither for analysing or interpreting scientific data. Furthermore, authors are responsible for reviewing and editing the text/images generated by AI tools to ensure their accuracy and correctness. In addition, AI tools should not be listed as authors, since they are not capable of taking any responsibility or accountability for the content of the manuscript intended to be published in terms of its integrity, accuracy or originality.
3.11 Standards of reporting
Incomplete reporting of experimental details, study rationale, methodological design and research outcomes can greatly compromise the validity, accuracy and reproducibility of scientific results. Thus, it is important for authors to ensure that their manuscript adheres to certain standards of reporting. Spandidos Publications encourage authors to refer to the standards set out by the EQUATOR Network. A list of standard reporting checklists used in biomedical/biological research can also be found at FAIRsharing.org.
Commonly used checklists are described in the Standards of reporting section of our website .
Reporting sex and gender information
Sex and gender are important factors that may influence the outcome and interpretation of scientific research. Whereas sex commonly means ‘biological sex’ (i.e. male or female), gender refers to ‘the socially constructed roles, expectations, relationships, behaviours, relative power, and other traits that societies ascribe to women, men and people of diverse gender identities’ (1).
Authors are encouraged to follow the Sex and Gender Equity in Research (SAGER) guidelines (2). As with other types of study designs, both positive and negative results should be described for all studies reporting sex and/or gender-based analyses. This applies even in cases where the sex and/or gender-based differences were not anticipated at the time the study was first conceived. Specifically:
- If sex-related differences may be expected (or were found) in the results, the study should be designed and carried out in a way that to addresses them.
- If gender-related differences may be expected (or were found) in the results, the study should be designed and carried out in a way that to addresses them.
- The Materials and methods section should clearly explain how the study design addresses sex and/or gender-based differences.
If a study included only one sex and/or gender, the manuscript should clearly emphasize this (for example, in the manuscript title and in the Abstract). The authors may also wish to address or comment on the lack of sex- and/or gender-based analysis in the Discussion.
In addition, the terms ‘sex’ (biological) and ‘gender’ (social or cultural) should not be used interchangeably and/or incorrectly.
Day, S., Mason, R., Lagosky, S., & Rochon, P. A. (2016). Integrating and evaluating sex and gender in health research. Health Res Policy Syst, 14(1), 75.
Heidari, S., Babor, T.F., De Castro, P. et al. Sex and Gender Equity in Research: rationale for the SAGER guidelines and recommended use. Res Integr Peer Rev 1, 2 (2016).
4. Proofs
- Authors must check their manuscript carefully before submission, after responding to peer-review, and when answering queries raised at the proof stage. Any errors will be faithfully transferred into the final PDF and print versions.
5. Reprints
- A reprint order form will accompany notification of acceptance of a manuscript and should be completed and returned immediately. For further information, please email contact@spandidos-publications.com.
6. Article charges
World Academy of Sciences Journal, the International Journal of Functional Nutrition, the International Journal of Epigenetics and Medicine International are free to publish.
7. Open access
- All articles in the International Journal of Functional Nutrition are published online as "Open access" articles and can be freely accessed at the journal's website by all users. The articles are made immediately available as Open access.
- Articles are licensed under the Creative Commons Attribution 4.0 International License.
- Spandidos Publications’ publishing policies enable authors to comply fully with the public access requirements of the major worldwide funding agencies (visit here for more information). Authors must take the necessary actions to obtain this compliance, including self-archiving, utilization of Spandidos Publications manuscript deposition service and selection of Open access publication under the correct license.
- Spandidos Publications makes articles freely available 12 months after the publication.
- Authors wishing to purchase immediate Open access for International Journal of Oncology, Oncology Reports, International Journal of Molecular Medicine, Molecular Medicine Reports, Oncology Letters, Experimental and Therapeutic Medicine, Molecular and Clinical Oncology and Biomedical Reports must select option 2.
- Spandidos Publications will refund an article processing charge (APC) if an error on our part has resulted in a failure to publish an article under the Open access terms selected by the authors. This may include a failure to make an article openly available on the journal platform, or publication of an article under a different Creative Commons licence from that selected by the authors. If you become aware of an error in the Open access status or licensing of your article, please contact the appropriate journal immediately. Note that APCs will not be refunded in instances where articles are retracted or removed as a consequence of author error or misconduct. Neither we will be liable if delays in the processing of the article result from changes made to the authorship on the article, or in cases where delays result from editorial decisions.
8. Compliance with funding agencies
All manuscripts that are agency-funded (e.g. NIH, HHMI, Cancer Research UK, Wellcome Trust, the Chinese Academy of Sciences (CAS), the National Natural Science Foundation of China (NSFC), etc.) and paid for under the Open access οption will be deposited automatically by the publisher and become publicly available in PubMed Central and Europe PMC. Alternatively, agency-funded articles without Open access purchase will be deposited and made publicly available in PubMed Central and Europe PMC 6-12 months following publication, depending on the public access policy of the agency.
9. Funding body policies and agreements
- Spandidos Publications has obtained agreements and constructed policies to enable authors to comply with manuscript archiving requirements of the following funding bodies specified in conditions of researcher grant awards.
- Arthritis Research Campaign (UK)
- Austrian Science Fund (FWF)
- Biotechnology and Biological Sciences Research Council (BBSRC)
- British Heart Foundation (UK)
- Cancer Research (UK)
- Chief Scientist Office (UK)
- Chinese Academy of Sciences (CAS)
- Department of Health (UK)
- Dunhill Medical Trust
- Economic and Social Research Council (ESRC)
- Howard Hughes Medical Institute (US)
- Medical Research Council (UK)
- National Institutes of Health (US)
- National Natural Science Foundation of China (NSFC)
- Research Councils (UK)
- Telethon (Italy)
- Wellcome Trust (UK)
- This enables authors to follow their funding body's policies on archiving without breaching their publishing agreements with Spandidos Publications.
10. Author self-archiving
Authors are encouraged to submit the final publisher’s version PDF (Version of Record) to their institution’s repository immediately following publication, as well as to their funding body’s archive. A link to the published version on the Spandidos Publications website must be included with full citation details and acknowledgement of the journal as the original source. In addition, authors are allowed to archive their articles pre-print (i.e., pre-refereeing). Pre-print can be on author's personal website or pre-print server.
11. English language editing, manuscript formatting and figure preparation
If English is not your first language, you may decide to have your manuscript proofread or edited by an English speaker, prior to submission. A colleague or a professional service may do this. Clear and concise language enables editors and reviewers to concentrate on the scientific content of your manuscript. This facilitates the peer review process and ensures that your article will not be rejected based on English language alone. Spandidos Publications offers a number of Manuscript Services including an English Language Editing service, through which the use of English in your manuscript may be checked and refined. The editors of this service will focus on the spelling, grammar, clarity and style of your manuscript.
For more information on this and other services, please access: www.spandidos-publications.com/languageediting.
Please be aware that an author's use of this or any other service in no way guarantees that his or her submission will be accepted by a Spandidos journal or any other. Any arrangement an author enters into will be exclusively between the author and their chosen company. Using an editing service is neither a requirement for nor a guarantee of acceptance for publication. For further details, please refer to our disclaimer on our affiliated website.

























