Introduction
Urticaria is a skin disorder presenting with
localized edema due to vasodilatation and increased permeability of
the small blood vessels in the mucosae and skin. The chronic form
is defined as symptoms lasting for a minimum of 6 weeks. Chronic
urticaria (CU) is characterized by recurrent, transitory, itchy
wheals, which occur daily or almost daily (1). Several conditions are associated with
allergy and autoimmune disorders, as well as urticaria, in which
the clinical symptoms may be attributed to the release of histamine
and other vasoactive mediators induced by the binding of a specific
allergen to the IgE antibodies conjugated to the mast cell surface
receptors (2,3). However, in a substantial percentage of
cases, the allergic trigger cannot be determined and the urticaria
is classified as idiopathic (CIU), whereas ~35–40% of cases appear
to be of autoimmune origin (4,5). The
inflammatory response presenting with spontaneous wheals exhibits
pro-inflammatory characteristics, involving a prominent role for
lymphocytes with a mixed Th1/Th2 response (6). It was demonstrated that IL-10
production was elevated and IL-2 reduced in CIU patients compared
to controls (7).
Certain natural products, such as the polysaccharide
nucleic acid fraction of bacillus Calmette-Guérin (BCG-PSN), may
possess immunoregulatory properties (8–10).
BCG-PSN may increase CD4+ T cells and induce Th1-type
immunity (11) and, by contrast,
restrain Th2-type immune response, switching the balance of Th1/Th2
towards the Th1 side (12–15). A recent study by Luo et
al(16) demonstrated that BCG
priming and boosting twice with the AMM vaccine induced a potent
antigen-specific interferon (IFN)-γ and IL-2 production.
Th1 and Th2 are involved in the pathogenesis of CIU,
whereas IL-2 and IL-10 are prominent cytokines secreted by Th1 and
Th2 cells, respectively. This study was designed to investigate
whether the effects of BCG-PSN on the Th1/Th2 balance, determined
by its effects on IL-2 and IL-10 production by the lymphocytes of
CIU patients, may facilitate the management of CIU.
Materials and methods
Subjects
This study included 30 CIU patients (15 males and 15
females), aged 18–60 years (average, 38.13±12 years), recruited in
The Affiliated Hospital of Chengde Medical College (Chengde,
China). All the patients met the following selection criteria: age
18–65 years, sharply defined skin wheals persisting for ≥6 weeks,
small (<1 cm) to large (>8 cm), erythematous or white wheals
with an erythematous rim, round, oval, acriform, annular or
serpiginous, due to confluence and resolution in one area and
progression in another, without definite provocation, pruritic and
transient. Patients during pregnancy or lactation, with a history
of autoimmune disease or administration of antihistamine drugs,
glucocorticosteroids or immunomodulating drugs within the 4 weeks
preceding enrollment, were excluded. The 30 controls were healthy
donors without any history of allergies.
This study was conducted in accordance with the
Declaration of Helsinki and approved by the Ethics Committee of our
hospital. All the participants provided written informed consent
prior to enrollment.
Immunocytochemistry
Human peripheral blood mononuclear lymphocytes were
collected from the venous blood of the subjects by Ficoll-Paque
gradient centrifugation. The lymphocytes were adjusted to a final
concentration of 2×106/ml in RPMI-1640 medium with 10%
fetal bovine serum. The lymphocytes in the RPMI-1640 medium (100
μl, 2×106/ml) were added to a 96-well culture plate, in
which 10 μl of phytohemagglutinin (2 mg/ml) and differently diluted
(0, 5, 10, 20, 40 and 80 μl/ml) of BCG-PSN (0.35 mg BCG
polysaccharide with ≥30 μg/l nucleic acid) had been previously
added. The cells were cultured at 37°C in a humidified atmosphere
containing 5% CO2 for 96 h. BCG-PSN was obtained from
Zhejiang Wanma Pharmaceutical Co., Ltd., Hangzhou, China.
The culture supernatants were collected for ELISA,
whereas the cells were smeared on slides and fixed with cold
acetone for 5 min. The endogenous peroxidase activity was
inactivated with 3% H2O2 for 20 min and
blocking was performed for 30 min in 10% calf serum. Cells were
incubated overnight at 4°C with the primary antibody against IL-2
(Santa Cruz Biotechnology Inc., Dallas, TX, USA) or IL-10 (Santa
Cruz Biotechnology Inc.), rinsed and incubated with the appropriate
horseradish peroxidase (HRP)-conjugated secondary antibody for 30
min at 37°C, followed by visualization with diaminobenzidine and
counterstaining with hematoxylin prior to mounting. Replacement of
the primary antibody with phosphate-buffered saline was used as
negative control. The slides were observed under a light
microscope. The expression of IL-2 and IL-10 was identified in the
cytoplasm and cell membrane as brown colour. The number of positive
cells in five separate fields was calculated with a balanced cell
number on every slide.
ELISA
The ELISA was performed using the ELISA kit
(NeoBioscience, Shenzhen, China) according to the manufacturer’s
instructions. In brief, the 96-well flat-bottom plate was precoated
with anti-human IL-2 or IL-10 antibody. The diluted standards and
samples were added to each coated well (100 μl/well) and incubated
at 36°C for 90 min. After 5 washes, biotin-conjugated anti-human
IL-2 or IL-10 antibody was added to each well and incubated at 36°C
for 60 min. After an additional 5 washes, 100 μl of HRP-conjugated
avidin was added to each well and incubated at 36°C in the dark for
30 min. The plate was washed 5 times and 100 μl of the
tetramethylbenzidine solution was added to the wells, followed by
incubation at 36°C in the dark for 15 min. The reaction was stopped
with 100 μl of 2 M sulphuric acid. The optical density was measured
at 450 nm using a microplate reader (Bio-Rad, Hercules, CA, USA).
The concentrations of IL-2 and IL-10 (pg/ml) were determined with a
standard curve derived from a known amount of the relevant
cytokines. The minimum detection level was 8 pg/ml for IL-2 and 1
pg/ml for IL-10.
Statistical analysis
The differences between the experimental and control
groups were compared with the Pearson’s Chi-square test for
categorical variables or one-way ANOVA followed by the Dunnett’s
t-test for continuous variables. The results are presented as
percentages for categorical variables, or means (± SD) for
continuous variables. P<0.05 indicated a statistically
significant difference.
Results
Effects of BCG-PSN on IL-2 production by
the lymphocytes of CIU patients
IL-2 is a prominent cytokine secreted by Th1 cells;
therefore, it may reflect Th1 function. It was demonstrated by
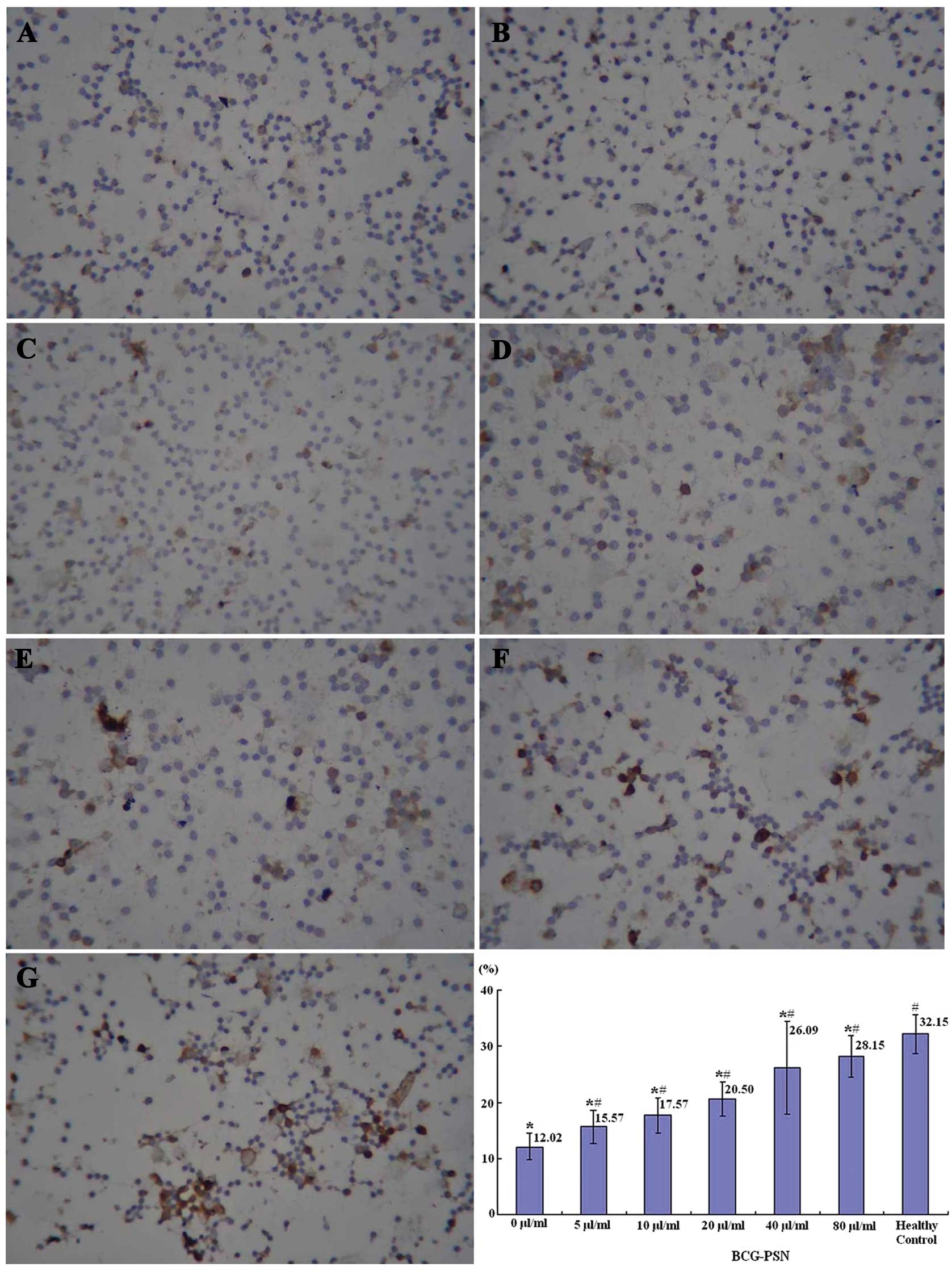
immunocytochemistry that the highest percentage of IL-2-positive
lymphocytes was observed in the healthy control group, the lowest
in the untreated CIU group (0 μg/ml BCG-PSN) and there was a
sequential increase with the elevation of the BCG-PSN concentration
(Fig. 1).
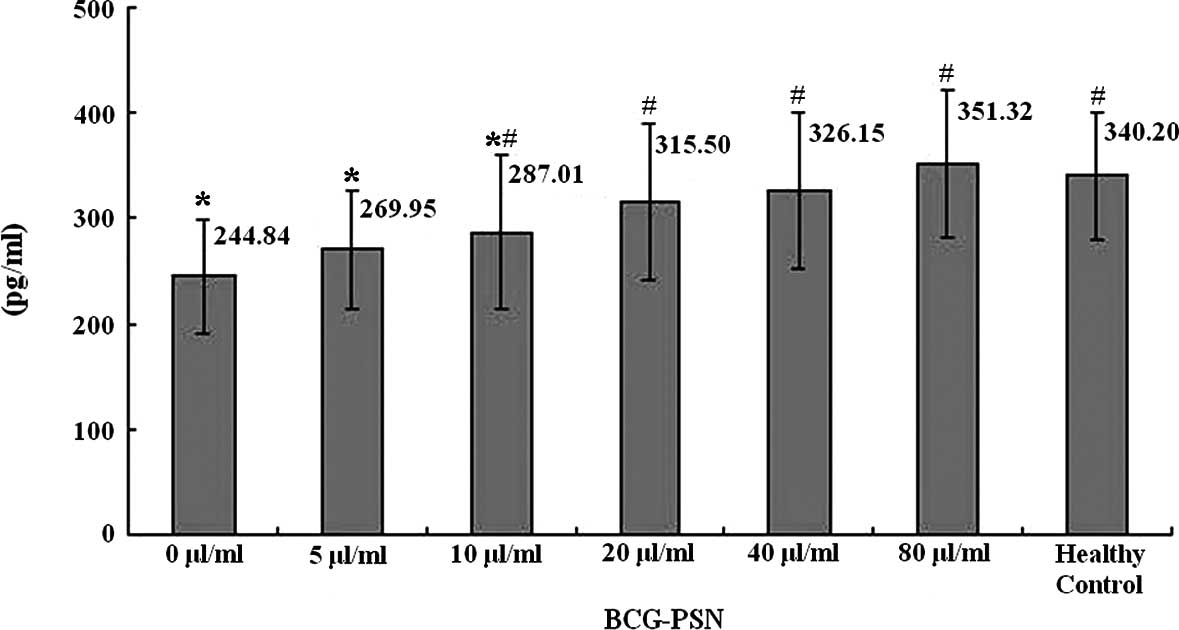
It was also demonstrated by ELISA that the levels of
IL-2 in the culture supernatant of the lymphocytes were the highest
in the healthy control group, the lowest in the untreated CIU group
(0 μg/ml BCG-PSN) and there was a sequential increase with the
elevation of the BCG-PSN concentration (Fig. 2).
Effects of BCG-PSN on IL-10 production by
the lymphocytes of CIU patients
IL-10 is a prominent cytokine secreted by Th2 cells;
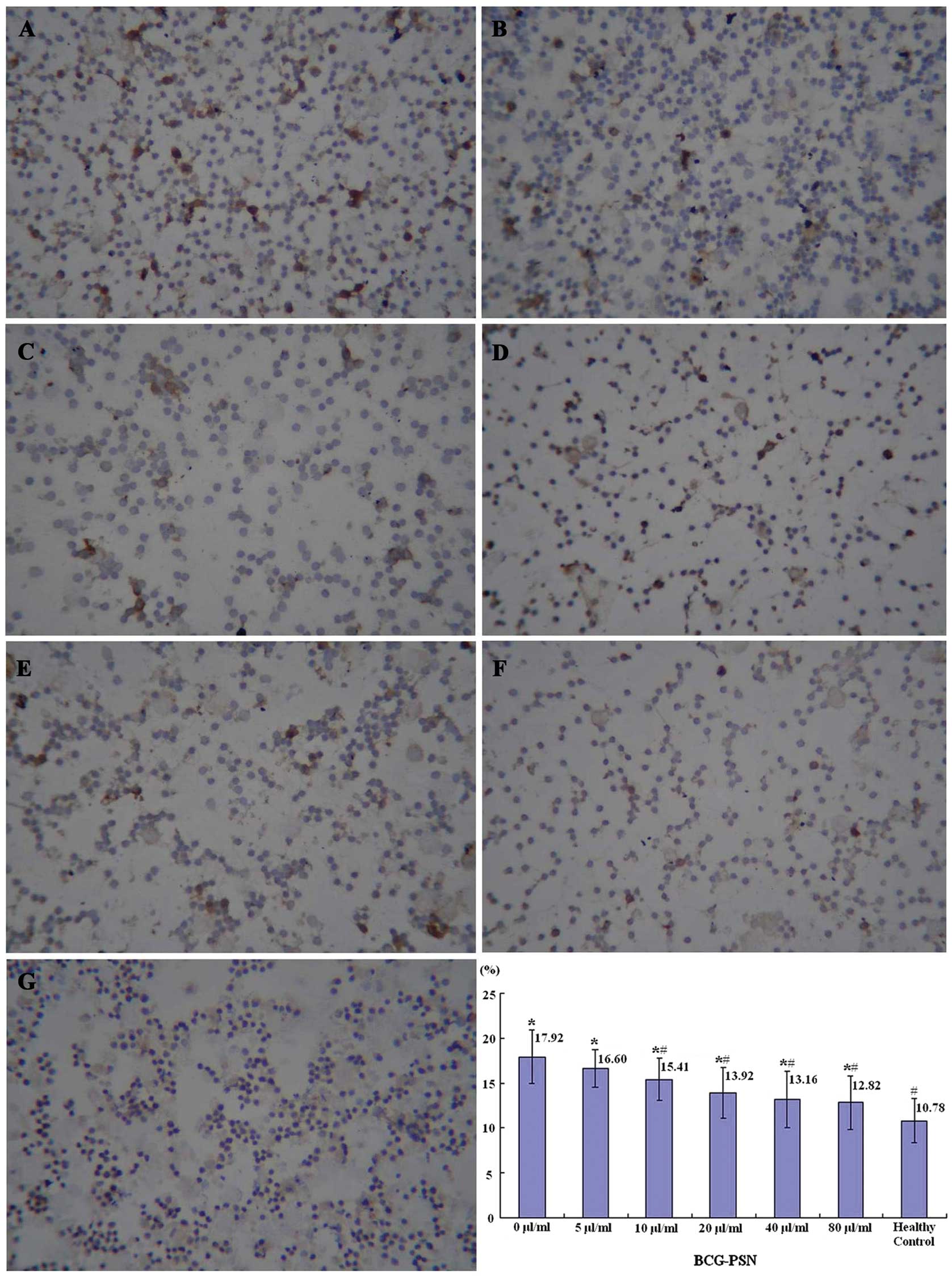
therefore, it may reflect Th2 function. It was demonstrated by
immunocytochemistry that the lowest percentage of IL-10-positive
lymphocytes was observed in the healthy control group, the highest
in the untreated CIU group (0 μg/ml BCG-PSN) and decreased
sequentially with the increase of the BCG-PSN concentration
(Fig. 3).
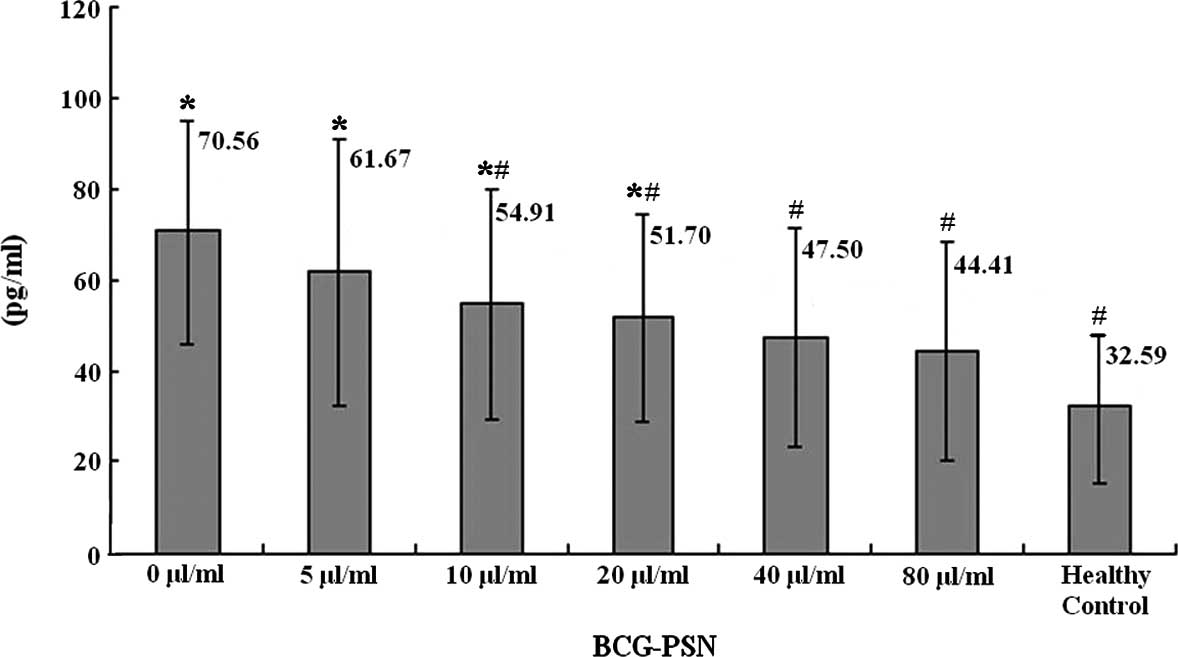
It was also demonstrated by ELISA that the levels of
IL-10 in the culture supernatant of the lymphocytes were the lowest
in the healthy control group, the highest in the untreated CIU
group (0 μg/ml BCG-PSN) and there was a sequential decrease with
the increase of the BCG-PSN concentration (Fig. 4).
Discussion
CU causes patients significant physical and mental
discomfort. CU has been defined as a daily or almost daily
occurrence of wheals and/or angioedema, occurring over a period of
≥6 weeks (17–19). Furthermore, the direct cost of CU,
in terms of healthcare visits, investigation and treatment, is high
(20). Several aetiological factors
have been associated with the onset of CU and it was suggested that
the condition has an autoimmune basis (21,22).
It was previously observed that IL-10 production was elevated and
IL-2 reduced in CIU patients compared to healthy subjects (7). Similar results were demonstrated by
the present study, suggesting that the decrease in IL-2 and
increase in IL-10 may be an important indicator of the Thl/Th2
imbalance associated with the occurrence and relapse of CIU.
Therefore, drugs that may restore the balance between the levels of
IL-2 and IL-10 may be an efficient strategy to treat patients with
CIU and prevent its recurrence.
BCG-PSN has the ability to regulate the CD4 and CD8
subsets of T cells and the Th1 and Th2 subtypes of helper T cells
(23). Following treatment with
BCG-PSN, CD4 and CD4/CD8 were significantly increased. In patients
with perennial allergic rhinitis, the cellular immunofunction was
modulated with an increase of the curative rate (11) and the levels of IL-4 in the serum of
patients with condylomata acuminata was reduced following treatment
with BCG-PSN (24). The IFN -γ and
IL-2 production may be induced by BCG (16). It may be concluded that BCG-PSN has
the ability to induce T-lymphocyte activation, IFN-γ and IL-2
production, IL-4 inhibition and Th1 promotion, thus regulating the
Th1/Th2 balance. It is important to elucidate the effect of BCG-PSN
on Th1/Th2 balance in the CU patients, due to the potential
application of BCG-PSN in the treatment of CU.
IgE has been frequently associated with CU (25). IL-4 causes a switch to IgE
production through B-cell differentiation; however, IFN-γ inhibits
that switch, preventing the production of specific IgE (26). IL-2, identified in 1976 as a T-cell
growth factor in the supernatants of activated T cells (27), may promote IFN-γ production
(28). IL-10 is known to
downregulate specific effector functions in monocytes, dendritic,
natural killer, B and Th1 cells and polymorphonuclear leukocytes
(29–31), which may result in the decreased
production of pro-inflammatory cytokines and chemokines. IL-10 may
inhibit the activity of IFN-γ, allowing the original IL-4 to
proceed in the IgE cascade (26).
Therefore, promotion of IL-2 and inhibition of IL-10 production may
result in the suppression of IgE production and restore the skin
abnormalities in the CIU patients.
In this study, it was demonstrated that the
secretion of IL-2 was increased while that of IL-10 was decreased
in the peripheral blood lymphocytes of patients with CIU following
treatment with BCG-PSN at different concentrations in vitro
for 96 h. In addition, with the increase of drug concentration, the
levels of IL-2 and IL-10 were more efficiently restored, suggesting
that BCG-PSN may restore the Th1/Th2 imbalance in patients with
CIU.
References
|
1
|
Di Campli C, Gasbarrini A, Nucera E,
Franceschi F, Ojetti V, Sanz Torre E, Schiavino D, Pola P,
Patriarca G and Gasbarrini G: Beneficial effects of Helicobacter
pylori eradication on idiopathic chronic urticaria. Dig Dis
Sci. 43:1226–1229. 1998.
|
|
2
|
Nagashima Y, Kako K, Kim JD and Fukamizu
A: Enhanced histamine production through the induction of histidine
decarboxylase expression by phorbol ester in Jurkat cells. Mol Med
Rep. 6:944–948. 2012.
|
|
3
|
Ouyang H, Shi Y, Liu Z, Feng S, Li L, Su
N, Lu Y and Kong S: Increased interleukin-9 and
CD4+IL-9+T cells in patients with systemic
lupus erythematosus. Mol Med Rep. 7:1031–1037. 2013.PubMed/NCBI
|
|
4
|
Greaves MW: Chronic idiopathic urticaria.
Curr Opin Allergy Clin Immunol. 3:363–368. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaplan AP: Chronic urticaria: pathogenesis
and treatment. J Allergy Clin Immunol. 114:465–474. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Caproni M, Giomi B, Volpi W, Melani L,
Schincaglia E, Macchia D, et al: Chronic idiopathic urticaria:
infiltrating cells and related cytokines in autologous
serum-induced wheals. Clin Immunol. 114:284–292. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Piconi S, Trabattoni D, Iemoli E, et al:
Immune profiles of patients with chronic idiopathic urticaria. Int
Arch Allergy Immunol. 128:59–66. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du Q, Gu X, Cai J, Huang M and Su M:
Chrysin attenuates allergic airway inflammation by modulating the
transcription factors T-bet and GATA-3 in mice. Mol Med Rep.
6:100–104. 2012.PubMed/NCBI
|
|
9
|
Gu X, Zhou L, Du Q, Jiang D, Yang X, Ji X
and Yin K: Hesperetin inhibits the maturation and function of
monocyte-derived dendritic cells from patients with asthma. Mol Med
Rep. 2:509–513. 2009.PubMed/NCBI
|
|
10
|
Sun LX, Lin ZB, Duan XS, Lu J, Ge ZH, Li
M, Xing EH, Lan TF, Jiang MM, Yang N and Li WD: Ganoderma
lucidum polysaccharides counteract inhibition on CD71 and FasL
expression by culture supernatant of B16F10 cells upon lymphocyte
activation. Exp Ther Med. 5:1117–1122. 2013.
|
|
11
|
Tang S, Zhao B, Zhang G, Liu L and Zhou T:
Immune modulatory and therapeutic effect of BCG polysaccharides
nucleic acid on perennial allergic rhinitis. J Clin
Otorhinolaryngol. 19:345–346. 2005.(In Chinese).
|
|
12
|
Song NN, Wang BX, Shi DZ, et al:
Dimo-thylidioctyl ammonium bromide-BCG polysaccharide nucleic acid
adjuvant enhanced the immunogenicity of a Mycobacterium
tuberculosis subunit vaccine. Chin J Tuberc Res Dis.
32:513–516. 2009.(In Chinese).
|
|
13
|
Luo Y, Wang B, Hu L, et al: Fusion protein
Ag85B-MPT64(190-198)-Mtb8.4 has higher immunogenicity than Ag85B
with capacity to boost BCG-primed immunity against Mycobacterium
tuberculosis mice. Vaccine. 27:6179–6185. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Q, Yu H, Zhang Y, et al: Immunogenicity
and protective efficacy of a fusion protein vaccine consisting of
antigen Ag85B and HspX against Mycobacterium tuberculosis
infection in mice. Scand J Immunol. 73:568–576. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu B, Qie Y, Wang J, et al: Chitosan
microspheres enhance the immunogenicity of an Ag85B-based fusion
protein containing multiple T-cell epitopes of Mycobacterium
tuberculosis. Eur J Pharm Biopharm. 66:318–326. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo Y, Jiang W, Da Z, et al: Subunit
vaccine candidate AMM down-regulated the regulatory T cells and
enhanced the protective immunity of BCG on a suitable schedule.
Scand J Immunol. 75:293–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grattan CEH and Humphreys F; British
Association of Dermatologists Therapy Guidelines and Audit
Subcommittee. Guidelines for evaluation and management of urticaria
in adults and children. Br J Dermatol. 157:1116–1123. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zuberbier T, Bindslev-Jensen C, Canonica
W, Grattan CEH, Greaves MW, Henz BM, et al: EAACI/GA2LEN/EDF
guideline: management of urticaria. Allergy. 61:321–331. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Henz BM and Zuberbier T: Most chronic
urticaria is food-dependent, and not idiopathic. Exp Dermatol.
7:139–142. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
DeLong KL, Culler Sd, Saini SS, Beck LA
and Chen SC: Annual direct and indirect care costs of chronic
idiopathic urticaria. Arch Dematol. 144:35–39. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tong LJ, Balakrishnan G, Kochan JP, Kinet
JP and Kaplan AP: Assessment of autoimmunity in patients with
chronic urticaria. J Allergy Clin Immunol. 99:461–465. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gruber BL, Baeza M, Marchese MJ, Agnello V
and Kaplan AP: Prevalence and functional role of anti-IgE
autoantibodies in urticarial syndromes. J Invest Dermatol.
90:213–217. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiong C, Li Q, Lin M, et al: The efficacy
of topical intralesional BCG-PSN injection in the treatment of
erosive oral lichen planus: a randomized controlled trial. J Oral
Pathol Med. 38:551–558. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu J and Chen H: The effect of BCG-PSN on
T-cell subsets and cytokines in vernal conjunctivitis. J Huazhong
Univ Sci Technolog Med Sci. 22:77–79. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Du Toit G, Prescott R, Lawrence P, Johar
A, Brown G, Weinberg EG, et al: Autoantibodies to the high-affinity
IgE receptor in children with chronic urticaria. Ann Allergy Asthma
Immunol. 96:341–344. 2006.PubMed/NCBI
|
|
26
|
Kidd P: Th1/Th2 balance: the hypothesis,
its limitations, and implications for health and disease. Altern
Med Rev. 8:223–246. 2003.PubMed/NCBI
|
|
27
|
Morgan DA, Ruscetti FW and Gallo R:
Selective in vitro growth of T lymphocytes from normal human bone
marrows. Science. 193:1007–1008. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bird JJ, Brown DR, Mullen AC, Moskowitz
NH, Mahowald MA, Sider JR, Gajewski TF, Wang CR and Reiner SL:
Helper T cell differentiation is controlled by the cell cycle.
Immunity. 9:229–237. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cassatella MA, Gasperini S, Bovolenta C,
Calzetti F, Vollebregt M, Scapini P, et al: Interleukin-10 (IL-10)
selectively enhances CIS3/SOCS3 mRNA expression in human
neutrophils: evidence for an IL-10-induced pathway that is
independent of STAT protein activation. Blood. 94:2880–2889.
1999.PubMed/NCBI
|
|
30
|
Moore KW, O’Garra A, de Waal Malefyt R,
Vieira P and Mosmann TR: Interleukin-10. Annu Rev Immunol.
11:165–190. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Buelens C, Willems F, Delvaux A, Piérard
G, Delville JP, Velu T and Goldman M: Interleukin-10 differentially
regulates B7-1 (CD80) and B7-2 (CD86) expression on human
peripheral blood dendritic cells. Eur J Immunol. 25:2668–2672.
1995. View Article : Google Scholar : PubMed/NCBI
|