Introduction
Since the identification of Helicobacter
pylori (H. pylori) in 1983 (1), the diagnosis and treatment of upper
gastrointestinal diseases, such as gastritis, peptic ulcer, gastric
carcinoma and mucosa-associated lymphoid tissue (MALT) lymphoma,
have undergone changes (2–7). The condition of H.
pylori-infected gastric mucosa is characterized as acute or
chronic inflammation, mucosal atrophy and intestinal metaplasia
(8–10). The endoscopic features of H.
pylori-induced gastritis include erythema and erosions, neither
of which are specific. Atrophic gastritis and intestinal metaplasia
are also observed secondary to H. pylori infection, but
these findings are not easy to correctly diagnose by conventional
endoscopy. In the present study, H. pylori infection was
evaluated in atrophic gastritis and its endoscopic features to
determine whether H. pylori-infected gastric mucosa can be
diagnosed through an endoscopically superficial vascular
network.
Materials and methods
Patients
This study was performed according to the principles
of the Declaration of Helsinki. Upper gastrointestinal tract
endoscopy was performed on 723 patients (510 males and 213
females), who had been screened for H. pylori infection
during the past one year at Katake and Fujio Clinic, Hyogo, Japan.
Any patients who had undergone H. pylori eradication therapy
were excluded. The patients provided written informed consent for
participation in the study. For the ethical procedure, linkable
anonymizing method was used to ensure study blindness was
maintained. Samples used in this study comprised materials for
biopsy obtained for diagnosis or treatment and not for research.
Medical disadvantage or risk of the patients did not increase by
patient participation in this study and was obtained strictly for
analysis of information as part of therapeutic intervention.
H. pylori infection status
The H. pylori status was determined in
patients who were subjected to a combined serological test and/or
histopathological examination. Serum samples were also tested for
total H. pylori antibodies using the Pyloriset Dry (Orion
Diagnostica, Espoo, Finland) latex agglutination test. Multiple
gastric biopsy specimens were removed for histopathological
examination (11,12). To detect H. pylori, the
samples were stained with hematoxylin and eosin, together with any
accompanying special stains (Giemsa, Warthin-Starry) and
immunohistochemical stains (Fig.
1). The biopsies were examined independently by two
pathologists (K.I. and T.F.) who were unaware of the serological
H. pylori status. If the serological test and/or
histopathological examination results of H. pylori were
positive, patients were diagnosed as being infected with H.
pylori.
Observation by endoscopy
Endoscopic observation using high-resolution
electronic endoscopy with an endoscopic video information system
(Olympus Optical Co., Ltd., Tokyo, Japan and Fujifilm Corporation,
Saitama, Japan) was carried out by two endoscopists (Y.K. and C.F.)
who were unaware of the serological H. pylori status. The
patients were closely observed after undergoing routine endoscopic
examination. An endoscopic atrophic border was regarded as present
or absent according to the Kimura-Takemoto classification (13). Following conventional endoscopy, the
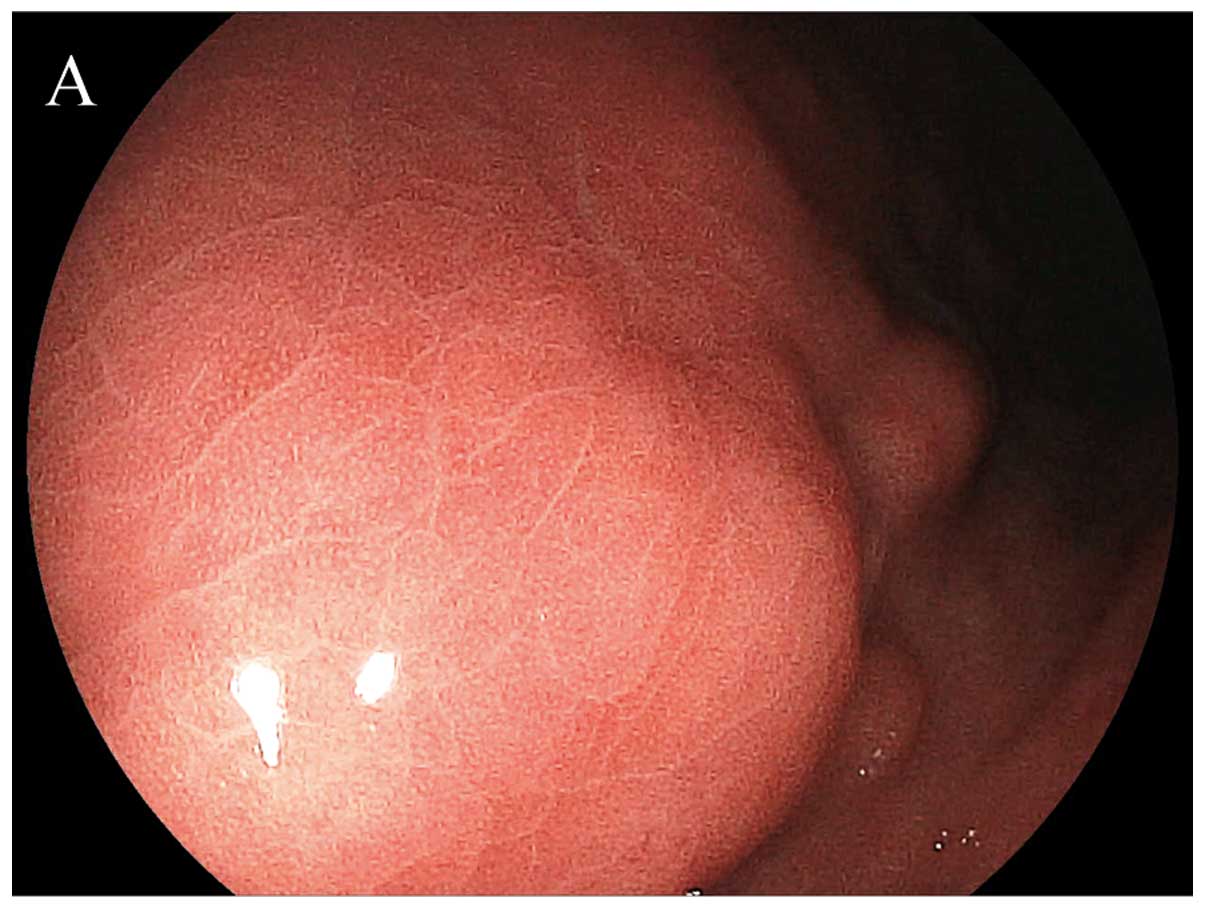
observed morphology of the capillary network structure was divided
into two patterns: RAC, regular arrangement of collecting venules
(Fig. 2) and IRAC, irregular
arrangement of collecting venules (Fig.
3) (14). A RAC pattern was
defined as numerous minute red points of similar size present at
regular intervals throughout the viewing area. By contrast, an IRAC
pattern was defined as an irregular or absent distribution of red
points.
Statistical analysis
The sensitivity, specificity, positive and negative
predictive values, likelihood ratios and accuracy were calculated
with standard formulas (15).
Results
H. pylori infection and endoscopic
atrophic border
The H. pylori infection rates were 95.4%
(455/477) in the group that had an endoscopic atrophic border [mean
age ± standard deviation (SD), 57.3±12.4 years] and 22.3% (55/246)
in the group without an endoscopic atrophic border (mean age ± SD,
42.6±11.8). In the diagnostic validity check, presence of an
endoscopic atrophic border had a sensitivity of 89.2 and a
specificity of 89.7%. The positive predictive value was 95.4, while
the negative predictive value was 77.6%. The positive likelihood
ratio was 8.638, while the negative likelihood ratio 0.120. The
accuracy was found to be 89.3% (Table
I).
 | Table I.Correlation between Helicobacter
pylori infection and endoscopic atrophic border. |
Table I.
Correlation between Helicobacter
pylori infection and endoscopic atrophic border.
| Helicobacter
pylori infection
| |
|---|
| Positive | Negative | Total |
|---|
| Atrophic border | | | |
| Present | 455 | 22 | 477 |
| Absent | 55 | 191 | 246 |
| Total | 510 | 213 | 723 |
H. pylori infection and capillary network
patterns
The H. pylori infection rates were 95.5%
(506/530) in the IRAC group (mean age ± SD, 56.2±13.2) and 2.1%
(4/193) in the RAC group (mean age ± SD, 48.9±12.9). In the
diagnostic validity check, IRAC had a sensitivity of 99.2% and a
specificity of 88.7%. The positive predictive value was 95.5%,
while the negative predictive value was 97.9%. The positive
likelihood ratio was 8.805, while the negative likelihood ratio was
0.009. The accuracy was found to be 96.1% (Table II).
 | Table II.Correlation between Helicobacter
pylori infection and capillary network patterns. |
Table II.
Correlation between Helicobacter
pylori infection and capillary network patterns.
| Helicobacter
pylori infection
| |
|---|
| Positive | Negative | Total |
|---|
| IRAC | 506 | 24 | 530 |
| RAC | 4 | 189 | 193 |
| Total | 510 | 213 | 723 |
Discussion
It would be useful to diagnose H. pylori
status on the basis of endoscopic appearance alone in patients with
H. pylori-related diseases such as gastritis, peptic ulcer,
gastric carcinoma and MALT lymphoma (2–7). There
has been some debate over whether H. pylori status can be
diagnosed by endoscopy before biopsies and serological tests are
performed (16–18). Previous studies have demonstrated
that the extent of atrophic gastritis is a valuable endoscopic
finding that helps in the diagnosis of H. pylori infection
(8–10). Previously, Yagi et
al(14,19) reported that RAC in the gastric
corpus seen by close observation essentially excluded H.
pylori infection. There were also several reports that
supported their seminal study (20–24).
Moreover, it has been reported that magnifying narrow-band imaging
(NBI) is useful for predicting H. pylori infection (25).
H. pylori infection can be diagnosed by two
main methods. Invasive tests that require endoscopy and
non-invasive or minimally invasive tests that do not require
endoscopy. The invasive tests include rapid urease tests, culture,
histopathological examination including immunohistochemistry and
polymerase chain reaction (PCR)-based methods, while the
non-invasive tests include serology, H. pylori stool antigen
test and urea breath test. With the exception of the PCR-based
methods, these tests were recommended for the diagnosis of H.
pylori infection prior and subsequent to eradication therapy in
the guidelines for the management of H. pylori infection in
Japan (26). However, the
guidelines did not include a description of endoscopic findings
that may be helpful in diagnosing H. pylori infection. The
rapid urease test and histopathological examination with biopsy are
accurate methods for identifying H. pylori. However, these
methods are more invasive and expensive tests as compared to
endoscopy without biopsy.
In this study, we confirmed that there was good
agreement between endoscopic findings and H. pylori status.
Our results indicate that the presence of an endoscopic atrophic
border and IRAC pattern were significant indicators of an H.
pylori-infected gastric mucosa. Thus, these findings are the
most reliable criteria for the diagnosis of H. pylori
infection. The absence of an endoscopic atrophic border and/or the
presence of a RAC pattern suggests that in such cases biopsy for
histopathological examination and rapid urease test to detect H.
pylori infection would be unnecessary. Therefore, we believe
that H. pylori screening by endoscopic examination without
biopsy is an excellent test of high diagnostic accuracy and
cost-effectiveness.
In conclusion, the presence of an endoscopic
atrophic border and IRAC are highly indicative of an H.
pylori-infected gastric mucosa.
Addendum
This article is based on a study first reported in
the Stomach and Intestine 2002; 37: 331–336 (Japanese paper with
English abstract, non-inclusion of MEDLINE). Since the definition
of regular arrangement of collecting venules (RAC) was not clearly
determined in the first report, it was expressed as ‘red spot
pattern’. Therefore, we performed a re-design of this study, and
attempted a secondary publication in English according to
conditions for acceptable secondary publications as stated in
Uniform Requirements for Manuscripts Submitted to Biomedical
Journals (International Committee of Medical Journal Editors).
Additionally, the results of the re-analysis, including additional
cases from the Fujio clinic, were similar to those in the first
report. Therefore, a secondary version of the initial report
includes content that faithfully reflects the data of the primary
version, and is not a ‘meat-expander’ article.
Duplicate publication has been an issue for debate
worldwide. However, the importance of secondary publications has
been suggested, mainly in Northern Europe. The World Medical
Association has adopted the Declaration of Helsinki with regard to
the ethics of research. As to the ethics of publication, however,
the conditions for acceptable secondary publications are described
in the Uniform Requirements for Manuscripts Submitted to Biomedical
Journals: Writing and Editing for Biomedical Publication Updated
April 2010 (http://www.icmje.org/) as follows: i)
the authors have received approval from the editors of both
journals (the editor concerned with the secondary publication is
required to have a photocopy, reprint, or manuscript of the primary
version), ii) the priority of the primary publication is respected
by a publication interval of at least 1 week (unless specifically
negotiated otherwise by both editors), iii) the paper for secondary
publication is intended for a different group of readers; an
abbreviated version is regarded as sufficient, iv) the secondary
version faithfully reflects the data and interpretations of the
primary version, v) the footnote on the title page of the secondary
version informs readers, peers, and documenting agencies that the
paper has been published in whole or in part and states the primary
reference. A suitable footnote might read: ‘This article is based
on a study first reported in the (title of journal, with full
reference).’ Permission for such secondary publication should be
free of charge: vi) the title of the secondary publication should
indicate that it is a secondary publication (complete
republication, abridged republication, complete translation or
abridged translation) of a primary publication. Of note, the
National Library of Medicine (NLM) does not consider translations
to be ‘republications’ and does not cite or index translations when
the original article was published in a journal that is indexed in
MEDLINE, vii) editors of journals that simultaneously publish in
multiple languages should understand that NLM indexes the primary
language version. When the full text of an article appears in more
than one language in a journal issue (such as Canadian journals
with the article in both English and French), both languages are
indicated in the MEDLINE citation. These conditions have been
accepted widely in academic journals and they would also be adopted
in this journal. This report clarified conditions for acceptable
secondary publication in this journal. Significance of secondary
publication should be considered from an international perspective
according to the rule previously described, instead of the manner
as in the proverb: ‘A scalded cat fears cold water’, in order for a
study to be appraised internationally.
Acknowledgements
The authors would like to thank Dr
Ichiro Hirata (Department of Gastroenterology, Fujita Health
University Hospital, Aichi, Japan), Dr Chikao Shimamoto and Dr
Kenichi Katsu (Second Department of Internal Medicine Osaka Medical
College, Osaka, Japan) for their help in the first Japanese study.
In addition, we thank Chiaki Matsuyama, Ayako Shimizu, Midori
Katayama, Atsuko Kikuchi and Shizuka Kidachi (Department of
Surgical and Molecular Pathology, Dokkyo University School of
Medicine, Tochigi, Japan) for their excellent technical and
secretarial assistance.
References
|
1.
|
Marshall B and Warren JR: Unidentified
curved bacilli in the stomach of patients with gastritis and peptic
ulceration. Lancet. 1:1311–1315. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
NIH consensus conference. Helicobacter
pylori in peptic ulcer disease. NIH consensus development panel
on Helicobacter pylori in peptic ulcer disease. JAMA.
272:65–69. 1994.
|
|
3.
|
Uemura N, Okamoto S, Yamamoto S, et al:
Helicobacter pylori infection and the development of gastric
cancer. N Engl J Med. 345:784–789. 2001. View Article : Google Scholar
|
|
4.
|
Parsonnet J, Friedman GD, Vandersteen DP,
et al: Helicobacter pylori infection and the risk of gastric
carcinoma. N Engl J Med. 325:1127–1131. 1991. View Article : Google Scholar
|
|
5.
|
Huang JQ, Sridhar S, Chen Y and Hunt RH:
Meta-analysis of the relationship between Helicobacter
pylori seropositivity and gastric cancer. Gastroenterology.
114:1169–1179. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Blaser MJ and Parsonnet J: Parasitism by
the ‘slow’ bacterium Helicobacter pylori leads to altered
gastric homeostasis and neoplasia. J Clin Invest. 94:4–8. 1994.
|
|
7.
|
Wotherspoon AC, Doglioni C, Diss TC, et
al: Regression of primary low-grade B-cell gastric lymphoma of
mucosa-associated lymphoid tissue type after eradication of
Helicobacter pylori. Lancet. 342:575–577. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Mihara M, Haruma K, Kamada T, et al: The
role of endoscopic findings for the diagnosis of Helicobacter
pylori infection: evaluation in a country with high prevalence
of atrophic gastritis. Helicobacter. 4:40–48. 1999.PubMed/NCBI
|
|
9.
|
Satoh K, Kimura K, Taniguchi Y, et al:
Distribution of inflammation and atrophy in the stomach of
Helicobacter pylori-positive and -negative patients with
chronic gastritis. Am J Gastroenterol. 91:963–969. 1996.PubMed/NCBI
|
|
10.
|
Kawaguchi H, Haruma K, Komoto K, et al:
Helicobacter pylori infection is the major risk factor for
atrophic gastritis. Am J Gastroenterol. 91:959–962. 1996.
|
|
11.
|
Ishida M, Terano A, Tabuchi M, et al: The
comparison with rapid urease test and histological examination in
detection of Helicobacter-pylori. Gastroenterol Endosco.
40:773–778. 1998.
|
|
12.
|
Smith SB, Snow AN, Perry RL and Qasem SA:
Helicobacter pylori: to stain or not to stain? Am J Clin
Pathol. 137:733–838. 2012. View Article : Google Scholar
|
|
13.
|
Kimura K and Takemoto T: An endoscopic
recognition of the atrophic border and its significance in chronic
gastritis. Endoscopy. 3:87–97. 1969. View Article : Google Scholar
|
|
14.
|
Yagi K, Nakamura A and Sekine A:
Characteristic endoscopic and magnified endoscopic findings in the
normal stomach without Helicobacter pylori infection. J
Gastroenterol Hepatol. 17:39–45. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Tanaka H, Ichikawa K, Fujimori T, et al:
Diagnostic validity of DNMT-1 and 3b immunoreactivity in
non-neoplastic epithelium of UC patients with and without
neoplasia. DJMS. 39:29–35. 2012.
|
|
16.
|
Laine L, Cohen H, Sloane R, et al:
Interobserver agreement and predictivevalue of endoscopic findings
for H. pylori and gastritis in normal volunteers.
Gastrointest Endosc. 42:420–423. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Khakoo SI, Lobo AJ, Shepherd NA and
Wilkinson SP: Histological assessment of the Sydney classification
of endoscopic gastritis. Gut. 35:1172–1175. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Bah A, Saraga E, Armstrong D, et al:
Endoscopic features of Helicobacter pylori-related
gastritis. Endoscopy. 27:593–596. 1995.
|
|
19.
|
Yagi K, Aruga Y, Nakamura A and Sekine A:
Regular arrangement of collecting venules (RAC): a characteristic
endoscopic feature of Helicobacter pylori-negative normal
stomach and its relationship with esophago-gastric adenocarcinoma.
J Gastroenterol. 40:443–452. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Nakayama Y, Horiuchi A, Kumagai T, et al:
Discrimination of normal gastric mucosa from Helicobacter
pylori gastritis using standard endoscopes and a single
observation site: studies in children and young adults.
Helicobacter. 9:95–99. 2004.PubMed/NCBI
|
|
21.
|
Anagnostopoulos GK, Yao K, Kaye P, et al:
High-resolution magnification endoscopy can reliably identify
normal gastric mucosa, Helicobacter pylori-associated
gastritis, and gastric atrophy. Endoscopy. 39:202–207. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Machado RS, Viriato A, Kawakami E and
Patricio FR: The regular arrangement of collecting venules pattern
evaluated by standard endoscope and the absence of antrum
nodularity are highly indicative of Helicobacter pylori
uninfected gastric mucosa. Dig Liver Dis. 40:68–72. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Gonen C, Simsek I, Sarioglu S and Akpinar
H: Comparison of high resolution magnifying endoscopy and standard
videoendoscopy for the diagnosis of Helicobacter pylori
gastritis in routine clinical practice: a prospective study.
Helicobacter. 14:12–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Alaboudy A, Elbahrawy A, Matsumoto S, et
al: Regular arrangement of collecting venules: Does patient age
affect its accuracy? World J Gastrointest Endosc. 3:118–123. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Okubo M, Tahara T, Shibata T, et al:
Usefulness of magnifying narrow-band imaging endoscopy in the
Helicobacter pylori-related chronic gastritis. Digestion.
83:161–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Asaka M, Kato M, Takahashi S, et al:
Guidelines for the management of Helicobacter pylori
infection in Japan: 2009 revised edition. Helicobacter. 15:1–20.
2010.
|