Introduction
In 1869, Ashworth (1) was the first to identify circulating
tumor cells (CTCs) in the peripheral blood of cancer patients.
Subsequent studies revealed that CTCs are of predictive value
regarding metastasis, recurrence and prognosis of melanoma, breast,
pancreatic, colorectal and lung cancer. CTCs are also predictive of
the patient response to antitumor treatment and may assist in
developing a customized treatment plan (2–5).
Primary hepatocellular carcinoma is one of the
malignant tumors that metastasize hematogenously. Previous studies
demonstrated that liver cancer cells enter the circulation at an
early stage and CTCs in the blood form a foremost condition leading
to recurrence and metastasis following liver cancer surgery.
Therefore, an effective method for the identification of CTCs in
the blood and the investigation of their biological characteristics
may promote the early diagnosis of liver cancer and prediction of
early metastasis.
Therefore, CTCs, as a potential independent
diagnostic index, have been extensively investigated. The
diagnostic value of circulating liver cancer cells for liver cancer
is currently under investigation worldwide. However, the detection
of circulating liver cancer cells cannot be used as a conventional
clinical screening approach due to the following reasons: i) the
sample size is relatively small; ii) the inspection technology
lacks standardization and automation and complicated sample
preparation procedures are required, which may lead to significant
differences among the results from different laboratories, or even
from the same laboratory; and iii) different reagents and methods
may exhibit different specificities and sensitivities (6).
Therefore, this meta-analysis aimed to collect
studies conducted in China and other countries that focused on the
diagnostic value of circulating liver cancer cells for liver
cancer. We aimed to summarize and analyze the studies and combine
the specific conditions of the related cases to create a sample
library and, furthermore, discuss the effect of circulating liver
cancer cells on the relevant indices of liver cancer, such as
stage, size, metastasis, recurrence, prognosis, survival time and
sensitivity to treatment from a statistical viewpoint.
Materials and methods
Study inclusion and exclusion
criteria
Inclusion criteria for the present study were: i)
Patients with aggressive liver cancer at preliminary diagnosis; ii)
patients with aggressive liver cancer with distinct pathological
evidence and evaluated as aggressive through α-fetoprotein
measurements, contrast-enhanced ultrasonography, computed
tomography (CT), or positron emission tomography-CT; and iii)
complete data records on liver cancer and CTCs.
The exclusion criteria were as follows: i)
non-cancer liver diseases, such as hepatitis or cirrhosis; ii)
liver metastasis from other malignant tumors; iii) novel detection
methods for CTCs; and iv) reviews of the literature.
Literature retrieval
Using ‘liver cancer’ and ‘circulating tumor cells’
as the keywords, 116 foreign studies published between 1983 and
2012 were retrieved from foreign language databases such as PubMed,
Springer Protocols and Web of Knowledge. Thirty-two studies
published in China between 2002 and 2011 were retrieved from
domestic databases, such as CCPD and VIP Information. In total, 148
studies were collected, excluding the duplicates. In addition,
Chinese studies on circulating liver cancer cells and full-text
references were manually retrieved. The related studies were
further tracked with a search engine and, if necessary, document
delivery service was employed for acquisition of the full text and
related data.
Data abstraction
The related studies were screened according to the
criteria mentioned above, the eligible studies were identified, the
full text was carefully read and data were abstracted, including
author, publication year, nationality, number of cases included in
the study, number of CTCs-positive cases with tumor diameters >5
or ≤ 5 cm, number of CTCs-positive cases with tumor stages I/II and
III/IV and number of CTCs-positive cases with or without
metastasis.
Data analysis and statistical
methods
The abstracted data were subjected to meta-analysis
by using Review Manager 5.1 software. The odds ratio (OR) was
analyzed as an efficacy parameter, with the 95% confidence interval
(CI) representing the variable. A two-sided P-value of <0.05 was
considered to indicate a statistically significant difference.
The statistical heterogeneity among the groups was
analyzed by the Q test. In the case of statistical homogeneity
among the groups (P>0.10 and I2<50%), the
fixed-effects model was selected for analysis; in the case of
statistical heterogeneity (P<0.10 and
50%<I2<70%), the random-effects model was selected
instead.
A funnel plot was drawn with the software for
assessing the publication bias.
Results
Study retrieval results
A total of 136 references, excluding the duplicates,
were electronically and manually retrieved from relevant databases.
In total, 108 apparently relevant studies were rejected after
reading the abstracts and 28 studies entered the next assessment
process. Twenty-two studies were rejected after reading the
abstracts due to reasons such as incomplete tumor data and
incomplete data on CTC positivity. Ultimately, 5 clinical
comparative studies published between 2004 and 2011 were included
in the meta-analysis.
Study information
Five clinical controlled trials conducted on a total
of 535 patients were included, with 4 trials having been completed
in China and 1 trial in France. The study information is presented
in Table I.
 | Table IStudies included in the
meta-analysis. |
Table I
Studies included in the
meta-analysis.
| Author | Year | Country | Liver cancer samples
(n) | Control samples
(n) | Refs. |
|---|
| Liu | 2007 | China | 75 | 25 | (7) |
| Zuo | 2008 | China | 56 | 30 | (8) |
| Yu | 2011 | China | 126 | 0 | (9) |
| Xu | 2010 | China | 235 | 57 | (10) |
| Vona et
al | 2004 | France | 43 | 69 | (11) |
Correlation analysis of the abstracted
data
Analysis of the correlation between
CTCs level and tumor size
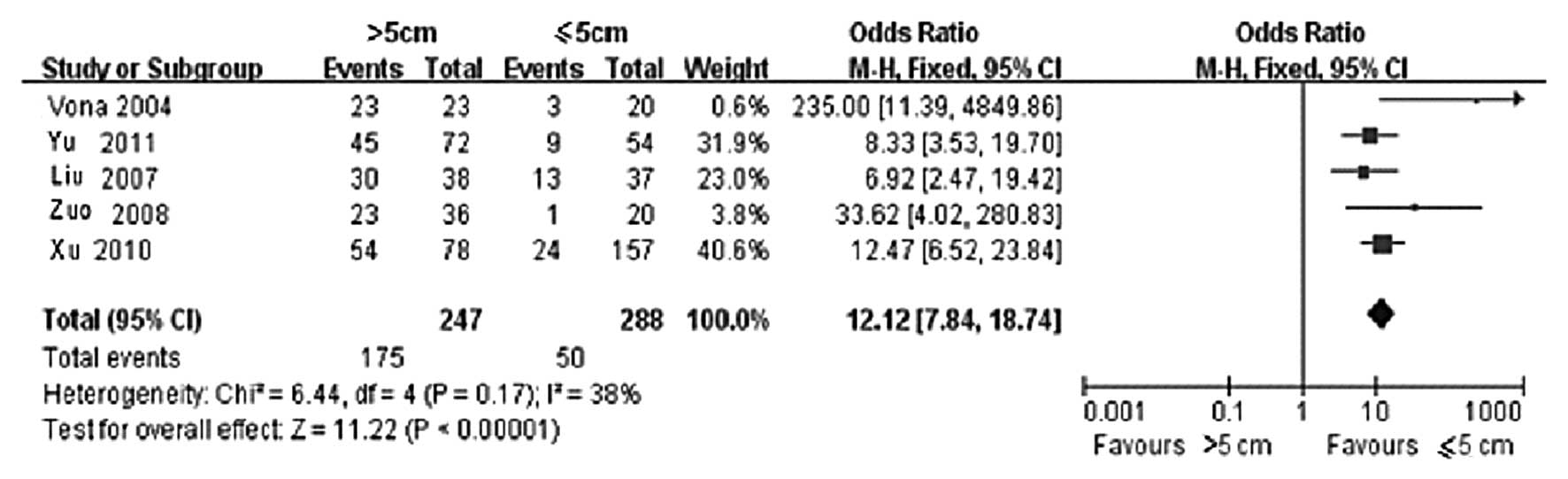
Among the included cases, 247 were classified in the
group with a tumor diameter of >5 cm, whereas 288 were
classified in the group with a tumor diameter of ≤5 cm. The
statistical results of CTCs-positive rate in the two groups are
presented in Table II. Of the 5
clinical controlled trials, the >5 cm group included 175
CTCs-positive cases, whereas the ≤5 cm group included 50 cases. The
result of the heterogeneity test is shown in Fig. 1, wherein χ2=6.44, degree
of freedom (DOF)=4, P=0.17 and I2=38%; therefore, the
fixed-effects model was applied. It was observed that the
difference in CTCs-positive rate in the peripheral blood between
the >5 and ≤5 cm groups was statistically significant (OR=12.12,
95% CI: 7.84–18.74 and P<0.00001). This finding demonstrated
that the CTCs-positive rate was directly correlated with tumor
size, i.e., the larger the tumor, the higher the CTCs-positive rate
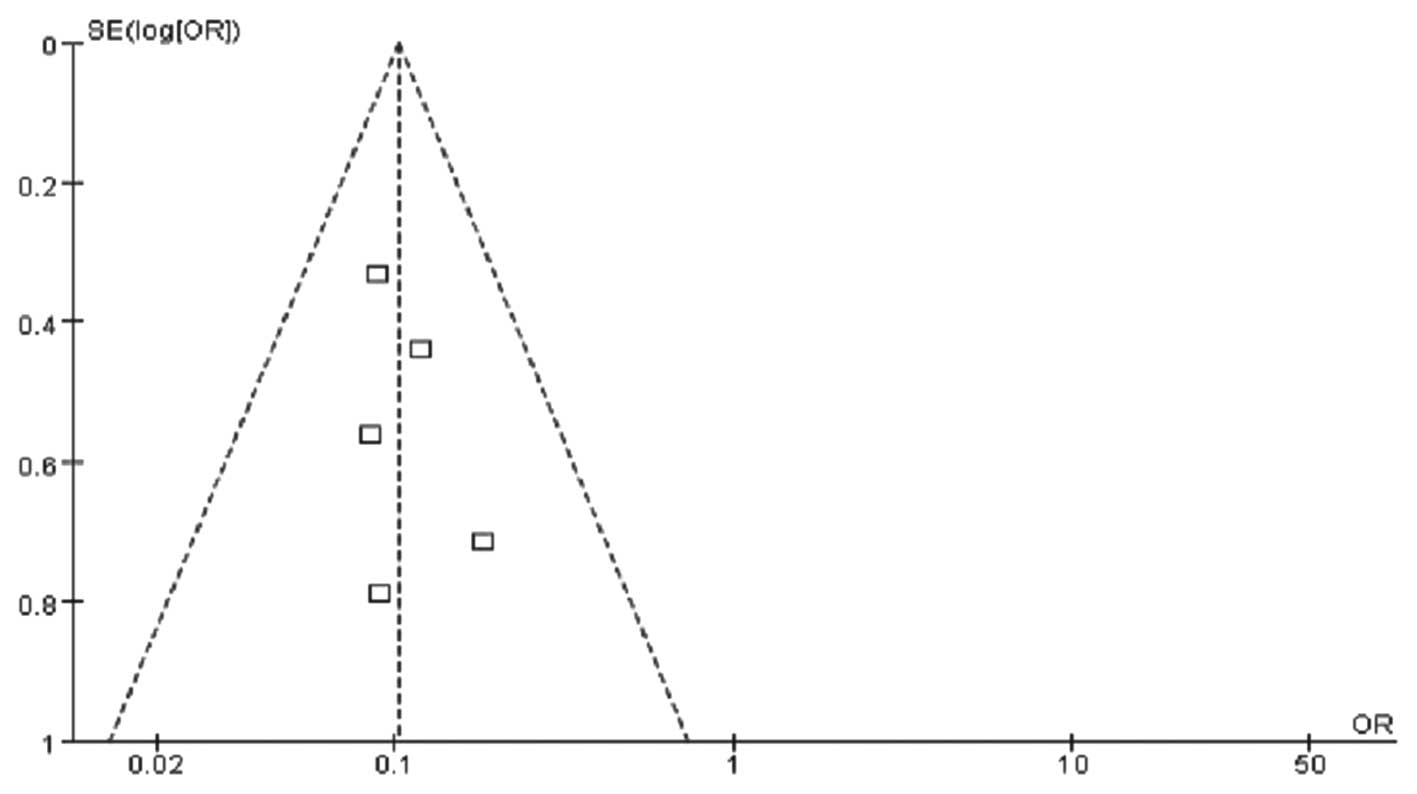
in the peripheral blood. The funnel plot demonstrated that the
bilateral scatter-plot distribution was generally symmetrical, with
no significant publication bias (Fig.
2).
 | Table IIAssociation of circulating tumor cells
(CTCs) level with >5 and ≤5 cm tumor size groups. |
Table II
Association of circulating tumor cells
(CTCs) level with >5 and ≤5 cm tumor size groups.
| | >5 cm group | ≤5 cm group | |
|---|
| |
|
| |
|---|
| Study | Year | Eventsa | Total | Eventsa | Total | Refs. |
|---|
| Liu | 2007 | 30 | 38 | 13 | 37 | (7) |
| Zuo | 2008 | 23 | 36 | 1 | 20 | (8) |
| Yu | 2011 | 45 | 72 | 9 | 45 | (9) |
| Xu | 2010 | 54 | 78 | 24 | 157 | (10) |
| Vona et
al | 2004 | 23 | 23 | 3 | 20 | (11) |
Analysis of the correlation between
CTCs level and tumor stage
Among the included cases, 258 were in the stage I/II
group and 277 were in the stage III/IV group. The statistical
results of the CTCs-positive rate in the two groups are presented
in Table III. Of the 5 clinical
controlled trials, the I/II group included 43 CTCs-positive cases,
whereas the III/IV group included 182 cases. According to the
results of the Q test, no significantly statistical heterogeneity
was observed between the two groups (χ2=1.11, DOF=4,
P=0.89 and I2=0%); therefore, the fixed effects model
was applied. The difference in CTCs-positive rates in the
peripheral blood between the two groups was found to be
statistically significant (OR=0.10, 95% CI: 0.07–0.16;
P<0.00001) (Fig. 3). The
comparison results revealed that the CTCs-positive rate in the
peripheral blood in the stage III/IV group was significantly higher
compared to that in the stage I/II group. This finding indicates
that the tumor stage was directly correlated with the presence of
CTCs in the peripheral blood, i.e., the more advanced the stage,
the higher the probability of CTCs detected in the peripheral
blood. The funnel plot revealed no significant publication bias
(Fig. 4).
 | Table IIIAssociation of circulating tumor cells
(CTCs) level with I/II and III/IV tumor stage groups. |
Table III
Association of circulating tumor cells
(CTCs) level with I/II and III/IV tumor stage groups.
| | I/II group | III/IV group | |
|---|
| |
|
| |
|---|
| Study | Year | Eventsa | Total | Eventsa | Total | Refs. |
|---|
| Liu | 2007 | 10 | 35 | 33 | 40 | (7) |
| Zuo | 2008 | 3 | 17 | 21 | 39 | (8) |
| Yu | 2011 | 9 | 54 | 45 | 72 | (9) |
| Xu | 2010 | 18 | 139 | 60 | 96 | (10) |
| Vona et
al | 2004 | 3 | 13 | 23 | 30 | (11) |
Analysis of the correlation between
CTCs level and tumor metastasis
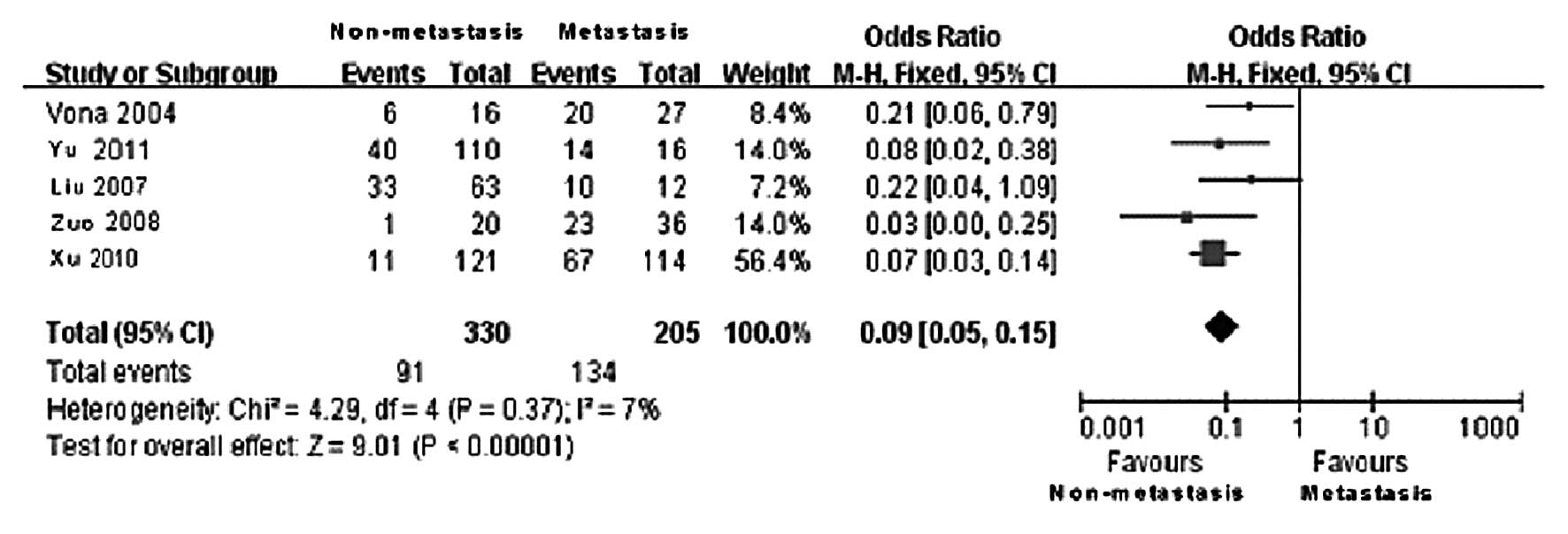
Among the included cases, 330 were classified in the
metastasis and 205 in the non-metastasis group. The statistical
results of CTCs-positive rates in the two groups are presented in
Table IV. Of the 5 clinical
controlled trials, the non-metastasis group included 91
CTCs-positive cases, whereas the metastasis group included 134
CTCs-positive cases. According to the results of the Q test, no
statistically significant heterogeneity was observed between the
two groups (χ2=4.29, DOF=4, P=0.37 and
I2=7%); therefore, the fixed-effects model was applied.
The difference in the CTCs-positive rate in the peripheral blood
between the metastasis and the non-metastasis group was found to be
statistically significant (OR=0.09, 95% CI: 0.05–0.15;
P<0.00001) (Fig. 5). The
comparison results revealed that the CTCs-positive rate in the
peripheral blood in the metastasis group was significantly higher
compared to that in the non-metastasis group. This finding
indicates that the tumor metastasis was directly correlated with
the presence of CTCs in the peripheral blood, i.e., patients with
tumor metastasis exhibited a higher probability of CTCs detected in
the peripheral blood. The funnel plot revealed no significant
publication bias (Fig. 6).
 | Table IVAssociation of circulating tumor cells
(CTCs) level with the metastasis and non-metastasis groups. |
Table IV
Association of circulating tumor cells
(CTCs) level with the metastasis and non-metastasis groups.
| | Non-metastasis
group | Metastasis group | |
|---|
| |
|
| |
|---|
| Study | Year | Eventsa | Total | Eventsa | Total | Refs. |
|---|
| Liu | 2007 | 33 | 63 | 10 | 12 | (7) |
| Zuo | 2008 | 1 | 20 | 23 | 36 | (8) |
| Yu | 2011 | 40 | 110 | 14 | 16 | (9) |
| Xu | 2010 | 11 | 121 | 67 | 114 | (10) |
| Vona et
al | 2004 | 6 | 16 | 20 | 27 | (11) |
Discussion
The diagnostic value of CTCs in liver cancer has
been attracting increasing attention. This study aimed to summarize
data from the literature published in China and other countries,
conduct a meta-analysis using Review Manager 5.1 software and
assess the correlation between CTCs level in the peripheral blood
and tumor size, stage and metastasis. The results demonstrated that
the CTCs-positive rate in the peripheral blood was directly
correlated with tumor size, stage and metastasis. However, due to
incomplete or missing data in the published studies, the effect of
CTCs on tumor recurrence monitoring, prognosis, survival time and
treatment customization could not be reviewed. However, considering
the employment of CTCs in the diagnosis of malignant solid tumor,
such as melanoma, breast, colorectal and prostate cancer, the
clinical application of CTCs in liver cancer diagnosis may become
more prominent with technological improvements (12).
CTC detection in the peripheral blood may be
considered a viable alternative to cancer diagnosis. CTC detection
assists in guiding molecular-targeted therapy and assessing
anticancer efficacy. Of note: i) the development of a CTC detection
method of high sensitivity and specificity is crucial for the
follow up in clinical applications; ii) the investigations on novel
CTC-specific markers may assist in improving the specificity and
sensitivity of the identification and quantification of CTCs; iii)
additional studies on the molecular and genetic constitution of
CTCs may assist in elucidating the molecular mechanisms of cancer
development, recurrence and metastasis; and iv) the role of cancer
stem cells in tumor metastasis and drug resistance is being
gradually emphasized. Follow-up studies in this field may assist in
elucidating the mechanisms underlying tumor metastasis and may lead
to the development of novel therapeutic interventions. Therefore,
studies focusing on this area may promote advances in cancer
biology and clinical cancer management, leading to improvement of
the quality of life and prolongation of the lifespan of cancer
patients.
References
|
1
|
Ashworth TR: A case of cancer in which
cells similar to those in the tumors were seen in the blood after
death. Aus Med J. 14:146–149. 1869.
|
|
2
|
Alemar J and Schuur ER: Progress in using
circulating tumor cell information to improve metastatic breast
cancer therapy. J Oncol. 2013:7027322013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ren C, Chen H, Han C, et al: Detection and
molecular analysis of circulating tumor cells for early diagnosis
of pancreatic cancer. Med Hypotheses. 80:833–836. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andreopoulou E and Cristofanilli M:
Circulating tumor cells as prognostic marker in metastatic breast
cancer. Expert Rev Anticancer Ther. 10:171–177. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cohen SJ, Punt CJ, Iannotti N, et al:
Relationship of circulating tumor cells to tumor response,
progression-free survival, and overall survival in patients with
metastatic colorectal cancer. Clin Oncol. 26:3213–3221. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun YF, Yang XR, Zhou J, Qiu SJ, Fan J and
Xu Y: Circulating tumor cells: advances in detection methods,
biological issues, and clinical relevance. J Cancer Res Clin Oncol.
137:1151–1173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu D: Detection of circulating tumor
cells in peripheral blood and its significance in patients with
hepatocellular carcinoma (unpublished PhD thesis). Fudan
University. 2007.
|
|
8
|
Zuo GH: Detection and biological
characteristics of circulating tumor cells in peripheral blood of
patients with liver cancer (unpublished PhD thesis). Third Military
Med Univ. 2008.
|
|
9
|
Yu F: Improvement of circulating liver
cancer cells detection and its preliminary study on the
postoperative recurrence prediction (unpublished PhD thesis).
Second Military Med Univ. 2011.
|
|
10
|
Xu W: Isolation/detection system and
clinical application research of circulating liver cancer cells
based on sialic acid glycoprotein receptor (unpublished PhD
thesis). Second Military Med Univ. 2010.
|
|
11
|
Vona G, Estepa L, Béroud C, et al: Impact
of cytomorphological detection of circulating tumor cells in
patients with liver cancer. Hepatology. 39:792–797. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ghossein R and Bhattacharya S: Molecular
detection and characterization of circulating tumor cells and
micrometastases in prostatic, urothelial, and renal cell
carcinomas. Semin Surg Oncol. 20:304–311. 2001. View Article : Google Scholar : PubMed/NCBI
|