Introduction
The processes of tumorigenesis and metastasis
involve cancer cell migration and penetration through the
extracellular matrix (ECM) (1).
Matrix metalloproteinases (MMPs) are a family of highly conserved
zinc-dependent proteolytic enzymes that are able to degrade
essentially all ECM components and regulate diverse cell behaviors
(2). MMPs play pivotal roles in
physiological ECM remodeling, such as tissue regeneration in
pregnancy, wound healing and angiogenesis (3). MMPs are also involved in pathological
conditions, such as cancer, arthritis, autoimmune diseases and
atherosclerosis (4).
Matrix metalloproteinase 7 (MMP7) (punctuated
metalloproteinase-1, PUMP-1, matrilysin) is coded by a gene
localized on chromosome 11q21-q22. MMP7 is capable of degrading
elastin, fibronectin, proteogylcans and type IV collagen (5) and of cleaving non-matrix substrates of
the cell surface, such as E-cadherin, pro-tumor necrosis factor and
Fas ligand. MMP7 is predominantly expressed in the epithelium of
various organs under physiological conditions and may be
overexpressed in a variety of cancers, such as cancers of the
colorectum, esophagus, stomach, kidney and breast (6,7).
Numerous molecular epidemiological studies on the
association of MMP7 polymorphisms with cancer susceptibility have
been conducted. However, the association between MMP7-81A/G
polymorphism and cancer risk has not been elucidated. Therefore, a
system review and meta-analysis was performed.
Materials and methods
Search strategy and eligibility
criteria
Two investigators (J. Wu and X. Guan) independently
conducted key word searches in PubMed, Web of Science and the China
National Knowledge Infrastructure databases to identify all
eligible studies between 2000 and 2013. The following terms were
used: ‘MMP7’ or ‘matrix matalloproteinase 7′ and ‘polymorphism’ and
‘cancer’, ‘tumor’, ‘neoplasm’ or ‘carcinoma’ (last search update,
February 20th, 2013). References cited in the publications were
also screened by hand. The following criteria were required to be
met: i) the publication was a case-control study; ii) the study
evaluated the association between MMP7-181A/G polymorphism and
cancer; iii) odds ratios (ORs) or available data for their
calculation were reported; and iv) the study was published in
English or Chinese.
Data extraction
The following basic data were collected: first
author, publication year, cancer type and ethnicity of study
populations. Two investigators conducted data extraction
independently and discrepancies were resolved through
discussions.
Statistical analysis
Odds ratios (ORs) and corresponding 95% confidence
intervals (CIs) were calculated to assess the association between
MMP7-181A/G polymorphism and cancer risk (8). Stratified analyses were also performed
by cancer type (if one cancer type was contained in less than three
single studies, it was classified in the ‘other’ group) and smoking
status. Two methods were utilized: the dominant genetic model
(GG/GA vs. AA) and allelic contrast (G allele vs. A allele).
The between-study heterogeneity was assessed through
Chi-square-based Q test (Cochran’s Q statistic) and I2
statistic (9,10). A fixed-effects model (the
Mantel-Haenszel method) was used when P>0.05 and
I2<50% (11).
Otherwise, the random-effects model [the DerSimonian and Laird
(12) method] was used.
Additionally, meta-regression and sensitivity analyses were
performed to investigate the sources of heterogeneity and assess
the stability of the results, respectively. The Hardy-Weinberg
equilibrium (HWE) in controls was recalculated and P<0.05 was
considered to indicate a statistically significant difference. The
Begg’s rank correlation method (13) was used to investigate publication
bias. All analyses were performed with Stata software version 12.0
(StataCorp LP, College Station, TX, USA).
Results
Study characteristics
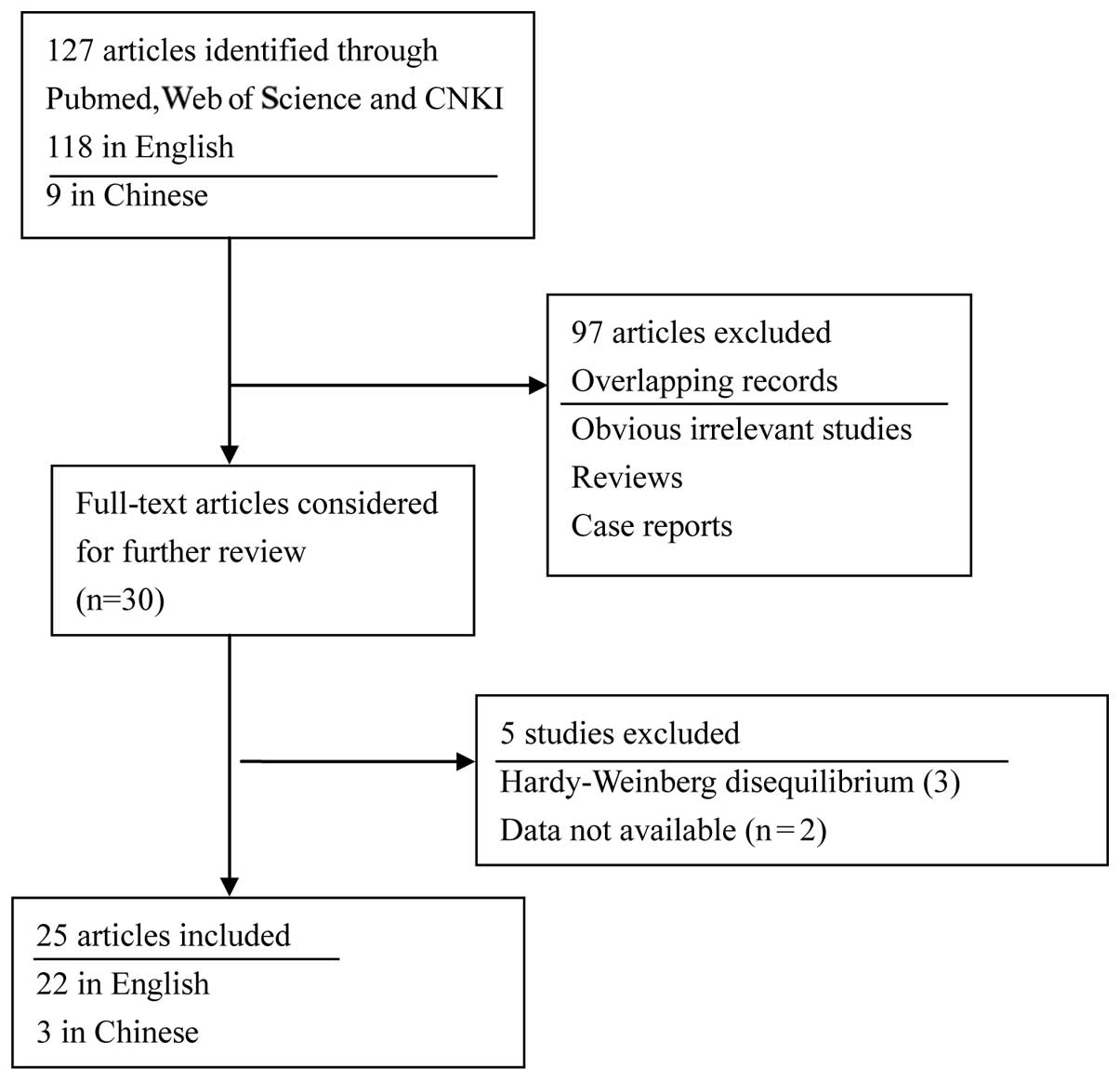
After comprehensive searching, 25 eligible
publications (6,7,14–36)
including 27 studies with a total of 6,166 cases and 7,195
controls, were included in this meta-analysis. Among these, Malik
et al published 2 studies (14,15)
involving 2 different cancer types, using the same control
populations. Additionally, Zhang et al(26) investigated 3 types of cancer in one
publication. The genotype distribution of the controls in all the
studies was consistent with Hardy-Weinberg equilibrium, except for
3 studies (34–36). Twenty studies used the polymerase
chain reaction-restriction fragment length polymorphism method to
analyse genotypes. The cases were histopathologically diagnosed in
the majority of the studies and the controls were free from cancer.
The characteristics of studies on MMP7-181A/G polymorphism are
provided in Table I and the flow
diagram of the literature search is shown in Fig. 1.
 | Table ICharacteristics of studies included in
the meta-analysis. |
Table I
Characteristics of studies included in
the meta-analysis.
| Author | Year | Country | Ethnicity | Cancer type | Sample size
(case/control) | PHWE | Refs. |
|---|
| Ghilardi et
al | 2003 | Italy | European | Colorectal | 58/111 | 0.129 | (27) |
| Li et al | 2008 | China | Asian | Gastric | 338/380 | 0.999 | (30) |
| Ohtani et
al | 2009 | Japan | Asian | Colorectal | 119/67 | 0.421 | (24) |
| Wu et al | 2011 | China | Asian | Cervical | 217/190 | 0.010 | (36) |
| Kubben et
al | 2006 | The Netherlands | European | Gastric | 79/169 | 0.000 | (34) |
| Vairaktaris et
al | 2007 | Germany/Greece | European | Oral | 159/120 | 0.018 | (35) |
| Li et al | 2006 | China | Asian | Ovarian | 138/160 | 0.714 | (29) |
| Woo et al | 2007 | Korea | Asian | Colorectal | 185/304 | 0.232 | (25) |
| Fang et
al | 2010 | China | Asian | Colorectal | 237/252 | 0.315 | (18) |
| Lu et al | 2006 | China | Asian | Adult
astrocytoma | 221/366 | 0.319 | (6) |
| de Lima et
al | 2009 | Brazil | other | Colorectal | 108/113 | 0.488 | (28) |
| Dziki et
al | 2011 | Poland | European | Colorectal | 184/205 | 0.295 | (17) |
| Lievre et
al | 2006 | France | European | Colorectal | 596/565 | 0.098 | (20) |
| Singh et
al | 2008 | India | Asian | Cervical | 150/162 | 0.750 | (22) |
| Srivastava et
al | 2010 | India | Asian | Bladder | 200/200 | 0.810 | (19) |
| Sugimoto et
al | 2008 | Japan | Asian | Gastric | 160/156 | 0.332 | (16) |
| Yi et
al | 2010 | Taiwan | Asian | Endometrial | 118/229 | 0.931 | (21) |
| Qiu et
al | 2008 | China | Asian | Hepatocellular | 425/475 | 0.338 | (23) |
| Beeghly-Fadiel
et al | 2008 | China | Asian | Breast | 1079/1082 | 0.236 | (7) |
| Tao et
al | 2010 | China | Asian | Cervical | 225/170 | 0.169 | (31) |
| Yao | 2011 | China | Asian | Cervical | 95/120 | 0.326 | (33) |
| Zhang et
al | 2010 | China | Asian | Epithelial
ovarian | 130/159 | 0.713 | (32) |
| Malik et
al | 2011 | India | Asian | Gastric | 108/195 | 0.547 | (14) |
| Malik et
al | 2011 | India | Asian | Esophageal | 135/195 | 0.547 | (15) |
| Zhang et
al | 2005 | China | Asian | Gastric | 201/350 | 0.888 | (26) |
| | | | NSCLC | 243/350 | 0.888 | |
| | | | ESCC | 258/350 | 0.888 | |
Quantitative synthesis
The MMP7-181G allele frequency varied widely among
the control subjects of the 27 case-control studies, ranging from
0.028 in Asian to 0.57 in European populations.
For the overall analysis of the 27 case-control
studies, a significantly increased cancer risk was found to be
associated with the G allele compared to the A allele (OR=1.34, 95%
CI: 1.17–1.53). A significantly increased cancer risk was also
found to be associated with the G allele carriers (GG/GA genotypes)
compared to the AA genotype in a dominant model (OR=1.33, 95% CI:
1.15–1.54). Similarly, an increased cancer risk was identified in
the 24 case-control studies under HWE [G vs. A: OR=1.43, 95% CI:
1.26–1.62 (Table III); GG/GA vs.
AA: OR=1.42, 95% CI: 1.23–1.63)].
 | Table IIIStratified analyses of the effect of
metalloproteinase 7-181A/G polymorphism on cancer risk
(Hardy-Weinberg equilibrium group). |
Table III
Stratified analyses of the effect of
metalloproteinase 7-181A/G polymorphism on cancer risk
(Hardy-Weinberg equilibrium group).
| | | GG/GA vs. AA | G vs. A |
|---|
| | |
|
|
|---|
| Variables | No.a | Cases/controls | OR (95% CI) | Pb | OR (95% CI) | Pb |
|---|
| Total | 24 | 5711/6716 | 1.42
(1.23–1.63) | 0.001 | 1.43
(1.26–1.62) | <0.001 |
| Cancer type |
| Gastric | 4 | 807/1081 | 1.72
(1.33–2.23) | 0.691 | 1.67
(1.35–2.08) | 0.925 |
| Colorectal | 7 | 1487/1617 | 1.02
(0.76–1.36) | 0.042 | 1.05
(0.85–1.32) | 0.036 |
| Cervical | 3 | 470/452 | 1.40
(1.07–1.84) | 0.961 | 1.45
(1.18–1.77) | 0.913 |
| Other | 10 | 2947/3566 | 1.64
(1.30–2.07) | 0.007 | 1.68
(1.36–2.08) | 0.003 |
| Quality
assessment |
| Low | 9 | 1616/1930 | 1.31
(0.94–1.84) | 0.001 | 1.37
(1.01–1.86) | 0.001 |
| High | 15 | 4095/4786 | 1.42
(1.23–1.65) | 0.059 | 1.41
(1.24–1.61) | 0.011 |
| Ethnicity |
| European | 3 | 838/881 | 1.30
(0.91–1.86) | 0.135 | 1.24
(0.97–1.59) | 0.091 |
| Asian | 20 | 4765/5722 | 1.45
(1.23–1.71) | 0.001 | 1.49
(1.29–1.72) | 0.001 |
| Source of
control |
| HB | 15 | 3111/3806 | 1.35
(1.11–1.64) | 0.003 | 1.41
(1.19–1.67) | <0.001 |
| PB | 8 | 2542/2799 | 1.54
(1.21–1.98) | 0.031 | 1.45
(1.18–1.79) | 0.029 |
| Smoking status |
| Smoking | 7 | 651/498 | 1.66
(1.21–2.28) | 0.204 | 1.56
(1.23–1.99) | 0.243 |
| Non-smoking | 6 | 742/929 | 1.57
(1.15–2.14) | 0.399 | 1.59
(1.25–2.00) | 0.895 |
Data were stratified according to the ethnicity of
the participants into European, Asian and other. A statistically
significant association was observed with the Asian but not the
European ethnicity, regardless of whether studies under
Hardy-Weinberg disequilibrium (HWD) were excluded (Tables II and III).
 | Table IIStratified analyses of the effect of
metalloproteinase 7-181A/G polymorphism on cancer risk (HWE/HWD
groups). |
Table II
Stratified analyses of the effect of
metalloproteinase 7-181A/G polymorphism on cancer risk (HWE/HWD
groups).
| | | GG/GA vs. AA | G vs. A |
|---|
| | |
|
|
|---|
| Variables | No.a | Cases/controls | OR (95% CI) | P-valueb | OR (95% CI) | P-valueb |
|---|
| Total | 27 | 6166/7195 | 1.33
(1.15–1.54) | <0.001 | 1.34
(1.17–1.53) | <0.001 |
| Cancer type |
| Gastric | 5 | 886/1250 | 1.34
(0.82–2.20) | 0.002 | 1.41
(0.97–2.03) | 0.007 |
| Colorectal | 7 | 1487/1617 | 1.02
(0.76–1.36) | 0.042 | 1.05
(0.85–1.32) | 0.036 |
| Cervical | 4 | 687/642 | 1.28
(1.02–1.59) | 0.666 | 1.38
(1.16–1.63) | 0.799 |
| Other | 11 | 3106/3686 | 1.56
(1.25–1.96) | 0.006 | 1.56
(1.21–2.01) | <0.001 |
| Quality
assessment |
| Low | 12 | 2071/2409 | 1.14
(0.85–1.54) | <0.001 | 1.18
(0.89–1.56) | <0.001 |
| High | 15 | 4095/4786 | 1.42
(1.23–1.65) | 0.059 | 1.34
(1.17–1.53) | 0.011 |
| Ethnicity |
| European | 5 | 1076/1170 | 1.03
(0.71–1.51) | 0.013 | 1.00
(0.74–1.34) | 0.001 |
| Asian | 21 | 4982/5912 | 1.42
(1.21–1.67) | 0.001 | 1.47
(1.28–1.69) | 0.001 |
| Source of
control |
| HB | 16 | 3190/3975 | 1.27
(1.03–1.57) | <0.001 | 1.35
(1.13–1.61) | <0.001 |
| PB | 10 | 2976/3220 | 1.40
(1.14–1.73) | 0.028 | 1.32
(1.05–1.65) | <0.001 |
| Smoking status |
| Smoking | 7 | 651/498 | 1.66
(1.21–2.28) | 0.204 | 1.56
(1.23–1.99) | 0.243 |
| Non-smoking | 6 | 742/929 | 1.57
(1.15–2.14) | 0.399 | 1.59
(1.25–2.00) | 0.895 |
Data were stratified according to cancer type into
gastric, cervical, colorectal and other types of cancer (Tables II and III). A significant association was
observed between MMP7-181A/G polymorphism and cervical and other
types of cancer, regardless of whether studies under HWD were
excluded. Of note, certain inconsistencies were oserved in the HWE
and HWE/HWD groups. In the former, a significantly elevated risk
for gastric cancer was observed (OR=1.72, 95% CI: 1.33–2.23 for
GG/GA vs. AA; OR=1.67, 95% CI: 1.35–2.08 for G vs. A), whereas no
significant association with gastric cancer was observed in the
latter (OR=1.34, 95% CI: 0.82–2.20 for GG/GA vs. AA; OR=1.41, 95%
CI: 0.97–2.03 for G vs. A). Additionally, no increased colorectal
cancer risk was found to be associated with the G allele or G
allele carriers in the HWE or the HWE/HWD group.
When smoking status was considered, a significantly
increased cancer risk was found to be associated with the G allele
and G allele carriers in the smoking as well as the non-smoking
groups. The stratified analyses of the eligible studies are
summarized in Tables II and
III.
Evaluation of heterogeneity
In the 27 case-control studies, it was observed that
variable ethnicity could explain 34.82% (G vs. A) and 13.54% (GG/GA
vs. AA) of the I2, whereas HWE could explain 43.93% (G
vs. A) and 35.28% (GG/GA vs. AA) of the I2. In the 24
case-control studies under HWE, none of these variables contributed
significantly to heterogeneity.
Sensitivity analysis
A sensitivity analysis was conducted to evaluate the
effect of each individual study on the pooled OR and removal of any
individual study imparted no significant difference in the HWE or
the HWE/HWD group.
Publication bias analysis
No evidence of publication bias for the association
of MMP7-181A/G polymorphism with cancer risk was identified in our
meta-analysis (HWE group, P=0.90 for GG/GA vs. AA and P=0.07 for G
vs. A; HWE/HWD group, P=0.97 for GG/GA vs. AA and P=0.09 for G vs.
A).
Discussion
Our meta-analysis was a systematic review of the
association between the MMP7-181A/G polymorphism and cancer
susceptibility, which provided evidence that the G allele and G
allele carriers were significantly associated with an increased
cancer risk (also in Asian ethnicity when race was considered).
Data were then stratified by cancer type. A significantly increased
risk of cervical and other types of cancer were observed in the HWE
and HWE/HWD groups (GG/GA vs. AA; G vs. A) and an increased risk of
gastric cancer was observed in the HWE group (GG/GA vs. AA; G vs.
A). However, we failed to identify any significant association
between MMP7-181A/G polymorphism and gastric cancer in the HWE/HWD
group. Therefore, we hypothesized that the results of the 24
case-control studies under HWE were reliable. However, further
studies on the effect of MMP7-181A/G polymorphism on cancer risk
are required to support this hypothesis.
Data were also stratified according to European and
Asian ethnicity. A statistically significant association was
observed in the Asian but not in the European populations. Although
the reason for this discrepancy is unclear, it may be attributed to
differences in genetic traits among different ethnic groups and a
potential reporting bias.
Tobacco is a well-known carcinogen, exposure to
which may lead to smoking-related cancers. Increased cancer
susceptibility was found to be associated with the G allele and G
allele carriers in the smoking as well as the non-smoking groups.
These findings indicate that MMP7-181A/G polymorphism may play a
pivotal role in cancer development.
Several limitations should be considered in our
meta-analysis. First, the majority of the eligible studies only
addressed the association of MMP7-181A/G polymorphism with cancer
risk. However, we believe that further studies assessing the effect
of gene-gene or gene-environment interactions may eventually
achieve a more comprehensive understanding. Second, publication
bias is likely to exist, despite the publication bias of low
significance or lack thereof in our meta-analysis. Third,
significant heterogeneity was detected in the overall comparisons
and some of the subgroup analyses. Therefore, a meta-regression
analysis was performed to identify the sources of heterogeneity and
it was observed that HWE and ethnicity could explain the
heterogeneity across studies, whereas other variables, such as
cancer type and source of controls could not, possibly due to the
heterogeneity resulting from other factors.
In conclusion, the MMP7-181A/G polymorphism is a
risk factor for cancer development, particularly cervical and other
types of cancer, in Asian populations. Further case-control studies
(including HWE or HWE/HWD) estimating the effect of gene-gene and
gene-environment interactions may eventually achieve a more
comprehensive understanding.
References
|
1
|
Wolf K and Friedl P: Mapping proteolytic
cancer cell-extracellular matrix interfaces. Clin Exp Metastasis.
26:289–298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chakraborti S, Mandal M, Das S, Mandal A
and Chakraborti T: Regulation of matrix metalloproteinases: an
overview. Mol Cell Biochem. 253:269–285. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malemud CJ: Matrix metalloproteinases
(MMPs) in health and disease: an overview. Front Biosci.
11:1696–1701. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
VanSaun MN and Matrisian LM: Matrix
metalloproteinases and cellular motility in development and
disease. Birth Defects Res C Embryo Today. 78:69–79. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wilson CL and Matrisian LM: Matrilysin: an
epithelial matrix metalloproteinase with potentially novel
functions. Int J Biochem Cell Biol. 28:123–136. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu Z, Wang Y, Zhang Q, et al: Association
between the functional polymorphism in the matrix
metalloproteinase-7 promoter and susceptibility to adult
astrocytoma. Brain Res. 1118:6–12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beeghly-Fadiel A, Long JR, Gao YT, et al:
Common MMP-7 polymorphisms and breast cancer susceptibility: a
multistage study of association and functionality. Cancer Res.
68:6453–6459. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Woolf B: On estimating the relation
between blood group and disease. Ann Hum Genet. 19:251–253. 1955.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cochran WG: The combination of estimates
from different experiments. Biometrics. 10:101–129. 1954.
View Article : Google Scholar
|
|
11
|
Mantel N and Haenszel W: Statistical
aspects of the analysis of data from retrospective studies of
disease. J Natl Cancer Inst. 22:719–748. 1959.PubMed/NCBI
|
|
12
|
DerSimonian R and Kacker R: Random-effects
model for meta-analysis of clinical trials: an update. Contemp Clin
Trials. 28:105–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malik MA, Zargar SA and Mittal B: Role of
the metalloproteinase-7 (181A>G) polymorphism in gastric cancer
susceptibility: a case control study in Kashmir valley. Asian Pac J
Cancer Prev. 12:73–76. 2011.
|
|
15
|
Malik MA, Sharma KL, Zargar SA and Mittal
B: Association of matrix metalloproteinase-7 (-181A>G)
polymorphism with risk of esophageal squamous cell carcinoma in
Kashmir Valley. Saudi J Gastroenterol. 17:301–306. 2011.
|
|
16
|
Sugimoto M, Furuta T, Kodaira C, et al:
Polymorphisms of matrix metalloproteinase-7 and chymase are
associated with susceptibility to and progression of gastric cancer
in Japan. J Gastroenterol. 43:751–761. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dziki L, Przybylowska K, Majsterek I,
Trzcinski R, Mik M and Sygut A: A/G polymorphism of the MMP-7 gene
promoter region in colorectal cancer. Pol Przegl Chir. 83:622–626.
2011.PubMed/NCBI
|
|
18
|
Fang WL, Liang WB, He H, et al:
Association of matrix metalloproteinases 1, 7, and 9 gene
polymorphisms with genetic susceptibility to colorectal carcinoma
in a Han Chinese population. DNA Cell Biol. 29:657–661. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Srivastava P, Gangwar R, Kapoor R and
Mittal RD: Bladder cancer risk associated with genotypic
polymorphism of the matrix metalloproteinase-1 and 7 in North
Indian population. Dis Markers. 29:37–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lievre A, Milet J, Carayol J, et al:
Genetic polymorphisms of MMP1, MMP3 and MMP7 gene promoter and risk
of colorectal adenoma. BMC Cancer. 6:2702006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yi YC, Chou PT, Chen LY, et al: Matrix
metalloproteinase-7 (MMP-7) polymorphism is a risk factor for
endometrial cancer susceptibility. Clin Chem Lab Med. 48:337–344.
2010.PubMed/NCBI
|
|
22
|
Singh H, Jain M and Mittal B: MMP-7
(-181A>G) promoter polymorphisms and risk for cervical cancer.
Gynecol Oncol. 110:71–75. 2008.
|
|
23
|
Qiu W, Zhou G, Zhai Y, et al: No
association of MMP-7, MMP-8, and MMP-21 polymorphisms with the risk
of hepatocellular carcinoma in a Chinese population. Cancer
Epidemiol Biomarkers Prev. 17:2514–2518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ohtani H, Maeda N and Murawaki Y:
Functional polymorphisms in the promoter regions of matrix
metalloproteinase-2, -3, -7, -9 and TNF-alpha genes, and the risk
of colorectal neoplasm in Japanese. Yonago Acta Medica. 52:47–56.
2009.
|
|
25
|
Woo M, Park K, Nam J and Kim JC: Clinical
implications of matrix metalloproteinase-1, -3, -7, -9, -12, and
plasminogen activator inhibitor-1 gene polymorphisms in colorectal
cancer. J Gastroenterol Hepatol. 22:1064–1070. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang J, Jin X, Fang S, et al: The
functional polymorphism in the matrix metalloproteinase-7 promoter
increases susceptibility to esophageal squamous cell carcinoma,
gastric cardiac adenocarcinoma and non-small cell lung carcinoma.
Carcinogenesis. 26:1748–1753. 2005. View Article : Google Scholar
|
|
27
|
Ghilardi G, Biondi ML, Erario M,
Guagnellini E and Scorza R: Colorectal carcinoma susceptibility and
metastases are associated with matrix metalloproteinase-7 promoter
polymorphisms. Clin Chem. 49:1940–1942. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Lima JM, de Souza LG, da Silva ID and
Forones NM: E-cadherin and metalloproteinase-1 and -7 polymorphisms
in colorectal cancer. Int J Biol Markers. 24:99–106.
2009.PubMed/NCBI
|
|
29
|
Li Y, Jin X, Kang S, et al: Polymorphisms
in the promoter regions of the matrix metalloproteinases-1, -3, -7,
and -9 and the risk of epithelial ovarian cancer in China. Gynecol
Oncol. 101:92–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li JY, Tian M and Zhao A: Polymorphism in
the promoter region of the metalloproteinase-7 increases
susceptibility and risk of metastasis of gastric adenocarcinoma.
Gastroenterology. 134:A6032008.
|
|
31
|
Tao HJ, Wu SH, Zhang L, et al: Correlation
of polymorphism of IL-8 and MMP-7 genes with the risk of early
stage cervical cancer in Shanxi. Chin Remedies and Clinics.
10:852–854. 2010.
|
|
32
|
Zhang Y, Du H, Kang S, Sun DL and Li Y:
Association of SNP in matrix metalloproteinase-7 polymorphism with
susceptibility of ovarian cancer. China J Mod Med. 20:2792–2798.
2010.
|
|
33
|
Yao N: Correlation of polymorphism of
MMP-7 gene with the risk of early stage cervical cancer. China J
Modern Med. 21:3519–3521. 2011.(In Chinese).
|
|
34
|
Kubben FJ, Sier CF, Meijer MJ, et al:
Clinical impact of MMP and TIMP gene polymorphisms in gastric
cancer. Br J Cancer. 95:744–751. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vairaktaris E, Serefoglou Z, Yapijakis C,
et al: High gene expression of matrix metalloproteinase-7 is
associated with early stages of oral cancer. Anticancer Res.
27:2493–2498. 2007.PubMed/NCBI
|
|
36
|
Wu S, Lu S, Tao H, et al: Correlation of
polymorphism of IL-8 and MMP-7 with occurrence and lymph node
metastasis of early stage cervical cancer. J Huazhong Univ Sci
Technolog Med Sci. 31:114–119. 2011. View Article : Google Scholar : PubMed/NCBI
|