Introduction
Ginkgo biloba is a dioecious tree and has
been used for thousands of years to treat a variety of ailments in
traditional Chinese medicine (1).
The Ginkgo biloba extract is a complex mixture that mainly
contains flavonoids (primarily quercetin, kaempferol and
isorhamnetin) and terpene lactones (ginkgolides and bilobalide)
(2). Extensive investigation of the
main bioactive constituents of the Ginkgo biloba extract
revealed several important pharmacological effects. It was
previously reported that the Ginkgo biloba extract exerts an
antioxidant effect by scavenging reactive oxygen species (3), reduces platelet aggregation and
exhibits neuroprotective properties (4). Previous studies demonstrated the
potential benefits of the Ginkgo biloba extract in the
treatment of Alzheimer’s disease (5), learning and memory deficits (6), cerebrovascular diseases (7), cardiovascular diseases (8), climacteric vasomotor symptoms and
postmenopausal syndrome (9,10). It was also demonstrated that the
Ginkgo biloba extract possesses antitumor properties
(11), may induce cancer cell
apoptosis and differentiation and inhibit the progression of human
colon cancer (12), hepatocellular
carcinoma (13), pancreatic
(14) and gastric cancer (15).
Cytochrome P450 (CYP) is a type of heme-thiolate
protein that is ubiquitously found in the biosphere. This enzyme
family is crucial in several biological processes, such as the
oxidative metabolism of exogenous and endogenous organic chemicals,
the biological transformation of drugs or xenobiotics, the
metabolism of chemical carcinogens and the biosynthesis of
physiologically crucial compounds, such as steroids and fatty acids
(16). Human CYPs are associated
with a number of diseases, such as hypertension, diabetes, obesity
and hepatic, infectious and inflammatory diseases (17,18).
Members of the CYP family have been identified in healthy and
cancerous extrahepatic tissue, such as breast cancer cells, and are
associated with tumor development and progression (19–24).
Three types of CYP1 enzymes are expressed in humans,
CYP1A1, CYP1A2 and CYP1B1. The members of this family are under the
transcriptional regulation of the aryl hydrocarbon receptor (AhR)
and are known to activate pro-carcinogens, such as polycyclic
aromatic hydrocarbons (PAHs) (25).
All the members of this family are expressed in extrahepatic
tissues. However, CYP1B1 is uniquely overexpressed in a wide range
of human cancers of different histogenetic types compared to normal
tissues (26–28). Several studies reported that the
Ginkgo biloba extract exhibits differential induction of
CYPs (29–31). The aim of this study was to assess
whether the Ginkgo biloba extract induces CYP1B1 expression
and affects the proliferation of MCF-7 and MDA-MB-231 human breast
cancer cells.
Materials and methods
Ginkgo biloba and Ginkgo biloba
extract
The Ginkgo biloba leaves and ginkgo fruit
were obtained from the Shenyang Agricultural University, without
prior exposure to chemical pesticides. This study was conducted in
accordance with the University’s ethic regulations concerning the
use of human related materials in scientific research and with the
approval of the ethics committtee of Shenyang Agricultural
University.
High-quality Ginkgo biloba leaves and ginkgo
fruit were used for the preparation of an aqueous extract.
Ginkgo biloba leaves and ginkgo fruit (50 g) were cut and
minced separately and 100 ml cold water was added to suspend the
minced Ginkgo biloba mixture. The mixture was maintained at
80°C for 40 min and was then subjected to negative pressure
filtration, followed by the addition of distilled water to the
clear supernatant to a final volume of 100 ml. The supernatant was
then filtered through a 0.45-μm membrane filter into a sterile
collection bottle and was kept at 2–8°C in a refrigerator as a
final extract for subsequent experimental use.
Cell lines and cell culture
The MCF-7 and MDA-MB-231 human breast cancer cell
lines were purchased from the cell bank of the Chinese Academy of
Sciences. MCF-7 and MDA-MB-231 cells were removed from the liquid
nitrogen and preheated for 1 min at 37°C. The cells were then
transferred to a 25-cm2 cell culture vessel, followed by
the addition of 10–15 ml RPMI-1640 cell culture medium (Hyclone,
Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS) and
1% antibiotics. The cells were then cultured at 37°C in an
atmosphere of 5% CO2 in a tissue culture apparatus. The
cell culture medium was changed after culture for 12 h. After
growing to 80–90% confluency, the cells were harvested and reseeded
for the treatment assay.
Experimental treatments
For the Ginkgo biloba extract treatment
assay, MCF-7 and MDA-MB-231 cells were divided into three treatment
groups: the control group, the Ginkgo biloba leaves extract
group and the ginkgo fruit extract group. Cells
(1–1.5×106 MCF-7 or 4–5×106 MDA-MD-231) were
plated in 10–15 ml of RPMI-1640 cell culture medium supplemented
with 10% FBS and 1% antibiotics. The MCF-7 cells were then
incubated with 500 μl extract of Ginkgo biloba leaves as the
first group and 500 μl extract of ginkgo fruit as the second group.
Cells without the addition of Ginkgo biloba extract served
as the control group. MDA-MB-231 cells were treated with Ginkgo
biloba extract at the same dose as MCF-7 cells. Cell morphology
was observed after 48 h under a confocal laser scanning microscope.
The cells of each group were then collected and counted and RNA was
extracted.
Qualitative reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) and the
first-strand complementary DNA (cDNA) was synthesized according to
the manufacturer’s instructions, using 1 μg total RNA with a random
primer and the Moloney murine leukemia virus reverse transcriptase,
RNase H minus [M-MLV RTase (RNase H−)] (GeneCopoeia,
Inc., Rockville, MD, USA). The first-strand cDNA was stored at
−20°C for later use.
The PCR primers for CYP1B1 and glyceraldehyde
3-phosphate dehydrogenase (GAPDH) were intron-spanning primer
sequences of CYP1B1 and GAPDH and were as follows: CYP1B1: sense,
5′-GGCTGGATTTGGAGAACGTA-3′ and antisense,
5′-GTTGATGAGGCCATCCTTGT-3′; GAPDH: sense, 5′-GGATTTGGTCGTATTGGG-3′
and antisense, 5′-GGAAGATGGTGATGGGATT-3′. The size of the PCR
products was 419 and 205 bp, respectively.
The PCR reactions were performed on a Bio-Rad S1000
thermal cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
PCR amplification was performed in a total reaction volume of 25
μl, containing 1 μl of cDNA sample, 10 pM of each primer, 2.5 mM of
deoxyribonucleotide, 10X EasyTaq buffer and 5 units of EasyTaq DNA
polymerase (TransGen Biotech, Beijing, China). The cycling
parameters were: initial denaturation at 95°C for 3 min, followed
by 27 cycles of denaturation at 95°C for 30 sec, annealing at 56°C
for 30 sec and a final extension at 72°C for 30 sec. The PCR
products were analyzed on 1.2% (w/v) agarose gels containing 0.5
μg/ml ethidium bromide and were visualized under UV light.
Quantitative PCR (qPCR)
qPCR was performed to detect CYP1B1 gene expression.
The 20-μl reaction mixture contained 4.6 μl
diethylpyrocarbonate-H2O, 1.0 μl cDNA, 2.0 μl (10 μM) of
each primer, 10 μl 2X All-in-One qPCR Mix and 0.4 μl 50X ROX
Reference Dye (GeneCopoeia). The thermal cycle program for PCR was
as follows: an initial step at 94°C for 10 min, followed by 40
cycles of PCR at 94°C for 10 sec, at 56°C for 20 sec and at 72°C
for 20 sec. The fluorescence signal was digitally collected after
each cycle of 72°C for 20 sec. Following PCR amplification, the
samples were subjected to a temperature ramp with continuous
fluorescence monitoring for melting curve analysis. LightCycler 480
analysis software (Roche Light Cycler 480, Hoffmann-La Roche, Ltd.,
Basel, Switzerland) was used to obtain the Ct values. The
2−ΔΔCt method (32) was
used to analyze the relative expression of CYP1B1 in MCF-7 or
MDA-MB-231 cells treated with Ginkgo biloba leaves extract
or ginkgo fruit extract.
Results
Effect of Ginkgo biloba extract on cell
growth
The cells were collected and counted after treatment
for 48 h. The number of MCF-7 cells in the control, first and
second groups was 4.2×106, 3.3×106 and
2.6×106, respectively. The number of MDA-MB-231 cells in
the control, first and second groups was 1.3×107,
0.9×107 and 0.6×107, respectively. The cell
numbers in the control groups were distinctly higher compared to
those in the treatment groups; in addition, the cell numbers in the
Ginkgo biloba leaves extract-treated group were
significantly higher compared to the ginkgo fruit extract-treated
group. These results indicated that treatment with Ginkgo
biloba extract significantly inhibited breast cancer cell
proliferation and the ginkgo fruit extract exerted a more potent
inhibitory effect compared to the Ginkgo biloba leaves
extract (Fig. 1).
The cell morphology in the Ginkgo biloba
extract and the control group were further examined under a
confocal laser scanning microscope after treatment for 48 h. As
shown in Fig. 1, in the Ginkgo
biloba leaves extract-treated group and the ginkgo fruit
extract-treated group, the cells were prone to grow in clusters
compared to the cells in the control group. Moreover, the ginkgo
fruit extract group exhibited more extensive cell morphology
changes compared to the Ginkgo biloba leaves extract group,
further indicating the more potent growth inhibitory effect of
ginkgo fruit extract treatment compared to the Ginkgo biloba
leaves extract treatment.
Induction of CYP1B1 expression in human
breast cancer cells treated with Ginkgo biloba extract
The gene expression of CYP1B1 was first confirmed by
RT-PCR amplification of the CYP1B1 cDNA (Fig. 2). To ascertain the mechanisms of the
inhibition effect of Ginkgo biloba extract on breast cancer
cell proliferation and growth, the CYP1B1 gene expression was
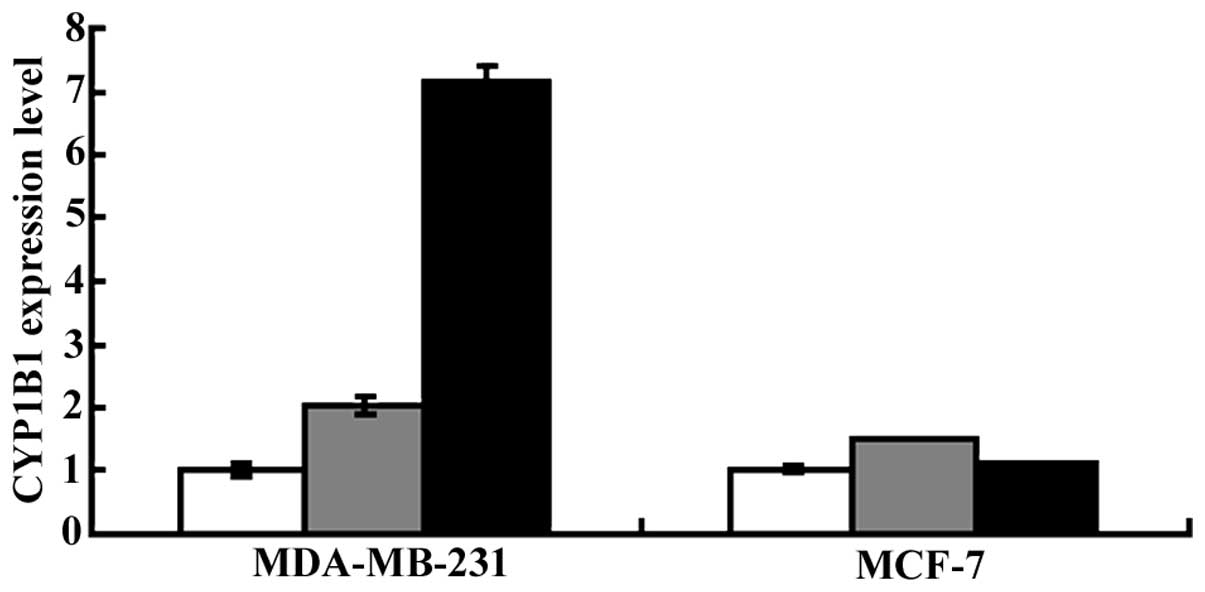
analyzed by qPCR. As shown in Fig.
3, the CYP1B1 expression in the Ginkgo biloba leaves
extract- and ginkgo fruit extract-treated MDA-MB-231 and MCF-7
cells was upregulated. In MCF-7 cells, the CYP1B1 expression was
1.5- and 1.1-fold higher in the ginkgo fruit extract- and in the
Ginkgo biloba leaves extract-treated group, respectively,
compared to that in the control group. However, in MDA-MB-231
cells, CYP1B1 expression was markedly enhanced, being 7.2- and
2.0-fold higher in the ginkgo fruit extract- and in the Ginkgo
biloba leaves extract-treated group, respectively, compared to
that in the control group.
Collectively, these results demonstrated that
treatment with the Ginkgo biloba extract enhanced CYP1B1
expression in MDA-MB-231 and MCF-7 cells. The results also
demonstrated that MDA-MB-231 cells were more sensitive to Ginkgo
biloba extract treatment compared to MCF-7 cells. Moreover, the
ginkgo fruit extract treatment resulted in a significantly higher
induction of CYP1B1 expression compared to the Ginkgo biloba
leaves extract, demonstrating the difference in the induction
potential of the two different types of Ginkgo biloba
extracts.
Discussion
In this study, we investigated the treatment effects
of the Ginkgo biloba leaves extract and ginkgo fruit extract
on breast cancer cell proliferation, cell morphology changes and
gene expression of CYP1B1. Our results demonstrated that the
treatment of MDA-MB-231 human breast cancer cells with either
Ginkgo biloba leaves extract or ginkgo fruit extract
significantly enhanced CYP1B1 gene expression. Moreover, treatment
with ginkgo fruit extract resulted in a more significant
enhancement of CYP1B1 gene expression in MDA-MB-231 cells. However,
the treatment of MCF-7 cells with either Ginkgo biloba
leaves extract or ginkgo fruit extract did not result in a
significant enhancement of CYP1B1 gene expression. Taken together,
these results indicate that the ginkgo fruit extract was the most
effective inducer of CYP1B1 gene expression in human MDA-MB-231
cells. In addition, MDA-MB-231 cell proliferation was also greatly
decreased following treatment with Ginkgo biloba leaves
extract or ginkgo fruit extract and the greatest decrease was
observed in the ginkgo fruit extract treatment group, in parallel
with the CYP1B1 gene expression changes.
CYP1B1 is an inducible enzyme regulated through the
AhR (33) and is activated by PAHs
and dioxin-like compounds (34).
CYP1B1 is regulated by several transcription factors, including the
AhR/AhR nuclear translocator complex (Ahr/ARNT), the Sp1
transcription factor, the cyclic AMP (cAMP)-response
element-binding protein (CREB) and the estrogen receptor (ER). An
estrogen-responsive element that is located in close proximity to
the Sp1 transcription factor binding site was recently shown to be
involved in the ERα regulation of CYP1B1 expression (35). Estrogen is required for the enhanced
AhR expression and constitutive induction of CYP1B1 expression in
MCF-7 cells (36), indicating the
possible involvement of estrogen in the induced expression of
CYP1B1 in breast tumor cells and the correlation of CYP1B1
expression with the ERα status (37). In ER-negative breast cancer cells,
the presence of estrogen was not able to stimulate the expression
of CYP1B1 (35), indicating the
dependence of CYP1B1 expression on ER. In addition, tamoxifen
treatment upregulated the expression of CYP1B1 in breast cancer
cells, suggesting that the induction of CYP1B1 expression may
reduce the treatment effects of tamoxifen (38). However, in some cells, the
expression of CYP1B1 does not depend on estrogen (39), suggesting that CYP1B1 expression may
be independent of estrogen and ER, although it may be induced
through alternative pathways.
7,12-Dimethylbenz(a)anthracene-induced carcinogenesis is an
extensively investigated model of PAH-induced mouse carcinogenesis
and a recent study demonstrated that CYP1B1 knockout mice exhibited
resistance to 7,12-dimethylbenz(a)anthracene-induced carcinogenesis
(40), demonstrating the metabolic
effects of CYP1B1. The results of those studies indicated that the
regulation of CYP1B1 expression is complex, involving transcription
factors, ER and AhR.
According to a previous study, treatment of
MDA-MB-231 or MCF-7 breast cancer cells with
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) inhibited cell growth,
accompanied by enhanced expression of CYP1B1 by 8-fold in MCF-7 and
30-fold in MDA-MB-231 cells (41).
It was previously demonstrated that in MCF-7 cells, the DNA
synthesis was significantly suppressed following treatment with
TCDD, suggesting an AhR-independent pathway of TCDD-induced
antiproliferation in breast cancer cells (42). Moreover, Larsen et
al(43) observed that TCDD
induced the expression of CYP1B1 mRNA in ER-negative primary human
breast epithelial cells, suggesting an alternative pathway of
TCDD-induced CYP expression in human breast cancer cells instead of
the ER-dependent one. Larsen et al(43) also demonstrated that in ER-positive
and ER-negative breast cancer cells, CYP1B1 was expressed
constitutively and was induced by TCDD (44). Results of the present study have
shown that in ER-negative breast cancer cells, the inhibitory
effects of Ginkgo biloba extract on MDA-MB-231 cell
proliferation were correlated with CYP1B1 expression induction,
which may occur through an ER and AhR-independent pathway. However,
the underlying mechanisms require elucidation through further
investigations.
Acknowledgements
This study was funded by the ‘Financial support for
selected researchers back from abroad (2011)’ project of the
Liaoning Province.
References
|
1
|
McKenna DJ, Jones K and Hughes K:
Efficacy, safety, and use of Ginkgo biloba in clinical and
preclinical applications. Altern Ther Health Med. 7:70–86. 88–90.
2001.PubMed/NCBI
|
|
2
|
No authors listed. EGb 761: Ginkgo
biloba extract, Ginkor. Drugs R D. 4:188–193. 2003. View Article : Google Scholar
|
|
3
|
Brunetti L, Orlando G, Menghini L,
Ferrante C, Chiavaroli A and Vacca M: Ginkgo biloba leaf
extract reverses amyloid beta-peptide-induced isoprostane
production in rat brain in vitro. Planta Med. 72:1296–1299. 2006.
View Article : Google Scholar
|
|
4
|
Maclennan KM, Darlington CL and Smith PF:
The CNS effects of Ginkgo biloba extracts and ginkgolide B.
Prog Neurobiol. 67:235–257. 2002.
|
|
5
|
Yao ZX, Han Z, Drieu K and Papadopoulos V:
Ginkgo biloba extract (Egb 761) inhibits β-amyloid
production by lowering free cholesterol levels. J Nutr Biochem.
15:749–756. 2004. View Article : Google Scholar
|
|
6
|
Gong QH, Wu Q, Huang XN, Sun AS, Nie J and
Shi JS: Protective effect of Ginkgo biloba leaf extract on
learning and memory deficit induced by aluminum in model rats. Chin
J Integr Med. 12:37–41. 2006.
|
|
7
|
Hrehorovska M, Burda J, Domorakova I and
Mechirova E: Effect of Tanakan on postischemic activity of protein
synthesis machinery in the rat brain. Gen Physiol Biophys.
23:457–465. 2004.PubMed/NCBI
|
|
8
|
Koltermann A, Hartkorn A, Koch E, Furst R,
Vollmar AM and Zahler S: Ginkgo biloba extract EGb 761
increases endothelial nitric oxide production in vitro and in vivo.
Cell Mol Life Sci. 64:1715–1722. 2007. View Article : Google Scholar
|
|
9
|
Oh SM and Chung KH: Estrogenic activities
of Ginkgo biloba extracts. Life Sci. 74:1325–1335. 2004.
View Article : Google Scholar
|
|
10
|
Oh SM and Chung KH: Antiestrogenic
activities of Ginkgo biloba extracts. J Steroid Biochem Mol
Biol. 100:167–176. 2006. View Article : Google Scholar
|
|
11
|
Feng X, Zhang L and Zhu H: Comparative
anticancer and antioxidant activities of different ingredients of
Ginkgo biloba extract (EGb 761). Planta Med. 75:792–796.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen XH, Miao YX, Wang XJ, et al: Effects
of Ginkgo biloba extract EGb761 on human colon
adenocarcinoma cells. Cell Physiol Biochem. 27:227–232. 2011.
|
|
13
|
Chao JC and Chu CC: Effects of Ginkgo
biloba extract on cell proliferation and cytotoxicity in human
hepatocellular carcinoma cells. World J Gastroenterol. 10:37–41.
2004.
|
|
14
|
Zhang Y, Chen AY, Li M, Chen C and Yao Q:
Ginkgo biloba extract kaempferol inhibits cell proliferation
and induces apoptosis in pancreatic cancer cells. J Surg Res.
148:17–23. 2008. View Article : Google Scholar
|
|
15
|
Xu AH, Chen HS, Sun BC, et al: Therapeutic
mechanism of Ginkgo biloba exocarp polysaccharides on
gastric cancer. World J Gastroenterol. 9:2424–2427. 2003.
|
|
16
|
Bernhardt R: Cytochromes P450 as versatile
biocatalysts. J Biotechnol. 124:128–145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mao P, Qiao DJ and Ma XR: Cytochrome P450
and iatrology. Chin J Antibiot. 36:93–101. 2011.
|
|
18
|
Orellana M and Guajardo V: Cytochrome P450
activity and its alteration in different diseases. Rev Med Chil.
132:85–94. 2004.(In Spanish).
|
|
19
|
Oyama T, Kagawa N, Kunugita N, et al:
Expression of cytochrome P450 in tumor tissues and its association
with cancer development. Front Biosci. 9:1967–1976. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murray GI, Weaver RJ, Paterson PJ, Ewen
SW, Melvin WT and Burke MD: Expression of xenobiotic metabolizing
enzymes in breast cancer. J Pathol. 169:347–353. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mace K, Bowman ED, Vautravers P, Shields
PG, Harris CC and Pfeifer AM: Characterisation of
xenobiotic-metabolising enzyme expression in human bronchial mucosa
and peripheral lung tissues. Eur J Cancer. 34:914–920. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Murray GI, McFadyen MC, Mitchell RT,
Cheung YL, Kerr AC and Melvin WT: Cytochrome P450 CYP3A in human
renal cell cancer. Br J Cancer. 79:1836–1842. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Murray GI: The role of cytochrome P450 in
tumour development and progression and its potential in therapy. J
Pathol. 192:419–426. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hashizume T, Imaoka S, Mise M, et al:
Involvement of CYP2J2 and CYP4F12 in the metabolism of ebastine in
human intestinal microsomes. J Pharmacol Exp Ther. 300:298–304.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bruno RD and Njar VC: Targeting cytochrome
P450 enzymes: a new approach in anti-cancer drug development.
Bioorg Med Chem. 15:5047–5060. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Murray GI, Taylor MC, McFadyen MC, et al:
Tumor-specific expression of cytochrome P450 CYP1B1. Cancer Res.
57:3026–3031. 1997.PubMed/NCBI
|
|
27
|
Gibson P, Gill JH, Khan PA, et al:
Cytochrome P450 1B1 (CYP1B1) is overexpressed in human colon
adenocarcinomas relative to normal colon: implications for drug
development. Mol Cancer Ther. 2:527–534. 2003.PubMed/NCBI
|
|
28
|
Tokizane T, Shiina H, Igawa M, et al:
Cytochrome P450 1B1 is overexpressed and regulated by
hypomethylation in prostate cancer. Clin Cancer Res. 11:5793–5801.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deng Y, Bi HC, Zhao LZ, et al: Induction
of cytochrome P450s by terpene trilactones and flavonoids of the
Ginkgo biloba extract EGb 761 in rats. Xenobiotica.
38:465–481. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He N, Cai HB, Xie HG, Collins X, Edeki TI
and Strom SC: Induction of cyp3a in primary cultures of human
hepatocytes by ginkgolides A and B. Clin Exp Pharmacol Physiol.
34:632–635. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li L, Stanton JD, Tolson AH, Luo Y and
Wang H: Bioactive terpenoids and flavonoids from Ginkgo
biloba extract induce the expression of hepatic
drug-metabolizing enzymes through pregnane X receptor, constitutive
androstane receptor, and aryl hydrocarbon receptor-mediated
pathways. Pharm Res. 26:872–882. 2009.PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCTmethod. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bhattacharyya KK, Brake PB, Eltom SE, Otto
SA and Jefcoate CR: Identification of a rat adrenal cytochrome P450
active in polycyclic hydrocarbon metabolism as rat CYP1B1.
Demonstration of a unique tissue-specific pattern of hormonal and
aryl hydrocarbon receptor-linked regulation. J Biol Chem.
270:11595–11602. 1995. View Article : Google Scholar
|
|
34
|
Shimada T, Oda Y, Gillam EM, Guengerich FP
and Inoue K: Metabolic activation of polycyclic aromatic
hydrocarbons and other procarcinogens by cytochromes P450 1A1 and
P450 1B1 allelic variants and other human cytochromes P450 in
Salmonella typhimurium NM 2009. Drug Metab Dispos.
29:1176–1182. 2001.PubMed/NCBI
|
|
35
|
Tsuchiya Y, Nakajima M, Kyo S, Kanaya T,
Inoue M and Yokoi T: Human CYP1B1 is regulated by estradiol via
estrogen receptor. Cancer Res. 64:3119–3125. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Spink DC, Katz BH, Hussain MM, Pentecost
BT, Cao Z and Spink BC: Estrogen regulates Ah responsiveness in
MCF-7 breast cancer cells. Carcinogenesis. 24:1941–1950. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sasaki M, Tanaka Y, Kaneuchi M, Sakuragi N
and Dahiya R: CYP1B1 gene polymorphisms have higher risk for
endometrial cancer, and positive correlations with estrogen
receptor α and estrogen receptor β expressions. Cancer Res.
63:3913–3918. 2003.PubMed/NCBI
|
|
38
|
Brockdorff BL, Skouv J, Reiter BE and
Lykkesfeldt AE: Increased expression of cytochrome p450 1A1 and 1B1
genes in anti-estrogen-resistant human breast cancer cell lines.
Int J Cancer. 88:902–906. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Berge G, Mollerup S, Øvrebø S, et al: Role
of estrogen receptor in regulation of polycyclic aromatic
hydrocarbon metabolic activation in lung. Lung Cancer. 45:289–297.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Buters JT, Sakai S, Richter T, et al:
Cytochrome P450 CYP1B1 determines susceptibility to
7,12-dimethylbenz[a]anthracene-induced lymphomas. Proc Natl Acad
Sci USA. 96:1977–1982. 1999.
|
|
41
|
Dohr O, Vogel C and Abel J: Different
response of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-sensitive
genes in human breast cancer MCF-7 and MDA-MB 231 cells. Arch
Biochem Biophys. 321:405–412. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yoshioka H, Hiromori Y, Aoki A, et al:
Possible aryl hydrocarbon receptor-independent pathway of
2,3,7,8-tetrachlorodibenzo-p-dioxin-induced antiproliferative
response in human breast cancer cells. Toxicol Lett. 211:257–265.
2012. View Article : Google Scholar
|
|
43
|
Larsen MC, Angus WG, Brake PB, Eltom SE,
Sukow KA and Jefcoate CR: Characterization of CYP1B1 and CYP1A1
expression in human mammary epithelial cells: role of the aryl
hydrocarbon receptor in polycyclic aromatic hydrocarbon metabolism.
Cancer Res. 58:2366–2374. 1998.
|
|
44
|
Angus WG, Larsen MC and Jefcoate CR:
Expression of CYP1A1 and CYP1B1 depends on cell-specific factors in
human breast cancer cell lines: role of estrogen receptor status.
Carcinogenesis. 20:947–955. 1999. View Article : Google Scholar : PubMed/NCBI
|