Introduction
Bone mass is maintained by the balance of bone
formation and resorption, involving a number of regulatory
pathways. Bone formation is performed by osteoblasts, while bone
resorption is carried out by osteoclasts. Disruptions of these
processes are likely to induce hyperosteogeny or osteopenia
(1,2). Numerous clinical studies reported that
patients with osteoporosis showed iron accumulation in the bone,
potentially leading to pathological damages, such as occurred
femoral neck fractures in patients of African descent (3–5).
Ovariectomized rats with an increased iron level
showed a higher tendency to have osteoporosis (6). Tsay et al(7) reported that the iron overload
(IO)-induced bone loss in mice was correlated with the inflammatory
bone resorption and oxidative stress. Using in vitro cell
assays, iron treatment was demonstrated to inhibit osteoblast
formation, proliferation and mineralization (8–10),
while promoting osteoclast differentiation and increased
osteoclastic function (11).
Therefore, IO is believed to disrupt bone metabolism and induce
bone loss.
Iron accumulation in tissue is likely to provoke the
formation of reactive oxygen species (ROS) (12), while the iron excess is likely to
induce an increased expression of tumor necrosis factor-α (TNF-α)
(13). However, the contribution of
oxidative stress to osteoporosis was also recognized (14,15).
ROS induced increased TNF-α expression and activated several
signaling systems involved in the osteoclastic differentiation,
especially the nuclear factor-κB (NF-κB) pathway. TNF-α was found
to synergize strongly with the receptor activator of NF-κB ligand
in osteoclast formation and activation (16,17).
Therefore, osteoclastic hyperresorption was highly involved in ROS-
and TNF-α-induced bone loss. However, whether IO is linked to
induced bone function impairment has yet to be investigated.
In this study, a mouse model with IO was developed
and was used to determine whether the excess iron undermined bone
strength and load-bearing capacity through TNF-α induction and
osteoclastic function promotion.
Materials and methods
Animal experiments
Six-week-old female BALB/c mice were fed under
sterile conditions and divided into two groups. The iron excess
(i.e., IO), group was injected with iron-dextran (Sigma, St. Louis,
MO, USA) at 250 mg/kg. The control mice were injected with equal
amounts of phosphate-buffered saline (PBS) twice a week for four
weeks. Subsequent to sacrificing the animals at 48 h after the
final injection, blood and femur samples were collected for
analysis.
Assessments of bone metabolic
markers
Blood samples were collected from the heart and
allowed to clot at room temperature. Serum samples were separated
from the blood by centrifugation at 3,000 rpm for 10 min. Serum
osteocalcin, C-telopeptide of type I collagen (CTX-1) and TNF-α
were measured by ELISA (Rapidbio; RapidBio, West Hills, CA, USA;
R&D Systems, Minneapolis, MN, USA), according to the
manufacturer’s instructions.
Bone biomechanical analysis
Biomechanical properties of femurs were measured by
the three-point bending test performed using a universal material
test machine (AG-1S; Shimadzu Co., Kyoto, Japan) at room
temperature, as described in previous studies (18–20).
Briefly, the diaphysis was placed on two supports with a span of 10
mm, while the loading pin was centered above two supports at a
displacement rate of 2 mm/min, until fracture occurred.
Statistical analysis
The two-tailed Student’s t-test was used to analyze
experimental data. The data were presented as the means ± standard
error (SE). P<0.05 was considered to indicate a statistically
significant difference.
Results and Discussion
Mechanical loading sends signals which are essential
for bone mass maintenance and strength depending on the stimulus
(21). To determine whether IO
undermines the bone strength and function, the biomechanical
parameters of the bone were measured. Maximal bending stress is the
maximum load per unit of bone area in the plastic deformation
stage, while the modulus of bending elasticity involves the
resistance to elastic deformation of the bone, reflecting the inner
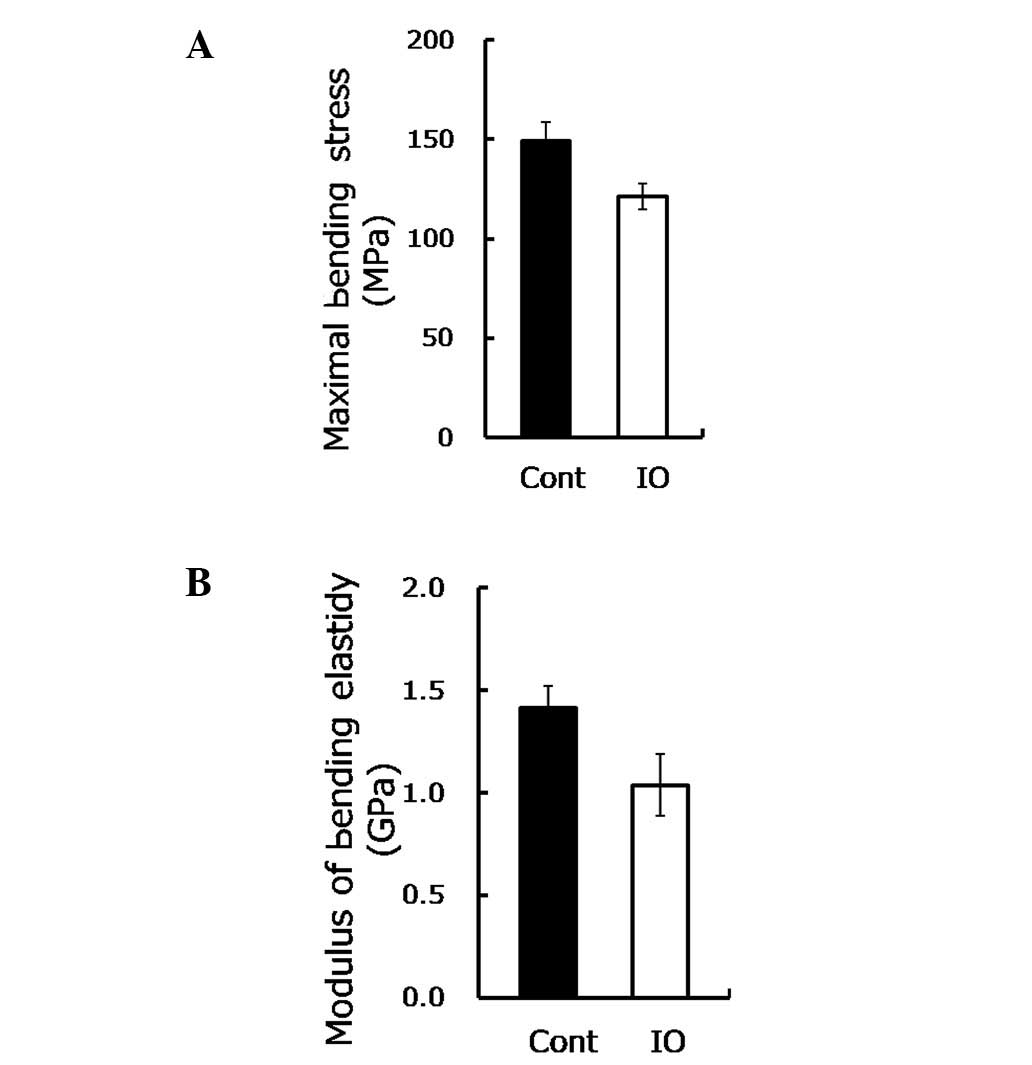
strength of the bone, without size effect. As shown in Fig. 1A, the maximal bending stress was
reduced approximately by 20% (P=0.03) under IO conditions, while
the modulus of bending elasticity was reduced by 30% (P=0.05,
Fig. 1B). These findings suggested
that IO led to a significant reduction of bone load-bearing
capacity and thus increased the risk of fractures.
Bone load-bearing capacity is dependent on bone
formation, as well as bone resorption. To gain insight into the
mechanism underlying the IO-mediated impairment to bone
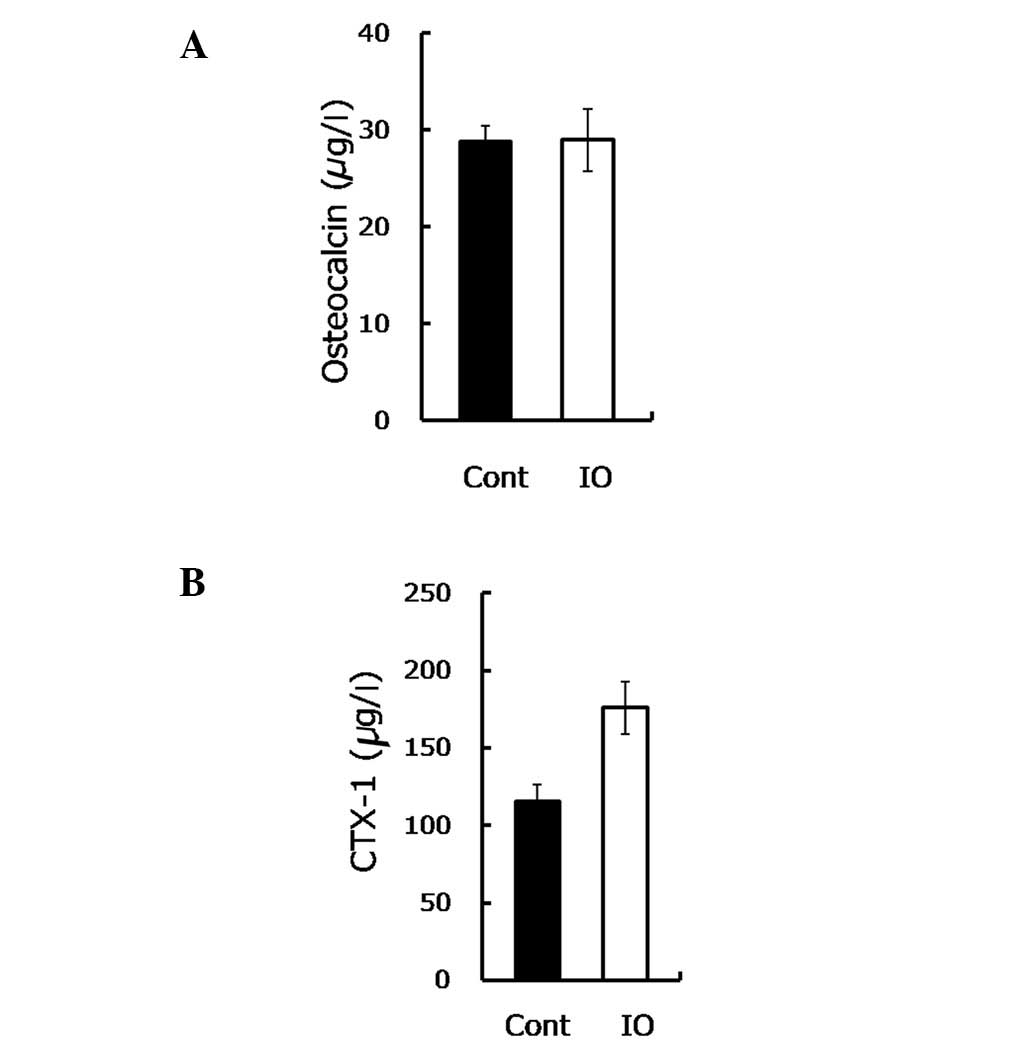
load-bearing capacity, the osteocalcin and CTX-1 were measured.
Osteocalcin is secreted by osteoblasts in the maturation stage to
sustain mineralization and may be used as a marker of bone
formation in the evaluation of bone mass. The findings of this
study showed no statistically significant difference in the
osteocalcin content between the mice with IO and the control group
(28.82 vs. 28.99, P=0.963, Fig.
2A). In contrast to these findings, in their study, Yamasaki
et al(8) demonstrated that
excess iron reduced the mineralization of rat calvarial
osteoblast-like cells by showing a decreased number of mineralized
nodules (9,10). This discrepancy may be due to the
in vitro sensitivity of osteoblasts being higher compared to
the in vivo, when directly exposed to iron. Thus, consistent
with another in vivo study (7), our results demonstrated that IO had
little effect on bone formation.
CTX-1 is the degraded product of type I collagen
with an enhanced osteoclastic activity, used to evaluate the bone
resorptive process. In the present study, the CTX-1 content was
observed to be notably increased (∼50%) in the IO group compared to
the control group (P=0.008, Fig.
2B), suggesting an elevated bone resorption.
The increased bone resorption indicated that the
osteoclastic activity was stimulated. Since IO was demonstrated to
induce the TNF-α expression that further triggers osteoclastic
function (13,22,23),
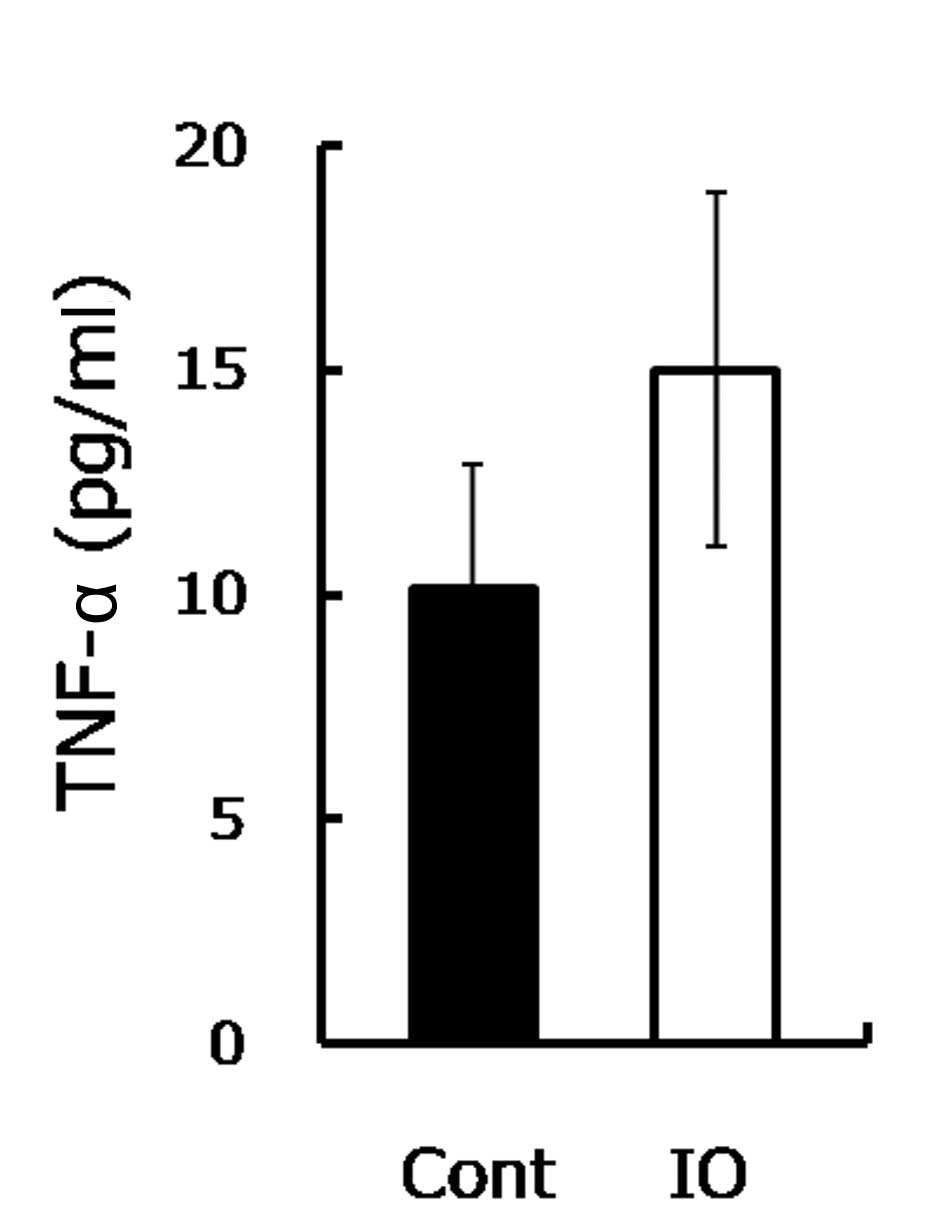
the TNF-α level was measured. Consistent with a previous study
(13), the TNF-α level in the IO
mice was approximately 1.5-fold higher compared to the control
group (Fig. 3), as shown. TNF-α was
demonstrated to be involved in the enhancement of the development
of osteopenia and osteoporosis (14,15).
TNF-α promoted osteoclastogenesis through synergizing with a
permissive level of the receptor activator of NF-κB ligand (RANKL),
as well as through directly affecting osteoclasts (16,17).
In the current study, a high serum TNF-α level was observed in mice
with IO. The increased TNF-α is likely to promote the osteoclastic
function and enhance inflammatory osteolysis, resulting in advanced
bone resorption (24). Therefore,
upon IO exposure, although increased TNF-α was not involved in
mineralization inhibition, it accelerated bone loss and eventually
reduced bone strength.
In conclusion, the findings of the present study
have shown that IO undermined bone load-bearing capacity through
the enhancement of TNF-α secretion, which facilitated osteoclastic
differentiation and promoted the bone-resorbing activity (Fig. 4). Therefore, decreasing the iron
burden is likely to be a promising approach for the treatment of
bone metabolic diseases, such as osteopenia and osteoporosis.
Acknowledgements
This study was financed by grants from
the Chinese Academy of Sciences (KZCX2-EW-404) and the National
Natural Science Foundation of China (nos. 21077128, 20921063 and
21177151). The authors would like to thank the laboratory staff
members for their assistance with the experiments and reagents.
References
|
1.
|
Mountzios G, Dimopoulos MA, Bamias A,
Papadopoulos G, Kastritis E, Syrigos K, Pavlakis G and Terpos E:
Abnormal bone remodeling process is due to an imbalance in the
receptor activator of nuclear factor-kappa B ligand
(RANKL)/osteoprotegerin (OPG) axis in patients with solid tumors
metastatic to the skeleton. Acta Oncol. 46:221–229. 2007.
View Article : Google Scholar
|
|
2.
|
Fohr B, Dunstan CR and Seibel MJ: Clinical
review 165: markers of bone remodeling in metastatic bone disease.
J Clin Endocrinol Metab. 88:5059–5075. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Maurer J, Harris MM, Stanford VA, Lohman
TG, Cussler E, Going SB and Houtkooper LB: Dietary iron positively
influences bone mineral density in postmenopausal women on hormone
replacement therapy. J Nutr. 135:863–869. 2005.PubMed/NCBI
|
|
4.
|
Schnitzler CM, Schnaid E, MacPhail AP,
Mesquita JM and Robson HJ: Ascorbic acid deficiency, iron overload
and alcohol abuse underlie the severe osteoporosis in black African
patients with hip fractures - a bone histomorphometric study.
Calcified Tissue Int. 76:79–89. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Valenti L, Varenna M, Fracanzani A, Rossi
V, Fargion S and Sinigaglia L: Association between iron overload
and osteoporosis in patients with hereditary hemochromatosis.
Osteoporosis Int. 20:549–555. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Liu G, Men P, Kenner GH and Miller SC:
Age-associated iron accumulation in bone: implications for
postmenopausal osteoporosis and a new target for prevention and
treatment by chelation. Biometals. 19:245–251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Tsay J, Yang Z, Ross FP,
Cunningham-Rundles S, Lin H, Coleman R, Mayer-Kuckuk P, Doty SB,
Grady RW, Giardina PJ, Boskey AL and Vogiatzi MG: Bone loss caused
by iron overload in a murine model: importance of oxidative stress.
Blood. 116:2582–2589. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Yamasaki K and Hagiwara H: Excess iron
inhibits osteoblast metabolism. Toxicol Lett. 191:211–215. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Messer JG, Kilbarger AK, Erikson KM and
Kipp DE: Iron overload alters iron-regulatory genes and proteins,
down-regulates osteoblastic phenotype, and is associated with
apoptosis in fetal rat calvaria cultures. Bone. 45:972–979. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Yang Q, Jian J, Abramson SB and Huang X:
Inhibitory effects of iron on bone morphogenetic protein 2-induced
osteoblastogenesis. J Bone Miner Res. 26:1188–1196. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Ishii KA, Fumoto T, Iwai K, Takeshita S,
Ito M, Shimohata N, Aburatani H, Taketani S, Lelliott CJ,
Vidal-Puig A and Ikeda K: Coordination of PGC-1beta and iron uptake
in mitochondrial biogenesis and osteoclast activation. Nat Med.
15:259–266. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Galaris D and Pantopoulos K: Oxidative
stress and iron homeostasis: mechanistic and health aspects. Crit
Rev Clin Lab Sci. 45:1–23. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Andrews M and Arredondo M: Hepatic and
adipocyte cells respond differentially to iron overload, hypoxic
and inflammatory challenge. Biometals. 25:749–759. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Lean JM, Davies JT, Fuller K, Jagger CJ,
Kirstein B, Partington GA, Urry ZL and Chambers TJ: A crucial role
for thiol antioxidants in estrogen-deficiency bone loss. J Clin
Invest. 112:915–923. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Jagger CJ, Lean JM, Davies JT and Chambers
TJ: Tumor necrosis factor-alpha mediates osteopenia caused by
depletion of antioxidants. Endocrinology. 146:113–118. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Fuller K, Murphy C, Kirstein B, Fox SW and
Chambers TJ: TNFalpha potently activates osteoclasts, through a
direct action independent of and strongly synergistic with RANKL.
Endocrinology. 143:1108–1118. 2002.PubMed/NCBI
|
|
17.
|
Lam J, Takeshita S, Barker JE, Kanagawa O,
Ross FP and Teitelbaum SL: TNF-alpha induces osteoclastogenesis by
direct stimulation of macrophages exposed to permissive levels of
RANK ligand. J Clin Invest. 106:1481–1488. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Mattila P, Knuuttila M, Kovanen V and
Svanberg M: Improved bone biomechanical properties in rats after
oral xylitol administration. Calcif Tissue Int. 64:340–344. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Zhang L, Liu Y, Wang D, Zhao X, Qiu Z, Ji
H and Rong H: Bone biomechanical and histomorphometrical investment
in type 2 diabetic Goto-Kakizaki rats. Acta Diabetol. 46:119–126.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Niu YB, Li YH, Kong XH, Zhang R, Sun Y, Li
Q, Li C, Liu L, Wang J and Mei QB: The beneficial effect of Radix
Dipsaci total saponins on bone metabolism in vitro and in vivo and
the possible mechanisms of action. Osteoporos Int. Apr 26–2012,
(Epub ahead of print). View Article : Google Scholar
|
|
21.
|
Rubin C, Turner AS, Mallinckrodt C, Jerome
C, McLeod K and Bain S: Mechanical strain, induced noninvasively in
the high-frequency domain, is anabolic to cancellous bone, but not
cortical bone. Bone. 30:445–452. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Blair HC and Zaidi M: Osteoclastic
differentiation and function regulated by old and new pathways. Rev
Endocr Metab Disord. 7:23–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Zhao B, Grimes SN, Li S, Hu X and Ivashkiv
LB: TNF-induced osteoclastogenesis and inflammatory bone resorption
are inhibited by transcription factor RBP-J. J Exp Med.
209:319–334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Vattakuzhi Y, Abraham SM, Freidin A, Clark
AR and Horwood NJ: Dual specificity phosphatase 1 null mice exhibit
spontaneous osteolytic disease and enhanced inflammatory osteolysis
in experimental arthritis. Arthritis Rheum. 64:2201–2210. 2012.
View Article : Google Scholar
|