Introduction
Androgen deprivation therapy is one of the standard
treatments for prostate cancer, since the growth and progression of
prostate cancer are primarily androgen-dependent (1). However, the progression of an
androgen-dependent prostate cancer to an androgen-independent state
is a well-established phenomenon (2). Zinc content, which is known to be
higher in the prostate compared to any other tissue, has been found
to be significantly lower in androgen-independent prostate cancers
compared to androgen-dependent ones (3,4). We
previously reported that androgen-insensitive AIDL cells, which
were established by long-term culture of androgen-sensitive LNCaP
cells in a hormone-deprived medium, exhibited a significantly lower
zinc content compared to LNCaP cells (5).
Metallothioneins (MTs) and zinc transporters
function to maintain cellular zinc homeostasis. MTs are small
metal-binding proteins that exist as four isoforms in mammals.
Metallothionein (MT)1 and MT2 are major isoforms expressed in the
majority of tissues, whereas MT3 and MT4 are minor isoforms
normally found in a limited number of tissues (6). The MT3 protein was first isolated as a
growth-inhibiting factor from brain neurons and was found to be
expressed at high levels in prostate cancer tissues (7). Zinc transporters are classified as the
ZnT and ZIP families, belonging to the solute-linked carrier (SLC)
gene families SLC30 and SLC39, respectively.
SLC30A1 and SLC30A4 expression was reported to be
decreased in patients with prostate cancer compared to those with
benign prostatic hyperplasia (8,9).
SLC39A1 and SLC39A2 expression in prostate tumor
tissues obtained from African-American patients was lower compared
to that in Caucasian patients (10).
Since zinc levels in the prostate are known to
decrease with the progression of prostate cancer during androgen
deprivation therapy, we hypothesized that zinc exerts a
preventative effect on cancer progression. We previously reported
that zinc suppressed the invasiveness of prostate cancer cells and
increased the sensitivity of cells to cytotoxic agents (11–13).
Therefore, we investigated the effect of androgen on the gene
expression of MTs and zinc transporters to elucidate the regulation
of zinc levels by androgen in LNCaP cells and demonstrated that
MT3 expression is downregulated by androgen.
Materials and methods
Materials
Dihydrotestosterone (DHT) was purchased from Wako
Pure Chemical Industries, Ltd. (Osaka, Japan). Bicalutamide was
obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Charcoal-stripped fetal bovine serum (CS-FBS) was obtained from
Invitrogen (Carlsbad, CA, USA). Phenol red-free RPMI-1640 medium
was purchased from Sigma-Aldrich (St. Louis, MO, USA). All other
chemicals were of analytical grade.
Cell culture
LNCaP human prostatic carcinoma cells and PC-3 cells
were obtained from American Type Culture Collection (Rockville, MD,
USA). Androgen-low-sensitive LNCaP-E9 cells have been previously
described (14). Cells were
cultured in RPMI-1640 medium containing 10% fetal bovine serum
(FBS) in a humidified atmosphere with 5% CO2 at
37°C.
Quantitative real-time reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen) and first-strand complementary DNA was synthesized
from 5 μg of total RNA using SuperScript III (Invitrogen) as
previously described (14).
Real-time monitoring of PCR reactions was performed using the
Thermal Cycler Dice Real-Time system (Takara, Otsu, Japan) with the
SYBR Premix Ex Taq (Takara). At the end of the reaction, a
dissociation curve analysis was performed to assess the specificity
of the product. PCR was performed under the following conditions:
35 cycles of 30 sec at 95°C, 30 sec at 58°C and 30 sec at 72°C for
MT1A, MT2A and MT3; 35 cycles of 30 sec at
95°C, 20 sec at 58°C and 30 sec at 72°C for MT1G and
MT1X; 35 cycles of 60 sec at 94°C, 60 sec at 55°C and 60 sec
at 72°C for SLC30A1 and SLC39A1; 35 cycles of 30 sec
at 95°C, 30 sec at 61°C and 30 sec at 72°C for SLC30A2; and
35 cycles of 10 sec at 95°C and 20 sec at 60°C for glyceraldehyde
3-phosphate dehydrogenase (GAPDH). GAPDH, a
housekeeping gene, was used for normalization of target mRNA
expression. The primers used in this study are listed in Table I.
 | Table I.Primer sequences designed for
real-time reverse transcription-polymerase chain reaction. |
Table I.
Primer sequences designed for
real-time reverse transcription-polymerase chain reaction.
| Gene name | Sequence |
|---|
| GAPDH | |
| Sense |
5′-CCAGCAAGAGCACAAGAGGA-3′ |
| Antisense |
5′-GCAACTGTGAGGAGGGGAGA-3′ |
| SLC30A1 | |
| Sense |
5′-TGTGAACTTGCCTGCAGAAC-3′ |
| Antisense |
5′-GCTTTAGTCCTCCTGGGCTT-3′ |
| SLC30A2 | |
| Sense |
5′-AGATGCAAGAGGGGAAACCT-3′ |
| Antisense |
5′-TGGCAGAGACAGACCTTGTG-3′ |
| SLC39A1 | |
| Sense |
5′-GTCCTGGTGATGGAGCAGAT-3′ |
| Antisense |
5′-CCGATGCCTAGAGGTGTCAT-3′ |
| MT1A | |
| Sense |
5′-CTCGAAATGGACCCCAACT-3′ |
| Antisense |
5′-ATATCTTCGAGCAGGGCTGTC-3′ |
| MT1G | |
| Sense |
5′-GCCAGCTCCTGCAAGTGCAA-3′ |
| Antisense |
5′-TCTCCGATGCCCCTTTGCAG-3′ |
| MT1X | |
| Sense |
5′-TCTCCTTGCCTCGAAATGGAC-3′ |
| Antisense |
5′-GGGGCACACTTGGCACAGC-3′ |
| MT2A | |
| Sense |
5′-CCGACTCTAGCCGCCTCTT-3′ |
| Antisense |
5′-GTGGAAGTCGCGTTCTTTACA-3′ |
| MT3 | |
| Sense |
5′-GCTGAGGCAGAAGCAGAGAAG-3′ |
| Antisense |
5′-TCATTCCTCCAAGGTCAGCA-3′ |
Plasmid construction
Genomic DNA from LNCaP cells was extracted using
TRIzol reagent (Invitrogen) according to the manufacturer's
instructions. The 5′-flanking region of the human MT3 gene
between −905 and +285 bp was amplified by PCR from genomic DNA
using primers containing restriction sites for KpnI and
NheI, respectively. The sequences of the primers were as
follows: sense, 5′-ACGGTACCTACTGCAGCCTCCTCAACCT-3′; anti-sense,
5′-ACGCTAGCGCTTCTCCAAGCAACTGGAC-3′. The fragment was ligated to a
pGL3-basic firefly luciferase reporter vector (Promega, Madison,
WI, USA).
Luciferase assay
LNCaP cells were seeded at a density of
2×105 cells/well into a 24-well culture plate (Nalge
Nunc International, Rochester, NY, USA). After 24 h, the medium was
changed to phenol red-free RPMI-1640 supplemented with DHT and/or
bicalutamide. Cells were then cotransfected with 0.7 μg of pGL3-MT3
(−905 to +285) firefly luciferase reporter plasmid and 0.1 μg of
Renilla luciferase plasmid pRL-CMV using
Lipofectamine® 2000 (Invitrogen), according to the
manufacturer's instructions. Forty-eight hours after transfection,
cell lysates were prepared and luciferase activities were measured
using the Dual-Luciferase Reporter Assay system (Promega). Firefly
luciferase activity was normalized to Renilla luciferase
activity.
Statistical analysis
Significance was assessed by a one-way ANOVA
followed by Dunnett's test using Prism4 software (GraphPad
Software, San Diego, CA, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
Effect of androgen on zinc transporter
and metallothionein expression in prostate cancer cells
The effect of DHT on the mRNA expression of zinc
transporters and MTs in prostate cancer cells was first assessed by
real-time RT-PCR analysis. As shown in Fig. 1, DHT decreased the expression of
MT3 in androgen-sensitive LNCaP cells in a dose-dependent
manner, but did not affect SLC30A1, SLC30A2, SLC39A1,
MT1A, MT1G, MT1X or MT2A expression. In
PC-3 cells lacking androgen receptors, DHT exerted no effect on the
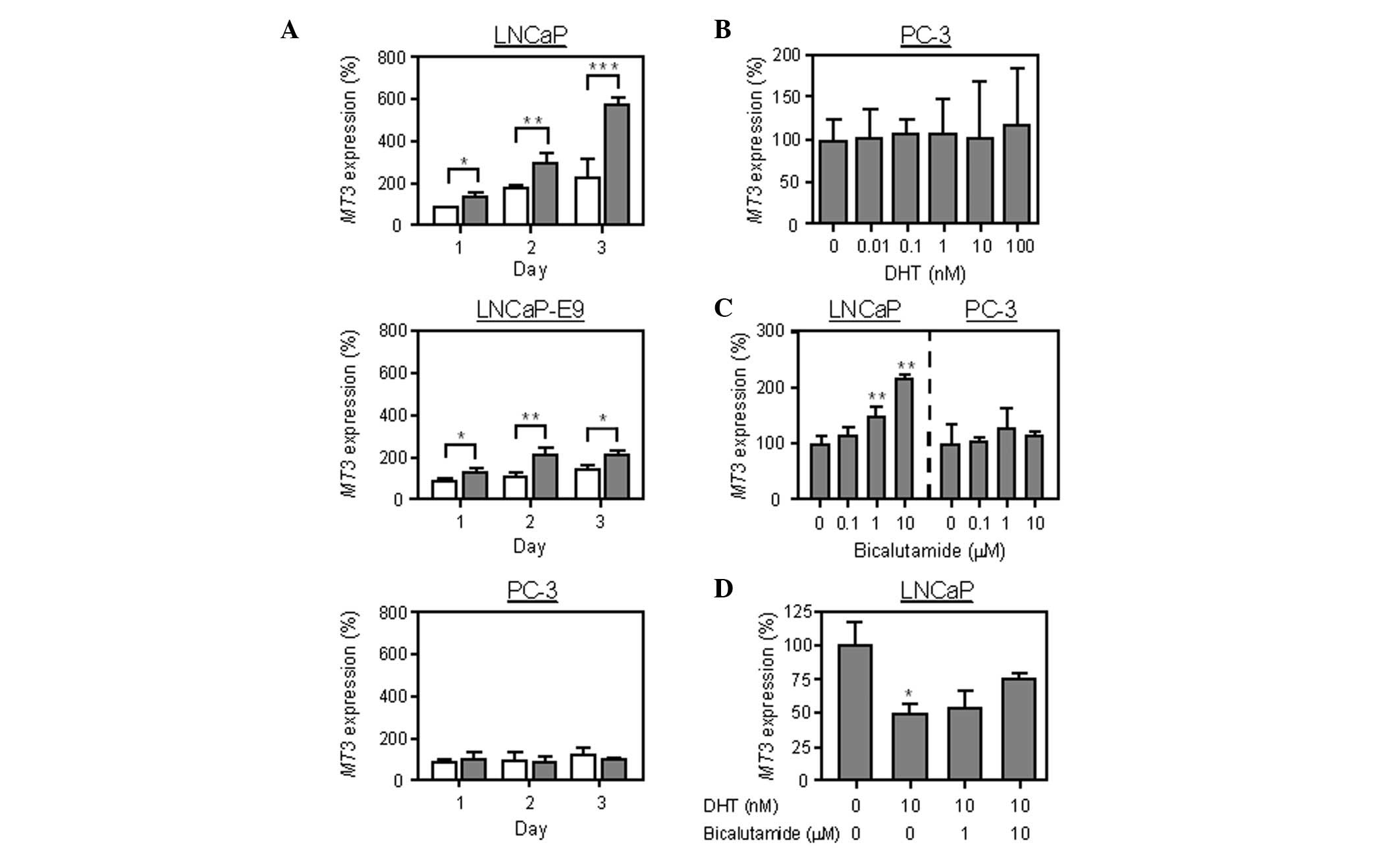
expression of MT3 (Fig. 2B).
In addition, MT3 expression in LNCaP cells was markedly
higher with the medium containing androgen-deficient CS-FBS
compared to the normal FBS (Fig.
2A). The MT3 expression in androgen-low-sensitive
LNCaP-E9 cells was significantly higher in the medium containing
CS-FBS compared to the normal FBS; however, the expression was less
pronounced compared to that in LNCaP cells. There were no
differences in MT3 expression between PC-3 cells in the
medium containing FBS and those in CS-FBS. The effect of
bicalutamide, an androgen receptor antagonist, on MT3
expression was then examined. Bicalutamide increased the mRNA
expression in LNCaP cells, whereas it exerted no effect on PC-3
cells (Fig. 2C). Moreover, the
suppression of MT3 expression triggered by DHT was inhibited
in the presence of bicalutamide (Fig.
2D). These results suggest that MT3 expression is
downregulated by androgen.
Androgen regulates MT3 transcriptional
activity in LNCaP cells
The luciferase reporter assay was then performed
using MT3 reporter constructs, to determine whether the
downregulation of MT3 expression by androgen is due to
transcriptional regulation. As shown in Fig. 3, DHT decreased luciferase activity
in LNCaP cells and this decrease was reversed by bicalutamide.
Taken together, these results suggest that MT3 expression
may be regulated by androgen transcriptional control in LNCaP
cells.
Discussion
In this study, we demonstrated that MT3
expression was regulated by androgen in prostate cancer cells.
Although DHT decreased MT3 expression in androgen-sensitive
LNCaP cells, bicalutamide exerted the opposite effect. The
induction of MT3 expression by androgen withdrawal was lower
in androgen-low-sensitive LNCaP-E9 cells compared to that in LNCaP
cells. In addition, no change was observed in MT3 expression
following androgen withdrawal or treatment with DHT or bicalutamide
in PC-3 cells lacking androgen receptors. These results suggest
that the expression of MT3 in LNCaP cells is downregulated
by androgen. The androgen receptor binds to specific DNA motifs
known as androgen-response elements (AREs) in the regulatory
regions of target genes. AREs comprise two 6-bp elements separated
by a 3-bp spacer, 5′-AGAACAnnnTGTTCT-3′ (15). The sequence (5′-TCTTGTnnnACAGGC-3′),
which is similar to the reverse sequence of ARE, is found upstream
in the MT3 gene at the position −871 to −857.
Previous studies suggested that the regulation of
MT3 expression appears to be complicated due to cell-type
specificity and differences between species (7,16–18).
Specifically, MT3 expression was observed to be upregulated
by androgen in rodent tissues (17,19).
In a previous study, we reported that MT3 expression in the
lateral lobe of the rat prostate was decreased following castration
(20), a finding inconsistent with
our present results, according to which MT3 mRNA expression
in LNCaP cells is downregulated by androgen. Therefore, the
5′-flanking region of the MT3 gene was compared between rats
and humans to investigate the opposite regulation of MT3
expression in these species. ARE and the reverse sequence of ARE
were not found within 3 kb of the 5′-upstream region of the
MT3 gene in rats, although the sequence exists in this 3-kb
region in humans. The existence of ARE and the reverse sequence of
ARE may be responsible for the differences in androgen-regulated
MT3 expression between the two species.
The mechanisms underlying the transition to the
aggressive phenotype following androgen deprivation therapy have
yet to be elucidated. However, one of the reasons considered is the
altered gene expression associated with alterations in the tumor
malignant potential triggered by androgen withdrawal. In this
study, we demonstrated that MT3 expression was downregulated
by androgen in LNCaP cells. MT3 has been shown to be involved in
growth inhibition, prevention of hypoxic damage and chemotherapy
resistance (21–23). These results suggest that the
androgenic regulation of MT3 expression may be the mechanism
underlying the progression of prostate cancer.
References
|
1
|
Huggins C and Hodges CV: Studies on
prostatic cancer. I. The effect of castration, of estrogen and of
androgen injection on serum phosphatases in metastatic carcinoma of
the prostate. Cancer Res. 1:293–297. 1941.
|
|
2
|
Emmett JL, Green LF and Papantoniou A:
Endocrine therapy in carcinoma of the prostate gland: 10-year
survival studies. J Urol. 83:471–484. 1960.
|
|
3
|
Shiina H, Igawa M and Ishibe T:
Estramustine-binding protein to dihydrotestosterone ratio in human
prostatic carcinoma: a new marker for predicting disease
progression. Br J Urol. 77:96–101. 1996. View Article : Google Scholar
|
|
4
|
Shiina H, Igawa M and Ishibe T: Clinical
study on estramustine binding protein (EMBP) in human prostate.
Prostate. 29:169–176. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iguchi K, Otsuka T, Usui S, Ishii K,
Onishi T, Sugimura Y and Hirano K: Zinc and metallothionein levels
and expression of zinc transporters in androgen-independent subline
of LNCaP cells. J Androl. 25:154–161. 2004.PubMed/NCBI
|
|
6
|
Hidalgo J, Chung R, Penkowa M and Vašák M:
Structure and function of vertebrate metallothioneins. In:
Metallothioneins and Related Chelators. Sigel A, Sigel H and Sigel
RKO: The Royal Society of Chemistry; Cambridge: pp. 279–317.
2009
|
|
7
|
Garrett SH, Sens MA, Shukla D, Nestor S,
Somji S, Todd JH and Sens DA: Metallothionein isoform 3 expression
in the human prostate and cancer-derived cell lines. Prostate.
41:196–202. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hasumi M, Suzuki K, Matsui H, Koike H, Ito
K and Yamanaka H: Regulation of metallothionein and zinc
transporter expression in human prostate cancer cells and tissues.
Cancer Lett. 200:187–195. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Henshall SM, Afar DE, Rasiah KK, Horvath
LG, Gish K, Caras I, Ramakrishnan V, Wong M, Jeffry U, Kench JG,
Quinn DI, Turner JJ, Delprado W, Lee CS, Golovsky D, Brenner PC,
O'Neill GF, Kooner R, Stricker PD, Grygiel JJ, Mack DH and
Sutherland RL: Expression of the zinc transporter ZnT4 is decreased
in the progression from early prostate disease to invasive prostate
cancer. Oncogene. 22:6005–6012. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rishi I, Baidouri H, Abbasi JA,
Bullard-Dillard R, Kajdacsy-Balla A, Pestaner JP, Skacel M, Tubbs R
and Bagasra O: Prostate cancer in African American men is
associated with downregulation of zinc transporters. Appl
Immunohistochem Mol Morphol. 11:253–260. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iguchi K, Hamatake M, Ishida R, Usami Y,
Adachi T, Yamamoto H, Koshida K, Uchibayashi T and Hirano K:
Induction of necrosis by zinc in prostate carcinoma cells and
identification of proteins increased in association with this
induction. Eur J Biochem. 253:766–770. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ishii K, Usui S, Sugimura Y, Yoshida S,
Hioki T, Tatematsu M, Yamamoto H and Hirano K: Aminopeptidase N
regulated by zinc in human prostate participates in tumor cell
invasion. Int J Cancer. 92:49–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishii K, Otsuka T, Iguchi K, Usui S,
Yamamoto H, Sugimura Y, Yoshikawa K, Hayward SW and Hirano K:
Evidence that the prostate-specific antigen
(PSA)/Zn2+axis may play a role in human prostate cancer
cell invasion. Cancer Lett. 207:79–87. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iguchi K, Ishii K, Nakano T, Otsuka T,
Usui S, Sugimura Y and Hirano K: Isolation and characterization of
LNCaP sublines differing in hormone sensitivity. J Androl.
28:670–678. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Verrijdt G, Haelens A and Claessens F:
Selective DNA recognition by the androgen receptor as a mechanism
for hormone-specific regulation of gene expression. Mol Genet
Metab. 78:175–185. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Belloso E, Hernandez J, Giralt M, Kille P
and Hidalgo J: Effect of stress on mouse and rat brain
metallothionein I and III mRNA levels. Neuroendocrinology.
64:430–439. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moffatt P and Séguin C: Expression of the
gene encoding metallothionein-3 in organs of the reproductive
system. DNA Cell Biol. 17:501–510. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei H, Desouki MM, Lin S, Xiao D, Franklin
RB and Feng P: Differential expression of metallothioneins (MTs) 1,
2, and 3 in response to zinc treatment in human prostate normal and
malignant cells and tissues. Mol Cancer. 7:72008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cyr DG, Dufresne J, Pillet S, Alfieri TJ
and Hermo L: Expression and regulation of metallothioneins in the
rat epididymis. J Androl. 22:124–135. 2001.PubMed/NCBI
|
|
20
|
Iguchi K, Morihara N, Usui S, Hayama M,
Sugimura Y and Hirano K: Castration- and aging-induced changes in
the expression of zinc transporter and metallothionein in rat
prostate. J Androl. 32:144–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dutta R, Sens DA, Somji S, Sens MA and
Garrett SH: Metallothionein isoform 3 expression inhibits cell
growth and increases drug resistance of PC-3 prostate cancer cells.
Prostate. 52:89–97. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gurel V, Sens DA, Somji S, Garrett SH,
Nath J and Sens MA: Stable transfection and overexpression of
metallothionein isoform 3 inhibits the growth of MCF-7 and Hs578T
cells but not that of T-47D or MDA-MB-231 cells. Breast Cancer Res
Treat. 80:181–191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang B, Wood IS and Trayhurn P: PCR arrays
identify metallothionein-3 as a highly hypoxia-inducible gene in
human adipocytes. Biochem Biophys Res Commun. 368:88–93. 2008.
View Article : Google Scholar : PubMed/NCBI
|