2. Studies involving humans
2.1 Ethics approval
Research performed on human subjects, materials or data must follow international and national regulations and be in agreement with the Declaration of Helsinki or any other relevant set of ethical principles. Generally, relevant human material includes any material made inside the human body, including all cellular material (unless it has divided in culture), body fluids (such as blood, urine, feces, saliva, pus) and fetal tissue (including amniotic fluid, umbilical cord, placenta and membrane). Primary cells maintained in culture are also relevant until their first division.
All manuscripts reporting such research studies must include the name of the ethics committee that approved the work and the reference number where appropriate. If a study is exempt from requiring ethics approval, this should also be detailed in the ‘Ethics approval and consent to participate’ section of the manuscript (including the name of the ethics committee that can confirm the exemption and the reason for the exemption). Additional information and official documentation to support this may be requested by the Editor. Please note that manuscripts that do not adhere to our ethics policies may be rejected at any stage of the editorial process.
If the study describes a new medical procedure or tool in patients, authors are required to justify their use, including a reason why the new procedure/tool was considered more appropriate than usual clinical practice for the purpose of patient treatment. Both ethics approval from a competent ethics committee and consent to participate in the study are required in such cases.
2.2. Embryonic stem cell research
Embryonic stem cell research must be subject to review and monitoring by a specialized Embryonic Stem Cell Research Oversight Committee (ESCRO). This includes all scientific research on human development at any pre-implantation stage, human embryos or embryo-derived cells or involving the production of human gametes in vitro (tested/used by fertilization or for the creation of embryos). The overseeing committee must be duly qualified and able to evaluate such studies for their adherence to the guidelines of the International Society for Stem Cell Research (ISSCR).
In particular, studies in Category 3 of these guidelines (heritable genome editing, transferring mtDNA-modified- not including MRT - embryos into a uterus, using gametes differentiated from human stem cells for reproduction, gestating human stem cell-based embryo models, human reproductive cloning, breeding human-animal chimeras where there may be human germ cells, transferring human-animal chimeric embryo(s) to a human or ape uterus, transferring human embryo(s), irrespective of origins, to an animal uterus) will not be considered for publication, as they are currently prohibited by the ISSCR due to their lack of safety and other scientific and ethical concerns (see link).
2.3. Retrospective ethics approval
For prospective studies, ethics approval must have been obtained prior to start of the study and cannot usually be sought retrospectively. In such cases, the manuscript may not be considered for peer review, and the decision on whether to proceed with the review will remain at the Editor's discretion.
2.4 Clinical trials
In the case of clinical trials, which are defined as "any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes", the trial registration number (TRN) and the date of the registration must be stated in the last line of the Abstract of the manuscript. Appropriate public registries are listed inn the ICMJE website and the WHO International Clinical Trials Registry Platform. If a clinical trial has not been registered prospectively, Spandidos Publications encourages retrospective registration to ensure that all results are fully available. In such cases, the last line of the Abstract of the manuscript should mention ‘retrospectively registered’ in addition to the TRN and date of registration.
2.5 Consent to participate
For all research involving human subjects or tissue, it must be stated that informed consent was obtained from all participants, for participation in the study or use of their tissue (or a parent/legal guardian in the case of children under 18 and patients otherwise considered minors under local legislation). Consent is also required for the procurement of biomaterials for stem cell research and translation, including gamete donors in IVF studies.
Studies involving vulnerable groups (i.e. individuals who are at higher risk of mistreatment or harm), with potential for coercion or exploitation (for example, prisoners or unconscious patients) or where consent may not have been fully informed (for example, due to a language barrier), will be considered at the Editor’s discretion under exceptional circumstances. Scientific research involving vulnerable groups can only be carried out if its aims and scope benefit those groups and meet their specific needs, and authors must be able to demonstrate this for their manuscript to considered for publication. For articles involving the use of human transplantation, authors must declare in the manuscript that no organs/tissues were obtained from prisoners. The institution(s) through which organs/tissues were obtained must be named.
2.6. Patient consent for publication
Patients have a right to anonymity and privacy, and authors have a legal and ethical responsibility to respect this right. Identifying information, including names, initials, date of birth or hospital numbers, images or statements should not be included in the manuscript unless the information is essential for scientific purposes and the patient (or parent or guardian) has provided written informed consent for the publication of this identifying information. It must be stated in the manuscript that the patient, or parent/guardian/next of kin (in case of minors or deceased patients) provided written informed consent for the publication of any data and/or accompanying images. The consent form may be requested by the Editor, in which case it will be treated confidentially by the editorial office. This applies to case reports or other studies in which case details, personal information or images are included and could allow an individual to be identified, either directly or indirectly. Authors should disclose to patients that personally identifiable material would be available via the Internet as well as in print after publication.
Under exceptional circumstances, the Editor will consider publication of clinical data without written consent if all identifying information is removed, if the risks and potential harm posed to the patient are far outweighed by the benefits of the study, if it is impossible to obtain permission or if it is considered that a reasonable individual would be unlikely to object to publication.
During clinical trials, informed consent for publication of clinical data should be sought from the participants at the point of recruitment to the trial. In cases where this is not possible, authors must demonstrate that the publication of the clinical data would not compromise anonymity and confidentiality, nor be in breach of any local data protection laws. The authors must determine whether the dataset might contain any patient identifiers, both direct and indirect. Before submission of the manuscript, the authors should also consult their local ethics committee or other appropriate regulatory body in order to confirm full patient anonymity. The manuscript must state whether informed consent was obtained for the publication of clinical data. If informed consent for publication was not obtained, authors must provide a compelling reason. The Editor may request a document from the relevant ethics committee, outlining the reason why the requirement for consent to publish was waived.
Please note that consent to participate and consent for publication are considered distinct at Spandidos Publications journals. Where applicable, the Editor may request evidence that both have been obtained. For example, in a study involving blood collection from a patient cohort, ‘consent to participate’ would be required, and the authors must be able to demonstrate that the participants consented to the use of their blood samples for the purpose of scientific research. By contrast, for a case report involving the publication of images, the authors must be able to demonstrate that both consent to participate and consent to publish was obtained, i.e. that the patient consented to the images being taken for the purpose of research and also consented to their publication.
3. Studies involving animals
The use of protected animals for the purpose of scientific research must follow internationally recognized guidelines on animal welfare, as well as local and national regulations, and be performed in accordance with the UK’s Animals (Scientific Procedures) Act 1986, and associated guidelines, the EU Directive 2010/63/EU for animal experiments, or the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. The name of the institutional ethics committee that approved the experiments undertaken, including the reference number where appropriate, should be stated in the manuscript. The ethics committee from which approval was obtained must be transparent in its functioning, independent of the researcher, the sponsor and any other unwarranted influence, and duly qualified.
All animal studies should also comply with the ARRIVE guidelines and the AVMA euthanasia guidelines 2020.
If a study is exempt from requiring ethics approval, this should also be stated in the ‘Ethics approval and consent to participate’ section of the manuscript (including the name of the ethics committee that can confirm the exemption and the reason for the exemption).
Animal welfare issues will be taken into account during peer review, and the Editor may reject a manuscript at any point during the editorial process if the research involves protocols that are inconsistent with commonly accepted norms of animal research. The Editor may request additional documentation, including approval forms and/or relevant citations from the literature if any of the experimental details described in the study are considered a deviation from common practice in animal research.
When rodents are used as in vivo cancer models, the tumor burden should not exceed the recommendations of the University of Pennsylvania Institutional Animal Care and Use Committee guidelines.
This animal welfare policy also applies to field studies, as well as other non-experimental research conducted on animals. If a study involves client-owned animals, the authors are also required to provide an informed consent from the client/owner of the animal. The research will also be expected to demonstrate adherence to the highest standards of veterinary care.
4. Studies involving plants
Studies involving the use or collection of plants or plant materials, either cultivated or wild, must adhere to institutional, national and international guidelines (such as the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora). Any field studies should be conducted in accordance with local legislation. The authors are required to include details of any appropriate licenses and/or permissions in the manuscript.
Voucher herbarium specimens must be deposited in a public, freely accessible collection (such as a museum or other institution). The manuscript must also contain information on the voucher specimen, including where it is stored and the name(s) of the individual(s) who identified it.
In the case of studies on Traditional Chinese Medicine herbs and plants, authors must provide the Latin names (scientific designation) of the plants in the manuscript. For studies involving the use of plant extracts, the scientific name of the plant from which the extract is derived must also be provided. For plant extracts containing multiple components, the scientific names of the major components should be provided, together with their relative concentrations.
5. Studies involving cell lines
For all manuscripts involving the use of cell lines, it is strongly advised to include the following information in the Materials and methods section of the manuscript:
- Whether mycoplasma testing has been carried out for the cell lines used
- Whether the cell lines used have been authenticated; if so, the methods used should be stated
- The name of the supplier (or if not obtained commercially, the name, title and affiliation of the individual who provided the cells) and, if available, catalogue number of all cell lines used the study.
The method used to maintain the cell lines in culture should be specified, according to good cell culture practice. For more information, please refer to the following links: fundamental techniques, mycoplasma contamination, passage number, etc.
Furthermore, information regarding misidentified or cross-contaminated cell lines must be provided and cross-checked from the International Cell Line Authentication Committee and ExPASy Cellosaurus databases in order to exclude any possible contamination with other cell lines or misidentification.
If any of the cell lines used in the study has been previously reported to be contaminated with another line and/or misidentified, the Editor may request an STR profile at any stage of the editorial process.
6. Standards of reporting
6.1. Study design
Incomplete reporting of experimental details, study rationale, methodological design and research outcomes can greatly compromise the validity, accuracy and reproducibility of scientific results. Thus, it is important for authors to ensure that their manuscript adheres to certain standards of reporting. Spandidos Publications encourage authors to refer to the standards set out by the EQUATOR Network. A list of standard reporting checklists used in biomedical/biological research can also be found at FAIRsharing.org.
Commonly used checklists are described in the following subsections. Where the authors made use of any of the checklists below, this should be duly acknowledged in Materials and methods, together with a reference (or URL) for the checklist used.
6.1.1. Systematic reviews/meta-analyses
Systematic reviews and meta-analyses are articles that discuss and/or re-analyze the findings of previously published studies, using a defined set of search and analysis parameters. For these types of articles, the authors may wish to refer to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist.
In addition, systematic reviews may be prospectively registered in an appropriate registry, such as the National Institute for Health Research’s PROSPERO database.
For more guidance on the design of search strategies for systematic reviews, the authors can also consult the Cochrane Reviewers' Handbook.
6.1.2. Observational studies
Observational studies are a type of epidemiological research that does not involve any intervention. For more information on the reporting standards for observational studies, the authors are invited to consult the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines.
6.1.3. Randomized clinical trials
In contrast to observational studies, randomized clinical trials involve the use of a specific intervention in a defined number of individuals who are specifically enrolled in the study using specific inclusion and exclusion criteria.
For all clinical trials, Spandidos Publications encourage authors to follow CONsolidated Standards Of Reporting Trials (CONSORT) standards. Specific trial protocols should also be described using the recommendations of Standard Protocol Items Recommendations for Interventional Trials (SPIRIT).
For diagnostic studies, the authors may wish to specifically refer to the Standards for Reporting Diagnostic accuracy studies (STARD) checklist. Similarly, for prognostic studies, the Transparent Reporting of studies on prediction models for Individual Prognosis Or Diagnosis (TRIPOD) recommendations may provide additional guidance.
6.1.4. Case reports
Case reports are short articles describing individual medical cases. Examples include conditions that are distinct from known diseases (such as variations or unique clinical presentations of a known disease, as well as rare and/or idiopathic conditions), clinical cases involving unique treatment regimens (if these are reported to benefit the patient) or unexpected clinical events (for example, a known disease with unusual progression or outcome).
For all case reports, authors must clearly highlight the reasons why the case is considered unique and useful to the field. The case report should be written to meet the standards set out by the CAse REports guidelines (CARE).
6.1.5. Pre-clinical animal studies
Authors are encouraged to refer to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines to identify the minimum information required for in vivo studies. Further guidance on best practices for the design and completion of animal studies can be found in the original ARRIVE 2.0. publication (1) (1) Percie du Sert N, Ahluwalia A, Alam S, et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020 Jul 14;18(7):e3000411.
6.1.6. Qualitative research an economic evaluation studies
Qualitative research encompasses all studies that do not involve the description of numerical (quantitative) data. Guidance for the reporting of qualitative results can be found in the Consolidated criteria for reporting qualitative research (COREQ) checklist.
For studies involving economic evaluation, the authors can consult the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) guidelines.
6.2. Reporting sex and gender information
Sex and gender are important factors that may influence the outcome and interpretation of scientific research. Whereas sex commonly means ‘biological sex’ (i.e. male or female), gender refers to ‘the socially constructed roles, expectations, relationships, behaviours, relative power, and other traits that societies ascribe to women, men and people of diverse gender identities’ (1).
Authors are encouraged to follow the Sex and Gender Equity in Research (SAGER) guidelines (2). As with other types of study designs, both positive and negative results should be described for all studies reporting sex and/or gender-based analyses. This applies even in cases where the sex and/or gender-based differences were not anticipated at the time the study was first conceived. Specifically:
- If sex-related differences may be expected (or were found) in the results, the study should be designed and carried out in a way that to addresses them.
- If gender-related differences may be expected (or were found) in the results, the study should be designed and carried out in a way that to addresses them.
- The Materials and methods section should clearly explain how the study design addresses sex and/or gender-based differences.
If a study included only one sex and/or gender, the manuscript should clearly emphasize this (for example, in the manuscript title and in the Abstract). The authors may also wish to address or comment on the lack of sex- and/or gender-based analysis in the Discussion.
In addition, the terms ‘sex’ (biological) and ‘gender’ (social or cultural) should not be used interchangeably and/or incorrectly.
- (1) Day, S., Mason, R., Lagosky, S., & Rochon, P. A. (2016). Integrating and evaluating sex and gender in health research. Health Res Policy Syst, 14(1), 75.
- (2) Heidari, S., Babor, T.F., De Castro, P. et al. Sex and Gender Equity in Research: rationale for the (SAGER) guidelines and recommended use. Res Integr Peer Rev 1, 2 (2016).
6.3. Statistical methods
The Materials and methods section of original research articles should contain a Statistical analysis subsection in which details of the statistical methods and measures used in the study are described. This includes the name, version and supplier of the statistical software used, the name of the statistical tests used, the number of experimental repeats and the P-value threshold considered to indicate statistical significance.
6.4. Gene nomenclature
For all gene names, manuscripts should only contain standardized gene nomenclature. A full list of human gene symbols and names can be found in the HUGO Gene Nomenclature Committee (HGNC) database.
6.5. Reporting of sequence variants
When describing sequence variants, authors are encouraged to use the Human Genome Variation Society Mutation Nomenclature.
The authors should submit any novel variants described in a manuscript to an appropriate publicly accessible gene/disease-specific database; a list is available here. The database URL and the unique identifier should be reported in the manuscript.
Descriptions of phenotypes should adhere to the standardized vocabulary provided by the (Human Phenotype Ontology).
6.6. Chemical and biomolecular characterization
Manuscripts submitted to Spandidos Publications must have sufficient information to substantiate the identity and purity of novel compounds. This must be supported by a statement, which validates the origin, identity and purity of these compounds, irrespective of their supplier or synthetic methodology.
6.6.1. Identity of novel compounds
The identity of compounds containing carbon-hydrogen bonds with or without a metal should be validated using spectroscopy. 1H NMR and proton-decoupled 13C NMR must be supplemented for novel compounds. Additional NMR information can be reported (31P NMR, 19F NMR) if necessary. Mass spectrometry results should be supplemented to confirm identification of novel compounds. Additional spectroscopic information (UV or IR) can be used to support the presence of specific functional groups. Melting-point data are required for crystalline compounds. The characterization of chiral compounds can be supported by rotational data. References can be provided, instead of comprehensive methods, for known compounds unless the authors followed a modification of the published methods.
6.6.2. Combinatorial compound libraries
Authors studying the use of combinatorial libraries must provide standard characterization results that correspond to a wide range of library components.
6.6.3. Biomolecular identity
If direct structural analysis of novel biopolymeric compounds (e.g. oligosaccharides, peptides, nucleic acids) by NMR spectroscopy is not possible, the identification of these compounds should be performed by sequence and mass spec characterization.
6.6.4. Biological constructs
Sequencing or functional data should be provided to support the identification of biological constructs (e.g. plasmids or recombinant proteins) upon request.
6.6.5. Sample purity
Data supporting sample purity are required for novel compounds. For compounds containing carbon-hydrogen bonds with or without a metal atom, purity can be confirmed by 1H NMR or 13C NMR, whereas elemental analysis is preferred for small-molecular weight molecules. Chromatography-based or electrophoretic methods can also be used to assess purity of polymers or small-molecular weight compounds.
6.6.6. Spectroscopic information
Comprehensive spectroscopic information for novel compounds should be supplemented in the Materials and methods section. Figures containing spectra must be made available to the Editor upon request. The authors should explain how specific, unambiguous NMR assignments were made in the Materials and methods section.
6.6.7. Crystallographic data for small molecules
Authors presenting novel structural data of small-molecular weight compounds from crystallography-based methods must be able to provide a standard crystallographic information file (.cif); structure factors for each structure; and a structural figure with probability ellipsoids upon request. The associated parameters and structural output should be checked using International Union of Crystallography checkCIF. The results of the crystallographic analysis should be deposited to a relevant public database and the deposition number must be referenced in the manuscript.
6.6.8. Macromolecular structural data
Manuscripts reporting new structures must include a summary in a table format of the statistical analysis of structural and refinement parameters, and the different programs used in the analysis should be mentioned and referenced. To assess the structural information, a stereo image of a portion of the electron density map (for crystallography papers), of the superimposed lowest energy structures (>10; for NMR papers), or of the entire structure (as a backbone trace) in case a new overall fold is presented, must be provided upon request. For cryo-EM structures, a characteristic micrograph indicating sole particles must be supplemented at submission. Protein structures should be deposited in the Protein Data Bank PDB and the deposition number must be referenced in the manuscript.

6.6.9. Chemical structures
Structures of compounds should be prepared using a drawing program, such as ChemDraw. Please ensure to use the following settings (ACS Style sheet in ChemDraw):
- Chain angle: 120º
- Space between bonds: 18% of width
- Fixed length: 14.4 pt (0.508 cm, 0.2 in.)
- Bold width: 2.0 pt (0.071 cm, 0.0278 in.)
- Line width: 0.6 pt (0.021 cm, 0.0084 in.)
- Margin width: 1.6 pt (0.056 cm, 0.0222 in.)
- Hash spacing: 2.5 pt (0.088 cm, 0.0347 in.)
- Font: Arial/Helvetica
- Size: 10 pt
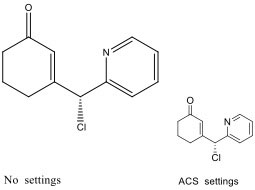
Figure 1: Representative molecule and lettering in ChemDraw default (left) and ACS (right) format.
6.7. Describing new taxa
6.7.1 Algal, fungal and botanical names
Any manuscript describing the names of new algal, fungal or botanical taxons must follow the guidelines outlined by the International Code of Nomenclature for Algae, Fungi and Plants. If the authors wish to describe new fungal taxa should register the names with a recognized repository, such as MycoBank, and request a unique digital identifier that should be included in the published article.
The publication of algal, fungal and botanical names in electronic form is currently considered a valid form of publication.
6.7.2. Prokaryotic names
The publication of new prokaryote names must comply with rules of the International Committee on Systematics of Prokaryotes (ICSP). The authors are required to submit the following to the International Journal of Systematic and Evolutionary Microbiology (IJSEM):
- i) A copy of the final, published article
- ii) Certificates of deposition of the type strain (for unrestricted distribution), in two or more internationally recognized, publicly accessible culture collections located in different countries.
The submissions are reviewed by the List Editor, and any names that conform to all of the rules of the International Code of Nomenclature of Prokaryotes (ICNP) will subsequently be added to the Validation List (i.e. validly published).
The publication of new prokaryote names in electronic form is considered valid according to the ICNP.
6.7.3. Viral names
All manuscripts describing new virus names must comply with the the International Code of Virus Classification and Nomenclature provided by the International Committee on Taxonomy of Viruses (ICTV).
The proposed, new viral taxa must be sent to the relevant ICTV study group for review.
7. Availability of data and materials
For all manuscripts submitted to Spandidos Publications, the authors must make provisions for all materials described in their manuscript, including all relevant raw data, to be freely available to any researchers who may wish to reuse or reanalyze them. If any conclusions made in the paper depend on a particular, dataset, then this dataset must be made available to the readers (unless it is already provided as part of the submitted article).
Spandidos Publications follow the guidance set out by COPE. Authors are encouraged to ensure their manuscript adheres to the FAIR principles (https://www.go-fair.org/fair-principles/), including the fundamental principles of Findability, Accessibility, Interoperability, and Reusability.
- Findable: Does the manuscript clearly state where the raw data may be found? The manuscript must include a data availability statement. If the data are available from the correspondence author, their details must be correct at the time of manuscript publication.
- Accessible: Are the datasets freely available to other researchers? Raw data must be made available to other researchers, at the very least, upon reasonable request. For example, for public datasets requiring deposition in curated databases, the data should not be password-protected (except under exceptional cases, such as identifiable data) and must be accessible, free of charge, to any interested reader.
- Interoperability: Are the raw data easily interpretable by other, interested researchers? For example, is the language used suitable to the audience? Is the format machine-readable?
- Reusability: Can the data easily be re-analyzed by other researchers who may wish to reuse them? For example, did the authors ensure that the reuse of the data would involve the use of user-friendly software or tools?
7.1. Data availability statements
‘All manuscripts should include a statement outlining to fellow researchers where the data generated during their study may be found. Authors can use any of the following standard statements (or combination of statements, where applicable).
- For data that are available upon request, the following statement must be used: ‘The data generated in the present study may be requested from the corresponding author’. For publicly available datasets, the authors can state that ‘The data generated in the present study may be found in the (name of database) under accession number (accession number) or at the following URL: (persistent, direct URL to datasets)’. If all the data are included in the manuscript (this includes all experimental repeats for all assays and analyses), then this also can be acknowledged as follows: ‘The data generated in the present study are included in the figures and/or tables of this article’.
- If the authors have a compelling reason to withhold their data then the following sentence should be included: ‘The data generated in the present study are not publicly available due (compelling reason why data are not public) to but may be requested from the corresponding author’. In case the manuscript does not contain data generated by the authors (such as review articles) the statement ‘Not applicable’ should be included. If the data are available from a third-party, then the following statement must be included: ‘The data generated in the present study may be requested from (name of third party), due to (reason why the data are available from third-party)’.
7.2. Public/previously published datasets
If public datasets are included in the study, the authors must include the following information in the ‘Availability of data and materials’ section of the article: the accession number of the dataset, the name of the repository in which it can be found, and a direct URL for the dataset. Authors who are unable to share their data must disclose this and provide a compelling explanation as to why the data are unavailable.
7.3. For large sequencing or proteomics datasets
Authors are required to deposit large datasets (such as high-throughput sequencing, X-ray crystallography and microarray datasets) in public curated repositories, unless there is a compelling reason for them not to do so (such as protection of patient privacy, pending or approved patents, biosecurity reasons or any other legislation prohibiting public data sharing). Authors who are unable to share their data must disclose this at submission on our online submission system. Please note that a manuscript may be rejected if the Editor considers that the manuscript does not comply with our data sharing policies, and the authors have failed to provide a compelling reason to withhold their data and materials.
The datasets must be described in the ‘Availability of data and materials’ section of the manuscript, and this must include the accession number of the dataset, the name of the repository in which it can be found, and a direct URL for the dataset. Although the choice of database is at the authors' discretion, we require the data to be freely available to readers upon publication of the manuscript. This means the data should not be embargoed or otherwise password-protected at the time of publication (unless there is a specific reason for that to be the case).
A list of recommended repositories is shown below; consulting the Registry of Research Data Repositories (http://www.re3data.org/) may also be useful in this regard.
7.4. List of suggested repositories
7.5. Software and code
For all manuscripts involving the use of new software application or custom code, these should be available for testing by reviewers in a way that respects their anonymity. The authors should provide clear instructions to allow the reviewers full access the unreported software application or custom code. This should include a direct link to the most recent version of the software/ code (as well as a links to the any archived versions referenced in the manuscript, where applicable). The software or code should be archived in an appropriate repository with a DOI or other unique identifier.
If the manuscript is published, the software application/custom code should be made freely available to any researchers who may wish to use it for non-commercial purposes. If the authors fail to make the software application/custom code freely available, then the manuscript should not discuss or describe the software/code itself in any detail.
8. Competing interests
Authors, reviewers and editors must disclose any competing interests that may exist with respect to the publication of a study. Competing interests are at play if the interpretation and presentation of the data, the conclusions or any other information provided by the authors can be influenced (or may be perceived as such) by their personal or financial relationship with other individuals or organizations, such as reimbursement for salaries, equipment or supplies, or a personal belief that may influence their objectivity and affect their interpretation of the data and associated conclusions as a result. Examples include, but are not limited to financial relationships, such as competing patents, grants, funding, bursaries and employment history, as well as personal relationships and strong ethical or personal beliefs. Competing interest statements for public funding sources, including government agencies, charitable or academic institutions, need not be included, as these should be described in the ‘Funding’ section instead. For example, if a charitable foundation sponsored the study and a pharmaceutical company provided the drugs, the former would be mentioned in the ‘Funding’ section, whereas the latter would be acknowledged in the ‘Competing interests’ section.
All competing interests must be disclosed, as they may affect (or be perceived to affect) the integrity of the research study and the reliability of its scientific contents. Authors are expected to disclose any competing interests at the time of submission in their cover letter and in the manuscript at the time of submission, even if the authors consider that these have not influenced their work. In addition, competing interests (or lack thereof) should also be mentioned in the manuscript. If no conflict exists, this must also be stated clearly in the 'Competing interests’ section, using the following format: “The authors declare that they have no competing interests”, and all authors should confirm its accuracy. If there is a conflict, please state so in the 'Competing interests' section. Examples of competing interest statements include ‘Dr Jones is a part-time employee at the ABC company’, ‘Recombinant protein Z was kindly provided by The ABC Company, London, United Kingdom’. Authors may be asked to confirm, update or provide further details regarding such disclosure statements following acceptance of the manuscript. For further information, please refer to the following link:www.icmje.org/conflicts-of-interest.
Prior to peer review, the external reviewers are also required to disclose any competing interests. If any competing interest is identified regarding a particular study, the reviewers are asked to notify Spandidos Publications and should decline to review the article if appropriate. If any competing interest are disclosed and the reviewers proceed with their evaluation, the Editor will make the final decision regarding whether or not the comments made by the reviewers should be recognised or reinterpreted.
9. Authorship
9.1 Authors’ contributions
The individual contributions of the authors of the manuscript should be specified in this section. The authors should be referred by their initials, for example: "MA carried out the high-throughput sequencing experiments and performed the bioinformatics analysis. VA performed the histological examination of the kidney and was a major contributor in writing the manuscript. MA and VA confirm the authenticity of all the raw data. All authors read and approved the final manuscript."
An 'author' is generally considered to be someone who has made meaningful intellectual contributions to a study. According to the ICMJE guidelines, to qualify as an author, one should have i) substantially contributed to the conception and the design of the study, or in the acquisition, analysis and interpretation of the data; ii) been contributed to manuscript drafting or critical revisions on the intellectual content; iii) approved of the final manuscript version to be published; and iv) agreed to be accountable for all aspects of the work, so that any questions relating to research integrity or scientific accuracy in any part of the study are appropriately investigated and resolved. Each author listed in the manuscript should have participated sufficiently in the study to be publicly responsible for the content (or portions of the content). Authors do not normally qualify for authorship if their sole contribution is, for instance, the procurement of funding, acquisition of data, or general study supervision.
Publication of a manuscript in Spandidos Publications journals requires at least two authors who can confirm the authenticity of all the raw data. These authors must be mentioned in the Authors’ contributions section of the declarations, using the following format: “MA and VA confirm the authenticity of all the raw data”.
Changes to authorship after manuscript acceptance are at the Editor’s discretion. If a manuscript requires a change in authorship, such as the addition or removal of an author, changes in the order of the authors, changes in affiliations or corresponding authors, the authors must must contact the Editor and provide reason to justify the change. In such cases, and if the changes are deemed appropriate, the Editor will request a letter of agreement, clearly stating the changes made, that has been signed by all authors (removed or added). Spandidos Publications will individually inform anyone who is added or removed from the author list.
If the wording of the ‘Authors’ contributions’ section does not reflect sufficient contribution from any of the authors to qualify them for authorship, the Editor may request the section to be clarified and/or updated at any point of the editorial process.
9.2. Acknowledgements
Any individuals who contributed to the study but do not meet the requirements to qualify for authorship may be listed in the ‘Acknowledgements’ section. For example, this may include a person who provided minimal technical assistance or writing advice, or the Chair/Head of department who provided general support. Where any other individual, such as a scientific or medical writer, assisted in preparing the contents of the manuscript, this should be explicitly acknowledged. Authors should obtain permission to acknowledge from all those mentioned in the Acknowledgements section. If there are no individuals to acknowledge, the authors should write “Not applicable” in this section. When the authors wish to thank a specific individual, their full name, title, affiliation and specific contribution must be stated. The acknowledgements section should not be used to thank companies (including language editing companies), patients, friends or family.
10. Funding
All sources of funding for the reported research should be declared in the ‘Funding’ section. If the funding body had any role in the design of the study or in the collection, analysis and interpretation of data, this should also be declared in this section. If there were no funding bodies supporting the study described in the manuscript, the ‘Funding’ section should state: “No funding was received”.
11. Citations
All articles must cite appropriate and relevant literature in support of the claims made. Authors must avoid excessive self-citation and would normally be encouraged to cite their previous work only if relevant to the current manuscript. Authors should consider the following guidelines when preparing their manuscript:
- Any statement in the manuscript that relies on external sources of information (i.e. a statement that does not reflect authors' own ideas or findings) should use a citation.
- Authors should avoid citing derivations of original work (e.g. authors should cite the original work rather than a review article that cites an original work).
- Authors should ensure that their citations are accurate (i.e. they should ensure the citation supports the statement made in their manuscript and should not misrepresent another work by citing it if it does not support the point the authors wish to make).
- Authors should not cite sources that they have not read.
- Authors should not preferentially cite their own, or their friends’, peers’ or institution’s publications.
- Authors should avoid citing work solely from one country.
- Authors should not use an excessive number of citations to support one point.
- Authors should cite sources that have undergone peer review where possible.
- Authors should not cite advertisements or advertorial material.
12. Duplicate publication
All submissions must be an account of new, original research. The authors are expected to submit their manuscript on the assumption that no similar work has been or will be submitted to any other journal for publication. Submitting identical manuscripts to more than one journal at a time is generally considered unethical. Multiple submissions of the same paper can damage the reputation of journals, and therefore, duplicate or redundant publications (re-packaging in different words of data already published by the same authors) will be rejected.
13. Plagiarism and other fraud
The authors are responsible for guaranteeing the originality of their work. Upon submission, all manuscripts are rigorously evaluated to identify any previously published material. Spandidos Publications uses the iThenticate, plagiarism software, as well as searches in PubMed and Google, and other search engines to screen submitted manuscripts against published studies and other relevant sources. In addition, images are routinely examined for the presence of duplicated, manipulated otherwise anomalous data.
The Editor may request the original data for comparison with the prepared figures, and therefore the authors are strongly advised to retain their unprocessed data and metadata files. If the original data cannot be produced, the manuscript may be rejected or, in the case of a published article, retracted. Any case in which the manipulation affects the interpretation of the data will result in rejection or retraction.
If the journal Editor has a reason to suspect misconduct, including plagiarism they reserve the right to raise their concerns to the institution which the authors are affiliated with and any other relevant bodies. Every suspected act of unethical publishing behaviour will investigated. In cases where plagiarism is suspected, a preliminary investigation will be conducted following the guidelines of the Committee on Publication Ethics (COPE) (https://publicationethics.org/resources/flowcharts) and the guidelines of ICMJE (http://www.icmje.org). If plagiarism is detected, the manuscript containing the plagiarism will be obviously marked on each page of the PDF and will be declined for publication. In the cases of published articles, the paper may also be formally retracted.
14. Image manipulation
All digital images in manuscripts considered for publication will be scrutinized for any indication of manipulation that is inconsistent with the following guidelines. Manipulation that violates these guidelines may result in delays in manuscript processing or rejection, or retraction of a published article:
- No specific feature within an image may be enhanced, obscured, moved, removed, or introduced.
- The grouping of images from different parts of the same gel, or from different gels, fields or exposures, must be made explicit by the arrangement of the figure and in figure legend.
- Adjustments of brightness, contrast or color balance may be acceptable if they are applied to every pixel in the image and as long as they do not obscure, eliminate or misrepresent any information present in the original, including the background. Non-linear adjustments (e.g. changes to gamma settings) must be disclosed in the figure legend.
Any questions raised during or after the peer review process will be referred to the Editor, who may request the original data from the author(s) for comparison with the prepared figures. If the original data cannot be produced, the manuscript may be rejected or, in the case of a published article, retracted. Any case in which the manipulation affects the interpretation of the data will result in rejection or retraction. Cases of suspected misconduct will be reported to the author(s)’ institution(s).
15. Misconduct
If misconduct by authors or reviewers is suspected, either pre- or post-publication action will be taken. In cases where misconduct, or otherwise unethical behaviour, on the part of the authors is suspected after a paper is published (even several years after the paper has been published), a preliminary investigation will be conducted following the guidance offered in the flowcharts of the Committee on Publication Ethics (COPE) (https://publicationethics.org/resources/flowcharts) and the guidelines of ICMJE (http://www.icmje.org/). An explanation will be sought from the party or parties considered to be involved. If the response is unsatisfactory, then an appropriate authority (ordinarily, the Head of the Department where the research was conducted) will be asked to investigate fully. Spandidos Publications will make all reasonable attempts to obtain a resolution in any such eventuality and correct the record or archive as necessary. In cases where misconduct is proven beyond all reasonable doubt, the paper will be formally retracted.
16. Peer review and confidentiality
All submissions made to Spandidos Publications journals are peer reviewed prior to acceptance for publication. The Editorial Board assesses the suitability of each manuscript to ensure its suitability with respect to the scopes and aims of the journal. The manuscript is then critically reviewed by a minimum of two academic reviewers. During the peer review process, the Editor reserves the right to decline the manuscript for publication or to return it to the authors for additional changes prior to manuscript acceptance.
For original articles or case reports, reviewers will generally be asked to comment on the following aspects of the submitted manuscripts:
- Significance of the study to the field and its novelty.
- Quality of data presented and controls/statistical analyses used.
- Compliance to ethics guidelines and regulations, where applicable
- Whether the conclusions are justified/supported by the data presented.
- How clearly the study is reported.
- Novelty of the research.
The peer review process for all Spandidos Publications journals is single-blinded. Reviewers are asked to objectively evaluate the merits of the reported research whilst respecting the authors’ own intellectual independence. Personal criticism is not appropriate under any circumstances. The reviewers must explain and provide evidence that supports their evaluation so that the authors can understand the reasoning behind the comments made.
The reviewers should mention any relevant, previously published studies that have not been cited in the manuscript. When commenting on any observations, deductions or arguments that have been previously reported, the reviewer should provide the relevant citation. If any substantial similarities are identified between the manuscript and any published article or any manuscript under consideration by another journal, this should be brought to the editor’s attention.
Spandidos Publications follows COPE’s Ethical Guidelines for Peer Reviewers. All manuscripts under review must be treated in strict confidence. The manuscript must not be shared or discussed with anyone, except in exceptional circumstances where additional expert advice is required from a specific individual. Where this is the case, the reviewer must disclose to the editor the identities of the individuals that have been consulted. Any unpublished information, argumentation or interpretation found in the manuscript under review must not be disclosed, unless consent from the authors is obtained.
If any competing interest is identified regarding a particular study, the reviewers are asked to notify Spandidos Publications and should decline to review the article if appropriate.
The final decision to accept or decline a manuscript for publication is made by the Editor(s) following consultation with the reviewers and the Editorial Board. For any manuscripts that do not meet the standards of Spandidos Publications journals, as outlined in our editorial policies, or that contain major scientific shortcomings, the reviewers will, to the best of their ability and wherever possible, advise the authors on how to further improve their work for publication.
The authors may be invited for reconsideration if a manuscript receives favorable comments from the reviewers but cannot be accepted immediately after the initial review. In such cases, the authors are expected to fully address the concerns and criticisms raised by the reviewers and/or the Editor. Manuscripts that are resubmitted after undergoing major revisions will be sent back for peer review. If the authors are not invited for resubmission by the Editor, the manuscript will not be reconsidered at any Spandidos Publications journal.
17. Corrections and retractions
If authors become aware of an error or fraudulent data (including, but not limited to, direct image manipulation, duplication or plagiarism) in their published article, they must notify the Editor. If the required changes can influence the interpretation of the data and associated conclusions without fully invalidating the entire study, the Editor will decide whether the changes may be corrected through the publication of an erratum, correction or notice of concern at the earliest possible date.
If the interpretation of the data and associated conclusions are severely undermined and may invalidate the study as a whole, the Editor reserves the right to have the published articles retracted. Spandidos Publications will follow the COPE guidelines in such cases. Retraction articles are indexed and linked to the original article.
18. Appeals and complaints
Spandidos Publications adheres to the COPE guidelines regarding appeals to editorial decisions and complaints. Authors who believe that the decisions to decline their manuscript was made in error may submit an appeal. The authors should contact Spandidos Publications and submit an appeal letter, clearly stating the reasons for the appeal. The authors should explain why they consider the decision made by the Editor to be incorrect and provide a detailed and specific response to each and any comment that led to the rejection. Following the receipt of the appeal letter, Spandidos Publications will seek the advice of the journal’s external Editorial Advisory Panel in order to determine whether the manuscript can be reconsidered for re-review.
19. Editorial responsibilities
For all Spandidos Publications journals, the Editor has complete responsibility and authority to accept or decline a submitted paper for publication. The Editor may confer with associate editors or reviewers in order make this decision.
The Editor will give prompt and unbiased consideration to all manuscripts submitted for publication. All manuscripts will be evaluated independently and fairly based on their own merits without consideration for race, sex, gender, religious belief, ethnic origin, citizenship or political philosophy of the authors. The Editor will respect the intellectual independence of the authors, and will avoid, to the best of their ability, any circumstances that may lead to competing interests (or perceived as such)
The Editor and the editorial office should not disclose to anyone any information concerning a manuscript under consideration at Spandidos Publications journals, with the exception of reviewers and potential reviewers. Unpublished information, arguments or interpretations disclosed in a submitted manuscript should not be used in an editor's own research except with the consent of the author. Any unpublished information, argumentation or interpretation found in the manuscript under review must not be used in the editor’s own research, unless consent from the authors is obtained.
If the Editor becomes aware of convincing evidence that the interpretations, arguments or conclusions made in a published paper are incorrect or invalid, they will, to the best of their ability, encourage the publication of a correction or retraction.
20. Transfers
The aims and scope of each Spandidos Publications journal are available from our website. The authors are strongly encouraged to carefully review the acceptance criteria for the journal they are considering and ensure that the study described in the manuscript adheres to the aims and scope of that journal. If a manuscript does not meet the aims and scope of a particular journal, it is possible to have it transferred to another, more suitable Spandidos Publications journal.
Transfers may be requested via email. Please note that a request for a manuscript transfer does not guarantee that the manuscript will be considered by the receiving journal. This process is only meant to facilitate transfer of manuscripts and associated files from journal to journal, without the need for withdrawal and/or resubmission.
21. Open access (optional)
All articles are published online with ‘open access’ and can be freely accessed from the journal’s website by all users. Articles may be licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, a Creative Commons Attribution-NonCommercial 4.0 International License or a Creative Commons Attribution 4.0 International License.
All publishing policies at Spandidos Publications allow full compliance with the public access requirements of the major worldwide funding agencies (see www.sherpa.ac.uk for more information). The authors must take all necessary actions to adhere to these policies and ensure full compliance to these requirements, including self-archiving, use of the Spandidos Publications manuscript deposition service and selection of open access publication under the correct license.
Authors who have published with ‘open access’ can add the final publisher's PDF file of their manuscript to the repositories of their institution and/or funding bodies immediately. A link to the published version on the Spandidos Publications website must be included with full citation details, acknowledging the journal as the original source.














