Introduction
β-glucans are some of the most abundant
polysaccharides found in the cell walls of bacteria and fungi
(1,2). They are present in water-soluble or
-insoluble forms, while certain β-glucans can be excreted into the
medium (3). β-glucans have been
proven to act as immunological modulators (4). Their water-soluble form has been shown
to have a more pronounced effect on immunity (5). They have the potential to directly
bind to specific receptors on immune cells and trigger innate
and/or adaptive immunity activation. Currently, a number of
β-glucan receptors have been identified. Dectin-1, located on
macrophages, mediates the β-glucan activation of phagogenesis and
the production of cytokines (6,7).
Activated complement receptors on natural killer cells, neutrophils
and lymphocytes have also been suggested to be associated with
cytotoxicity (8). Certain toll-like
receptors (TLRs) on macrophage cells were also demonstrated to
specifically bind with β-glucans and increase tumor necrosis factor
(TNF)-α and interleukin (IL)-12 production via nuclear factor-κB
(NF-κB) signaling (9). Two
additional receptors, scavenger (10,11)
and lactosylceramide (12), bind
with β-glucans and mediate signal pathways leading to immunological
activation.
The antitumor activity of β-glucans was first
demonstrated 50 years ago. A number of subsequent experiments
conducted in animals have confirmed the inhibitory effects of
certain β-glucans on tumor growth or metastasis (13). Several clinical trials have also
shown the potential benefits of β-glucans in the treatment of
cancer patients (14). Fungal
β-glucans have also been proven to have synergistic effects with
monoclonal antibodies, when used in the treatment of certain types
of cancer (15). Although one of
the most well-known anticancer mechanisms is stimulation of innate
immunity, direct action on tumor cells has also been suggested
(16). Therefore, fungal β-glucans
may exert multiple effects on cancer cells (4). Certain β-glucans have been clinically
used as a complementary therapy for cancer in Japan and China, due
to their ability to enhance host defense responses against various
tumors.
Fungal β-glucans are present as β-1→3-linked glucose
polymers with β-1→6-linked side chains of varying lengths and
distributions (2). Secreted yeast
β-glucans are important sources of β-glucans for potential medical
use, due to the fact that they can be recovered and purified more
easily (3). Black yeast
β-1,3-1,6-glucan belongs to the category of soluble glucans
(17). Previous studies have shown
a noteworthy stimulatory effect of this compound on the production
of IL-8 by peripheral blood mononuclear cells (18). However, a limited number of studies
has been conducted with a view to address the function of this
compound on tumor growth (17). In
this study, we demonstrated that gastric administration of purified
black yeast β-1,3-1,6-glucan significantly inhibited transplanted
sarcoma growth in mice. In addition to its proven role in immunity
stimulation, the microRNA (miRNA) expression profiles in
transplanted tumors also changed, due to β-1,3-1,6-glucan
administration.
Materials and methods
Materials
β-1,3-1,6-glucan purified from black yeast was
kindly provided by Gokei Trading Co., Ltd. (Tokyo, Japan).
RPMI-1640 medium, fetal bovine serum (FBS), miRVana RNA isolation
and mirVana™miRNA isolation kits were purchased from Life
Technologies (Carlsbad, CA, USA). Cytokine enzyme-linked
immunosorbent assay (ELISA) kits were purchased from R&D
(Minneapolis, MN, USA), while isoflurane was obtained from Abbott
Laboratories (North Chicago, IL, USA).
Cell line and animals
The mouse sarcoma S180 cell line was purchased from
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
Twenty male BALB/c mice (6-week-old; n=20) were purchased from
Shanghai Xipuerbikai Experimental Animal Co. Ltd. (Shanghai,
China). The mice were kept in cages (n=5 per group), at 22±1°C and
55±5% relative humidity, with an automatic 12-h light/dark cycle.
The experimental procedures were approved by the Animal Ethics
Committee of the Shanghai Jiaotong University (Shanghai,
China).
Animal experiment
Mice were randomized into the control and study
groups (n=10/group). Mouse sarcoma S180 cells were cultured in
RPMI-1640 medium containing 10% FBS. Cells growing in log phase
were trypsinized and resuspended in sterile saline and immediately
inoculated into mice by subcutaneous injection (2×106
cells in 0.2 ml saline/mouse). Immediately after the inoculation,
mice in the control and study groups were submitted to daily
intragastric administration of β-1,3-1,6-glucan (5 mg/100 g body
weight) or saline, respectively. Nine days after the implantation,
the engrafted tumor sizes were measured daily in vivo, using
external caliber and were calculated as previously reported
(19,20). Twenty-one days after treatment, the
mice were anaesthetized with 2% isoflurane and blood was drawn by
cardiac puncture. Serum was separated from the clot within 20 min
and stored at −20°C until use. The mice were then sacrificed and
engrafted tumors were dissected, weighed and frozen in liquid
nitrogen. Tumor sizes and weights were calculated using the
formula: A% = [(X–Y) / X] × 100, where A is the inhibition rate, X
is the average tumor weight of control group and Y is the average
tumor weight of the study group.
Measurement of cytokine content
The serum content of 10 cytokines including IL-2,
IL-4, IL-6, IL-8, IL-10, IL-12, interferon (IFN)-γ, TNF-α, TNF-β
and granulocyte-colony stimulating factor (G-CSF) were determined
using double antibody sandwich ELISA tests, following the
manufacturer’s instructions. Two-way ANOVA was used to determine
the significance of statistical differences in cytokine
concentrations in the control and study groups. P<0.05 was
considered to indicate a statistically significant difference.
RNA isolation, library construction and
miRNA sequencing
Total RNA was extracted from the dissected tumor
tissues using the miRVana RNA isolation kit, while miRNA-enriched
RNA fractions were isolated using the mirVana™miRNA isolation kit,
according to the manufacturer’s instructions. RNA integrity was
confirmed using Agilent 2100 Bioanalyzer small-RNA chips. Equal
amounts of miRNA-enriched RNA fractions from five samples within
the same group were combined and sent to the Genergy Biotech
Shanghai Co., Ltd. (Shanghai, China) for small RNA library
preparation. The libraries were then sequenced using the Solexa’s
Sequencing-By-Synthesis method, a massively parallel sequencing
technology. The 36-bp reads on the Illumina Genome Analyzer
(Illumina, Inc., Hayward, CA, USA) were used for sequencing.
Statistical analysis
miRNA expression levels were tested for
statistically significant differences in the study and control
group libraries using the Limma package (21). The Benjamini-Hochberg method
(BH-FDR) (22) was used to control
the false discovery rate. Statistical significance was determined
using the square root transformed templates per million (TPM)
expression values of the 5-prime and 3-prime miRNA transcripts.
Differences in expression with BH-FDR P≤0.05 and a minimal 1.5-fold
change were considered to indicate a statistically significant
difference.
Target selection and functional
clustering
Target mRNAs for differentially expressed miRNAs
were identified using miRWalk (http://www.rna.uni-heidelberg.de/apps/zmf/mirwalk/index_html;
last updated on March 15, 2011) in the validated targets module.
Curated targets were subjected to functional enrichment to identify
significantly over-represented biological functions using the DAVID
functional clustering software (http://david.abcc.ncifcrf.gov). Functional clusters
with statistical P-values ≤0.05 were reported.
Results
Inhibitory effect of β-1,3-1,6-glucan on
transplanted tumors
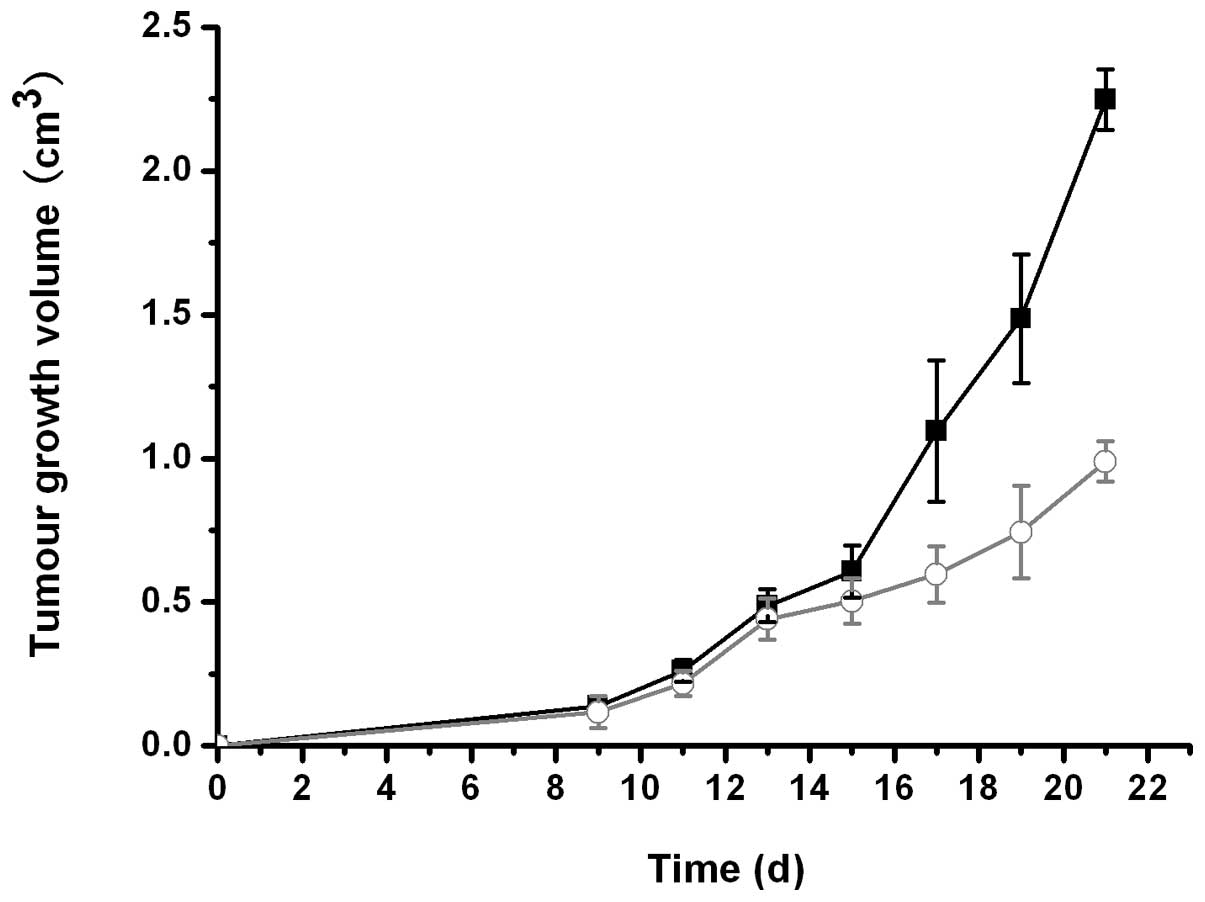
In the tumor intervention study, significant growth
inhibition was observed in mice treated with β-1,3-1,6-glucan vs.
the control mice, as demonstrated by slower tumor growth rates
(Fig. 1) and reduced sizes of
dissected tumors (Table I).
 | Table I.Correlation between body and tumor
weight.a |
Table I.
Correlation between body and tumor
weight.a
| Groups
|
|---|
| Variables | Control (n=10) | Study (n=10) |
|---|
| Body weight (g) | 25.31±2.41 | 23.60±1.46 |
| Tumor weight (g) | 3.081±0.998 | 0.898±0.396b |
| Actual body weight
(g) | 22.23±2.678 | 22.70±1.248 |
| Tumor weight/actual
body weight | 0.143±0.057 | 0.039±0.017a |
| Inhibition rate | - | 70.85 |
Changes in cytokine production
As shown in Table
II, mice administered β-1,3-1,6-glucan demonstrated a
significant increase in the levels of the tested cytokines,
including IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IFN-γ, TNF-α, TNF-β
and G-CSF. However, the elevation of serum IL-2 and IL-12 levels
was more significant.
 | Table II.Serum concentrations of cytokines. |
Table II.
Serum concentrations of cytokines.
| Groups (pg/ml)
|
|---|
| Cytokines | Control | Study |
|---|
| IL-2 | 216.576±66.004 |
912.878±72.036a |
| IL-4 | 30.257±8.327 | 80.620±16.163a |
| IL-6 | 34.515±2.999 | 82.946±13.628a |
| IL-8 | 57.285±8.785 |
166.316±12.630a |
| IL-10 | 404.768±35.001 |
673.272±40.305a |
| IL-12 | 0.995±0.198 | 5.284±0.660a |
| IFN-γ | 425.537±52.853 |
503.201±43.922b |
| TNF-α | 217.007±33.128 |
573.126±38.860a |
| TNF-β | 66.628±5.625 | 130.744±6.332a |
| G-CSF | 275.950±59.676 |
758.219±69.910a |
Changes in miRNA expression
Two datasets were obtained from two biological
replicates in the study or control groups. Differentially expressed
miRNAs with a minimum of 1.5-fold change were reported. Twelve
miRNAs were reproducibly found to be upregulated in two biological
replicates (Fig. 2).
Identification of target genes and
functional enrichment
A total of 308 validated target genes were
identified using the miRWalk database. These genes were classified
using the DAVID functional annotation clustering program with the
high-stringency enrichment algorithm. As a result, we retrieved 15
annotation clusters with P≤0.05. These functional gene clusters are
summarized in Table III. Notably,
induction of apoptosis may contribute to the decrease in tumor
growth and size.
 | Table III.Gene functional clusters enriched by
DAVID annotation. |
Table III.
Gene functional clusters enriched by
DAVID annotation.
| Cluster term | Gene count, na | P-value |
|---|
| 1. Regulation of
macromolecule biosynthetic process | 58 | 4.19E-25 |
| 2. Regulation of
apoptosis | 31 | 2.76E-15 |
| 3. Regulation of
transcription | 29 | 5.24E-9 |
| 4. Cell
migration | 22 | 3.60E-8 |
| 5. Stem cell
maintenance | 7 | 4.63E-6 |
| 6. Regulation of
protein kinase activity | 16 | 9.17E-6 |
| 7. Regulation of
caspase activity | 8 | 7.83E-5 |
| 8. Regulation of cell
division | 6 | 8.81E-4 |
| 9. Glucose metabolic
process | 12 | 1.82E-4 |
| 10. Cyclin-dependent
protein kinase inhibitor activity | 4 | 1.75E-4 |
| 11. Transforming
growth factor-β-related | 4 | 0.02 |
| 12. Peptide hormone
secretion | 4 | 0.03 |
| 13. DNA fragmentation
involved in apoptosis | 3 | 0.03 |
| 14. Cellular ion
homeostasis | 11 | 0.05 |
| 15. Mitochondrial
outer membrane | 5 | 0.05 |
Discussion
The antitumor effect of β-1,3-1,6-glucan has been
documented in previous studies. The antitumor effects have been
directly attributed to the activation of leukocytes. However, the
incurred molecular and biochemical changes in tumor cells have not
been fully elucidated. In this study, the antitumor efficacy of
β-1,3-1,6-glucan derived from black yeast was assessed in
subcutaneously-injected mice with mouse S180 sarcoma cells.
Intragastric administration of β-1,3-1,6-glucan significantly
decreased the growth rate of transplanted tumor cells and reduced
tumor size during the 21-day treatment period. Mice administered
β-1,3-1,6-glucan also demonstrated significantly higher levels of
cytokines, such as IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IFN-γ,
TNF-α, TNF-β and G-CSF, compared to the control mice. These
findings demonstrate that β-1,3-1,6-glucan clearly triggers
cytokine release in sarcoma xenograft-bearing mice. These findings
are consistent with the data obtained from a previous study on a
transplanted breast cancer model (23).
In addition, mice administered β-1,3-1,6-glucan also
showed significant changes in the expression of several miRNAs in
transplanted tumors. Previously, IL-6 was found to trigger the
expression of miR-21 in myeloma cells (24). IL-6 was also shown to modulate the
miR-17/92 cluster in human pulmonary artery endothelial (HPAE)
cells through the STAT3 transcription factor (25). However, none of these miRNA species
were found to be differentially expressed in the present study.
Functional annotation clustering demonstrated that the target genes
of these differentially expressed miRNAs are involved in the
regulation of apoptosis and cell division. These findings indicate
that β-1,3-1,6-glucan may exert its tumor-inhibition effects
through the activation of the immune system and the modulation of
miRNA expression in tumor cells.
Despite the limited number of studies available on
β-glucans purified from certain plants having direct cytotoxic
effects on cancer cells (16), the
majority of the studies have shown no direct cytotoxic effects of
fungal β-glucans on common cancer cell lines including carcinoma,
sarcoma, blastoma (1,26) or lymphoma (27). In the present study, we investigated
the direct effects of β-1,3-1,6-glucan on the S180 sarcoma cell
line in vitro and found no phenotypic changes in cell growth
and apoptosis (data not shown). Thus, β-1,3-1,6-glucan inhibits the
growth of transplanted tumors by direct activation of immunity and
that alteration of miRNA expression is potentially induced by
changes of cytokines indirectly. Previous studies have proven that
the β-glucans exhibit their antitumor activity through stimulation
of immune responses (26). The
findings of this study suggest that β-1,3-1,6-glucan, extracted
from black yeast, inhibits transplanted sarcoma through activation
of the immune system as well as the modulation of miRNA expression
in tumor cells. To the best of our knowledge, this is the first
study investigating the effects of β-1,3-1,6-glucan ingestion on
miRNA expression in transplanted tumor cells. The precise
mechanisms of β-1,3-1,6-glucan-induced changes of miRNA expression
remain to be fully elucidated.
References
|
1.
|
Chan GC, Chan WK and Sze DM: The effects
of beta-glucan on human immune and cancer cells. J Hematol Oncol.
2:252009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Tsoni SV and Brown GD: beta-Glucans and
dectin-1. Ann N Y Acad Sci. 1143:45–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Schmid F, Stone BA, McDougall BM, Bacic A,
Martin KL, Brownlee RT, Chai E and Seviour RJ: Structure of
epiglucan, a highly side-chain/branched (1→3;1→6)-β-glucan from the
micro fungus Epicoccum nigrum Ehrenb. ex Schlecht. Carbohydr
Res. 331:163–171. 2001.PubMed/NCBI
|
|
4.
|
Chen J and Seviour R: Medicinal importance
of fungal beta-(1→3),(1→6)-glucans. Mycol Res. 111:635–652.
2007.
|
|
5.
|
Rop O, Mlcek J and Jurikova T:
Beta-glucans in higher fungi and their health effects. Nutr Rev.
67:624–631. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ariizumi K, Shen GL, Shikano S, Xu S,
Ritter R, Kumamoto T, Edelbaum D, Morita A, Bergstresser PR and
Takashima A: Identification of a novel, dendritic cell-associated
molecule, dectin-1, by subtractive cDNA cloning. J Biol Chem.
275:20157–20167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Brown GD and Gordon S: Immune recognition.
A new receptor for beta-glucans. Nature. 413:36–37. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ross GD: Regulation of the adhesion versus
cytotoxic functions of the Mac-1/CR3/alphaMbeta2-integrin
glycoprotein. Crit Rev Immunol. 20:197–222. 2000.PubMed/NCBI
|
|
9.
|
Lebron F, Vassallo R, Puri V and Limper
AH: Pneumocystis carinii cell wall beta-glucans initiate macrophage
inflammatory responses through NF-kappaB activation. J Biol Chem.
278:25001–25008. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Acton SL, Scherer PE, Lodish HF and
Krieger M: Expression cloning of SR-BI, a CD36-related class B
scavenger receptor. J Biol Chem. 269:21003–21009. 1994.PubMed/NCBI
|
|
11.
|
Rice PJ, Kelley JL, Kogan G, Ensley HE,
Kalbfleisch JH, Browder IW and Williams DL: Human monocyte
scavenger receptors are pattern recognition receptors for
(1→3)-beta-D-glucans. J Leukoc Biol. 72:140–146. 2002.
|
|
12.
|
Zimmerman JW, Lindermuth J, Fish PA,
Palace GP, Stevenson TT and DeMong DE: A novel
carbohydrate-glycosphingolipid interaction between a
beta-(1-3)-glucan immunomodulator, PGG-glucan, and lactosylceramide
of human leukocytes. J Biol Chem. 273:22014–22020. 1998. View Article : Google Scholar
|
|
13.
|
Vetvicka V and Yvin JC: Effects of marine
beta-1,3 glucan on immune reactions. Int Immunopharmacol.
4:721–730. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Mizuno M, Minato K, Ito H, Kawade M, Terai
H and Tsuchida H: Anti-tumor polysaccharide from the mycelium of
liquid-cultured Agaricus blazei mill. Biochem Mol Biol Int.
47:707–714. 1999.PubMed/NCBI
|
|
15.
|
Cheung NK and Modak S: Oral
(1→3),(1→4)-beta-D-glucan synergizes with antiganglioside GD2
monoclonal antibody 3F8 in the therapy of neuroblastoma. Clin
Cancer Res. 8:1217–1223. 2002.
|
|
16.
|
Zhang M, Chiu LC, Cheung PC and Ooi VE:
Growth-inhibitory effects of a β-glucan from the mycelium of
Poria cocos on human breast carcinoma MCF-7 cells:
Cell-cycle arrest and apoptosis induction. Oncol Rep. 15:637–643.
2006.
|
|
17.
|
Kimura Y, Sumiyoshi M, Suzuki T and
Sakanaka M: Antitumor and antimetastatic activity of a novel
water-soluble low molecular weight beta-1, 3-D-glucan (branch
beta-1,6) isolated from Aureobasidium pullulans 1A1 strain
black yeast. Anticancer Res. 26:4131–4141. 2006.PubMed/NCBI
|
|
18.
|
Ikewaki N, Fujii N, Onaka T, Ikewaki S and
Inoko H: Immunological actions of Sophy beta-glucan (beta-1,3-1,6
glucan), currently available commercially as a health food
supplement. Microbiol Immunol. 51:861–873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Euhus DM, Hudd C, LaRegina MC and Johnson
FE: Tumor measurement in the nude mouse. J Surg Oncol. 31:229–234.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Tomayko MM and Reynolds CP: Determination
of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother
Pharmacol. 24:148–154. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Smyth GK: Linear models and empirical
bayes methods for assessing differential expression in microarray
experiments. Stat Appl Genet Mol Biol. 3:Article 3. 2004.PubMed/NCBI
|
|
22.
|
Reiner A, Yekutieli D and Benjamini Y:
Identifying differentially expressed genes using false discovery
rate controlling procedures. Bioinformatics. 19:368–375. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Vetvicka V, Vashishta A, Saraswat-Ohri S
and Vetvickova J: Immunological effects of yeast- and
mushroom-derived beta-glucans. J Med Food. 11:615–622. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Löffler D, Brocke-Heidrich K, Pfeifer G,
Stocsits C, Hackermüller J, Kretzschmar AK, Burger R, Gramatzki M,
Blumert C, Bauer K, et al: Interleukin-6 dependent survival of
multiple myeloma cells involves the Stat3-mediated induction of
microRNA-21 through a highly conserved enhancer. Blood.
110:1330–1333. 2007.PubMed/NCBI
|
|
25.
|
Brock M, Trenkmann M, Gay RE, Michel BA,
Gay S, Fischler M, Ulrich S, Speich R and Huber LC: Interleukin-6
modulates the expression of the bone morphogenic protein receptor
type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ
Res. 104:1184–1191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Gomaa K, Kraus J, Rosskopf F, Röper H and
Franz G: Antitumour and immunological activity of a beta 1→3/1→6
glucan from Glomerella cingulata. J Cancer Res Clin Oncol.
118:136–140. 1992.
|
|
27.
|
Harnack U, Kellermann U and Pecher G:
Yeast-derived beta-(1-3),(1-6)-D-glucan induces up-regulation of
CD86 on dectin-1-positive human B-lymphoma cell lines. Anticancer
Res. 31:4195–4199. 2011.PubMed/NCBI
|