Introduction
Myocardial infarction (MI) may induce severe
alterations of the cardiac contractile function that may, in turn,
lead to heart failure (HF). Patients with HF exhibit progressive
left ventricular (LV) dilation, ventricular remodeling and systolic
dysfunction (1,2). Ventricular remodeling, the main
pathological process underlying chronic heart failure (CHF), is one
of the determinants affecting morbidity and mortality. Therefore,
the inhibition of ventricular remodeling is crucial in the
prevention of HF. The ubiquitin-proteasome system (UPS) signaling
pathway plays a critical role in the modulation of the development
of cardiac remodeling. An increasing number of data indicate that
the UPS is suppressed in CHF (3).
The complexity of cardiac proteasomes provides dynamic regulatory
venues for proteasome function (4).
Detailed knowledge regarding proteasome dynamics in cardiac disease
phenotypes is essential for the development of therapeutic
strategies (4). Previous studies
demonstrated an effective reduction of LV hypertrophy via the
inhibition of proteasome function and proteasome inhibitors were
used to prevent or even induce a regression of LV hypertrophy in
animal models (5,6).
The renin-angiotensin-aldosterone system is involved
in the pathogenesis and progression of numerous cardiovascular and
renal pathological conditions, including structural cardiac
remodeling, MI, HF, hypertension and chronic kidney disease
(7). Accumulating evidence
indicates that angiotensin II is crucial in the transition from
compensated to decompensated cardiac hypertrophy or failure
(8,9). Experimental studies also demonstrated
that the inhibition of the renin-angiotensin system (RAS) with
angiotensin II type 1 receptor (AT1R)-blockers improves
cardiovascular function and remodeling and exerts beneficial
effects on survival in post-ischemic HF rats (10,11).
Therefore, the aim of this study was to investigate whether the UPS
signaling pathway is associated with the inhibition of cardiac
remodeling induced by irbesartan.
Materials and methods
Animals
A total of 93 male Sprague-Dawley rats, weighing
200–250 g, were purchased from the Animal Center of Bengbu Medical
College (Anhui, China). The rats were fed normal chow, had free
access to water and were housed at a constant temperature of 21±1°C
with a fixed 12-h light/dark cycle. The rats were anesthetized with
an intraperitoneal injection of 3.5% chloral hydrate sodium (1
ml/100 g) and were then intubated and ventilated with a rodent
respirator. A thoracotomy was performed at the fourth or fifth
intercostal space in order to expose the heart. A 5-0 suture was
placed under the left descending coronary artery and its circumflex
branch and the suture ends were threaded through polyethylene tubes
to form snares for reversible coronary artery occlusion. Perpetual
ligation of the left anterior descending artery was performed and
paleness of the anterior wall of the left ventricle indicated a
successful model. The mortality rate within 24 h following this
procedure was ~46%. Complete data regarding the infarct size were
obtained from 46 rats (the rats that died and those with an infarct
size occupying <35 or >55% of the heart were excluded). The
surviving rats were randomly divided into five groups: the placebo
(control) group (n=12), the Ir5 group (n=10, 5 mg/kg/day
irbesartan), the Ir25 group, (n=10, 25 mg/kg/day irbesartan), the
Ir50 group (n=10, 50 mg/kg/day irbesartan) and the sham-operated
group (n=8). Except the sham-operated group, there was no
difference in MI size and heart rate (HR) among the four remaining
groups. Irbesartan was obtained from Hangzhou Sanofi Minsheng
Pharmaceutical Co., Ltd. (Hangzhou, China). The sham-operated
control animals underwent the same procedure, without coronary
artery ligation. After the experiment, the rats were housed in
polyethylene cages (3–4 rats/cage), fed standard laboratory chow
and had free access to tap water, starting 24 h after the ligation
and for a total of 6 weeks.
At the end of this study, 24 h after the last
treatment, the rats were anesthetized with an intraperitoneal
injection of 0.4 g/kg chloral hydrate and deep anesthesia was
maintained with intermittent intraperitoneal injections of 0.016
g/kg chloral hydrate. A tracheotomy and endotracheal intubation
were performed with a cannula connected to an animal respirator.
The rats were ventilated with O2-enriched air at 70
breaths/min and maintained the tidal volume at 1.0 ml/100 mg body
weight (BW). The body temperature was sustained at 37°C using a
heating lamp. The haemodynamic parameters were measured
continuously by intubation of the left common carotid artery, using
the MedLab biological signal collecting and processing system
(Nanjing Medease Science and Technology Co., Ltd., Nanjing, China).
The standard electrocardiogram was also recorded continuously.
The hearts were arrested in diastole by
intraperitoneal injection of ~2–3 ml KCl and were removed from the
animals, cleaned, dried on filter paper and weighed. Subsequently,
the ventricles were separated and also weighed.
All the procedures were approved by the Ethics
Committee for the use of experimental animals in Bengbu Medical
College.
Determination of total cardiac
collagen
Mallory’s trichrome staining method was used to
measure total collagen deposition in post-MI hearts with and
without treatment. A total of 8 non-vascular areas were randomly
selected in each slide under a microscope. The average was
determined by a computer image analysis system.
TNF-α, 20S proteasome and ubiquitin
protein plasma concentrations
TNF-α, 20S proteasome and ubiquitin protein
detection was performed with the enzyme linked immunosorbent assay
(ELISA) kit (Shanghai Yu Bo Biological Technology Co., Ltd.,
Shanghai, China), according to the manufacturer’s instructions. The
minimum level for detection was 2 ng/l and the concentration of
TNF-α, 20S proteasome and ubiquitin protein were calculated based
on the standard curve.
Statistical analysis
Data are presented as means ± standard error of the
mean. The differences between the values obtained at 6 weeks were
evaluated using analysis of variance (ANOVA), followed by Dunnett’s
or Kruskal-Wallis test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Haemodynamic measurements
The haemodynamic parameters were measured in the
anaesthetized animals 6 weeks following MI (Table I). Compared to the sham-operated
group, the MI groups exhibited reduced values of left ventricular
systolic pressure (LVSP), maximum rate of left ventricular pressure
rise (dP/dtmax) and maximum rate of left ventricular
pressure decrease (dP/dtmin), accompanied by increased
values of HR and left ventricular end-diastolic pressure (LVEDP).
The MI rats receiving irbesartan exhibited dose-dependently
elevated values of LVSP, dP/dtmax and
dP/dtmin compared to the control group. In addition, HR
and LVEDP were dose-dependently decreased in the MI-Ir groups
compared to the MI control group.
 | Table IHaemodynamic parameters measured in
anaesthetized rats in the different experimental groups. |
Table I
Haemodynamic parameters measured in
anaesthetized rats in the different experimental groups.
| Groups |
|---|
|
|
|---|
| Parameters | Sham | MI-control | MI-Ir5 | Ml-Ir25 | Ml-Ir50 |
|---|
| HR (bpm) | 400±15 |
439±19a | 432±12 |
424±25b |
413±22b |
| LVSP (mmHg) | 90.21±1.40 |
67.15±4.8a | 70.76±2.31 |
75±1.83b |
85.13±0.85b |
| LVEDP (mmHg) | 6.41±0.18 |
27.23±0.43a | 25.09±1.06 |
19.82±2.27b |
12.4±1.73b |
| dP/dtmax
(mmHg/s) | 3876.84±382.25 |
1151.31±86.53a | 1297±46.80 |
1876.55±2.83b |
2493.32±447.12b |
| dP/dtmin
(mmHg/s) | 3576.54±315.71 |
934±265.68a | 987.98±8.81 |
1257±9.37b |
1532±273.35b |
Cardiac morphometric parameters
Although BW did not differ between sham-operated and
MI rats at the end of the treatment period, it was mildly reduced
following treatment with irbesartan (Table II), despite similar food
consumption among the five groups. Whereas in MI the heart weight
(HW) and the HW/BW ratio were significantly increased, treatment
with irbesartan significantly and dose-dependently reduced HW and
HW/BW. Collagen density (CD) was found to be significantly
increased in the infarcted myocardium and treatment with irbesartan
dose-dependently reduced the collagen content.
 | Table IIBody weight and cardiac morphometric
parameters measured in the different experimental groups. |
Table II
Body weight and cardiac morphometric
parameters measured in the different experimental groups.
| Groups |
|---|
|
|
|---|
| Parameters | Sham | MI-control | MI-Ir5 | Ml-Ir25 | Ml-Ir50 |
|---|
| BW (g) | 460±9 | 459±8 | 455±3 |
441±7b |
424±6b |
| HW (g) | 1276±14 |
1678±29a | 1629±46 |
1466±31b |
1282±26b |
| HW/BW | 2.77±1.56 |
3.69±3.63a | 3.60±15.3 |
3.32±4.43b |
3.02±4.33b |
| CD of infact
(%) | | 62±6 | 60±8 |
55±7b |
50±4b |
Myocardial concentration of TNF-α
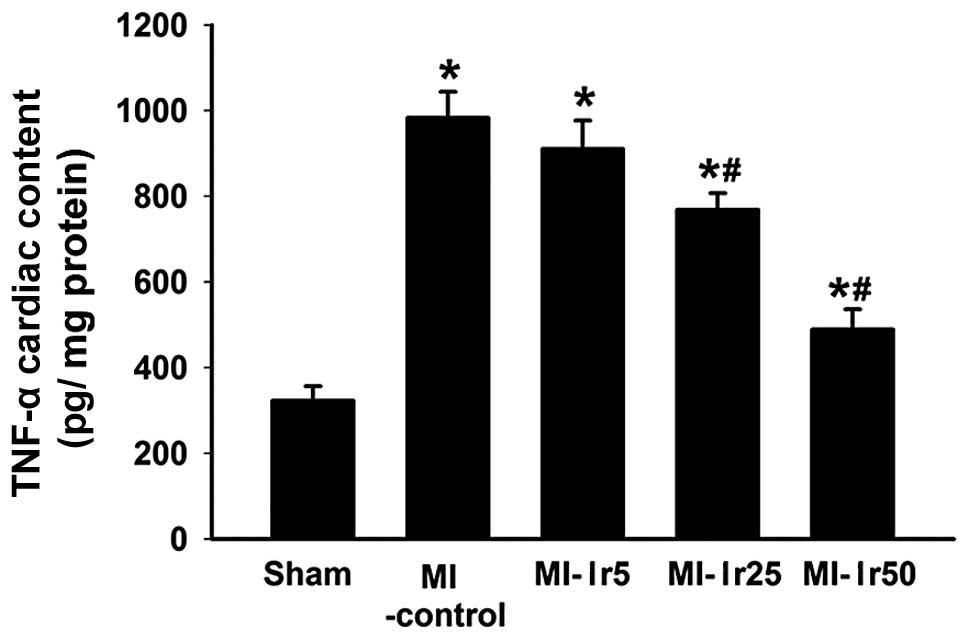
A TNF-α concentration of 313±32 pg/mg was detected
in the left ventricle of sham-operated rats (Fig. 1). This value was lower in the left
ventricle of rats in the MI, MI-Ir5, -Ir25 and -Ir50 groups
(998±139 pg/mg, P<0.01 vs. sham; 911±56 pg/mg, P<0.01 vs.
sham; 735±76 pg/mg, P<0.01 vs. sham; and 501±69 pg/mg, P<0.05
vs. sham, respectively). Treatment with irbesartan (25 and 50
mg/kg/day) attenuated TNF-α concentration compared to the
MI-control group (P<0.05). By contrast, no differences were
observed between the MI-control and MI-Ir5 groups.
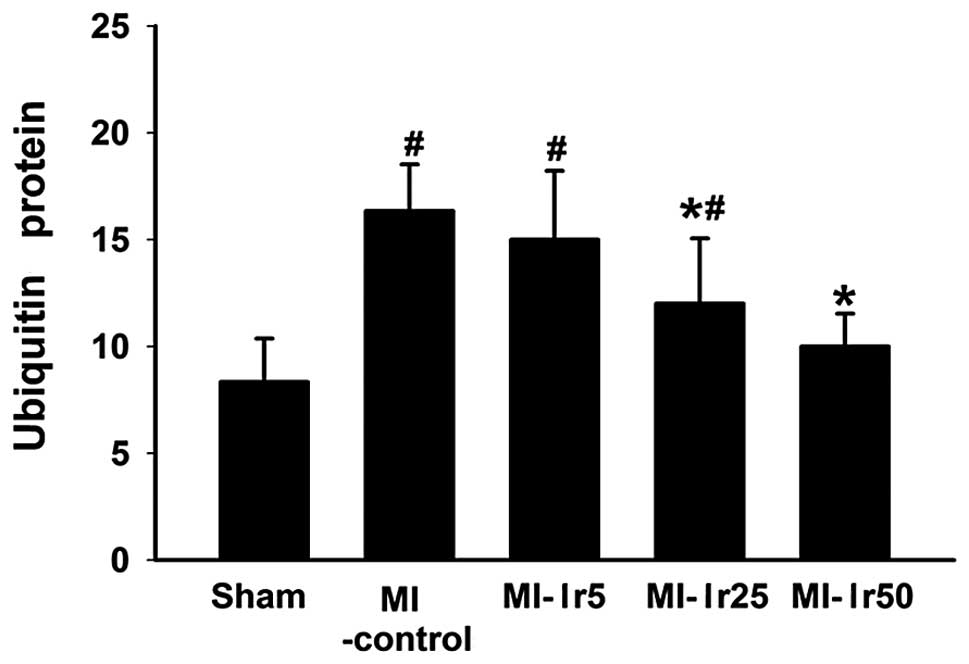
Ubiquitin protein concentration
Bar charts illustrating ubiquitin concentration in
the sham, MI-control, MI-Ir5, MI-Ir25 and MI-Ir50 groups are
presented in Fig. 2. In the sham
group, the concentration of ubiquitin was significantly lower
compared to that in the MI-control, MI-Ir5 and MI-Ir25 groups
(P<0.05). By contrast, no differences were observed between the
MI-control and MI-Ir5 groups, or between the sham and MI-Ir50
groups.
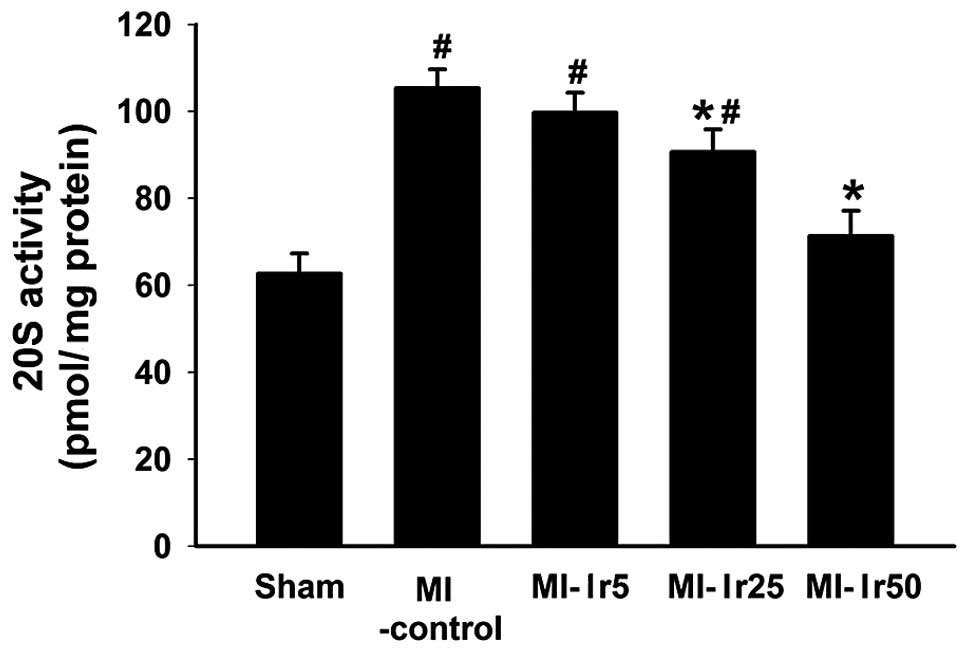
20S proteasome activity
Bar charts illustrating 20S proteasome activity in
the sham, MI-control, MI-Ir5, MI-Ir25 and MI-Ir50 groups are
presented in Fig. 3. In the sham
group, the 20S proteasome activity was significantly lower compared
to that in the MI-control, MI-Ir5 and MI-Ir25 groups (P<0.05).
By contrast, no differences were observed between the MI-control
and MI-Ir5 groups, or between the sham and MI-Ir50 groups.
Discussion
A major finding in the present study was the fact
that the AT1R antagonist irbesartan strongly and dose-dependently
improved the morphometric parameters and the haemodynamic status of
the animals in the post-infarction rat model of CHF. Irbesartan was
shown to prevent post-infarction ventricular remodeling through the
inhibition of ubiquitin protein expression and 20S proteasome
activity and to decrease TNF-α generation.
MI Sprague-Dawley rats were orally treated with
irbesartan for 6 weeks (5, 25 or 50 mg/kg/day). Subsequently,
morphology and cardiac haemodynamics were assessed. From the
morphological and cardiac haemodynamic point of view, the
post-infarction CHF rat model was characterized by an important
cardiac remodeling process including the HW/BW ratio and CD.
Compared to the sham-operated group, the HW/BW ratio, CD and LVEDP
were significantly increased in the MI control group, whereas LVSP
and LV contractility (dP/dt) were significantly reduced. These
pathophysiological alterations in the cardiac structure and
function were shown to be intimately associated with increased
morbidity and mortality (1,2).
An increased tissue RAS activity is likely to play a
key role in the development of cardiac hypertrophy, remodeling and
hypertension. The RAS components, including renin, constitute an
autocrine/paracrine system in the myocardium (12). The inhibition of the
renin-angiotensin-aldosterone system is therefore a therapeutic
target. Angiotensin-converting enzyme inhibitors block the
conversion of angiotensin I to angiotensin II and angiotensin
receptor blockers selectively antagonize angiotensin II at the
AT1Rs (13). Irbesartan, a specific
AT1R-blocker, was shown to prolong survival in CHF rats (14) by improving LV function and
haemodynamics. In addition, irbesartan was shown to interfere with
the cardiac remodeling process by preventing hypertrophy and the
development of pericoronary fibrosis (10). Our study demonstrated that
irbesartan strongly and dose-dependently reduced the HW/BW ratio
and CD and limited the elevation of LVEDP in MI rats. Furthermore,
irbesartan increased dP/dt and cardiac index values. Therefore,
treatment with the AT1R-blocker irbesartan attenuated the
development of cardiac remodeling. Our observations were also
consistent with the results of previous experimental studies, which
suggested that AT1R-blockers attenuated myocardial remodeling in
diabetic and post-infarcted hearts (15,16)
and demonstrated that irbesartan may significantly attenuate the
structural and functional remodeling induced by experimental
thyrotoxic cardiomyopathy (17).
A previous study demonstrated that TNF-α is elevated
in patients with acute MI (18).
Moreover, clinical and experimental data verified that the
increased expression of TNF-α may expedite the progress of
ventricular remodeling and further compromise cardiac function
(19). However, rat cardiac
function was shown to improve with TNF-α receptor knockout
(20). Our study demonstrated that,
compared to the sham-operated group, TNF-α was significantly
increased in the MI control group. It was previously reported that
an increase in AT1R density was observed in the peri-infarction and
infarction zones of the myocardium following MI in rats (21). It was also demonstrated that
AT1R-blockers were able to reduce soluble TNF-α levels in patients
with HF (22,23). Our study demonstrated that
irbesartan strongly and dose-dependently decreased TNF-α in MI
rats.
Protein components of the UPS have also been
identified in extracellular fluids, such as blood plasma and
cerebrospinal, epididymal and bronchoalveolar fluids (24). To elucidate whether irbesartan
reshapes the LV through the UPS signaling pathway, ubiquitin
protein expression and 20S proteasome activity were assessed by
ELISA. An increasing number of evidence indicates that patients
with unstable angina pectoris (25)
and those with CHF (26) exhibit
different degrees of activation of the UPS. The present study
demonstrated that ubiquitin and the 20S proteasome were
functionally active in post-infarction ventricular remodeling.
These observations were also consistent with previous experimental
results, which reported higher myocardial ubiquitin levels and
proteasome activity in type 2 diabetic subjects with MI (27). We also observed that irbesartan
mainly inhibited the activity of UPS, indicating that irbesartan
may exert a therapeutic effect on post-infarction ventricular
remodeling, partly via the inhibition of ubiquitin expression and
20S proteasome activity. This finding was consistent with those of
previous studies, which demonstrated that the inhibition of the
proteasome resulted in the prevention of or decrease in
pressure-induced hypertrophy in animals (6,28). UPS
is an important quality control system for myocardial protein and
an imbalance in this system may lead to HF (29). We concluded that an inappropriate
increase in proteasome activity and the resulting acceleration of
protein degradation may be the main mechanism underlying CHF.
Therefore, the UPS pathway is crucial in post-infarction
ventricular remodeling.
In conclusion, irbesartan delayed the exacerbation
of post-infarction HF in rats through reversing cardiac remodeling.
Cardiac UPS plays a critical role in the reversal of
post-infarction HF by irbesartan in rats. Our study may provide
novel insight into the mechanism underlying the beneficial effect
of irbesartan on post-infarction HF. However, the upstream factors
in the regulation pathway have yet to be determined. Therefore, the
precise mechanism responsible for myocardial remodeling remains to
be elucidated.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (81170046) and the Science
Foundation of Bengbu City of China (no. 201103033).
References
|
1
|
Jessup M, Abraham WT, Casey DE, Feldman
AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS,
Silver MA, Stevenson LW and Yancy CW: 2009 focused update: ACCF/AHA
guidelines for the diagnosis and management of heart failure in
adults: a report of the American College of Cardiology
Foundation/American Heart Association. Task Force on Practice
Guidelines: developed in collaboration with the International
Society for Heart and Lung Transplantation. Circulation.
119:1977–2016. 2009.
|
|
2
|
Dickstein K, Cohen-Solal A, Filippatos G,
McMurray JJ, Ponikowski P, Poole-Wilson PA, Strömberg A, van
Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M,
Priori SG and Swedberg K; ESC Committee for Practice Guidelines
(CPG). ESC guidelines for the diagnosis and treatment of acute and
chronic heart failure 2008: the Task Force for the Diagnosis and
Treatment of Acute and Chronic Heart failure 2008 of the European
Society of Cardiology. Developed in collaboration with the Heart
Failure Association of the ESC (HFA) and endorsed by the European
Society of Intensive Care Medicine (ESICM). Eur Heart J.
29:2388–2442. 2008.
|
|
3
|
Wohlschlaeger J, Sixt SU, Stoeppler T,
Schmitz KJ, Levkau B, Tsagakis K, Vahlhaus C, Schmid C, Peters J,
Schmid KW, Milting H and Baba HA: Ventricular unloading is
associated with increased 20s proteasome protein expression in the
myocardium. J Heart Lung Transplant. 29:125–132. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Drews O, Tsukamoto O, Liem D, Streicher J,
Wang Y and Ping P: Differential regulation of proteasome function
in isoproterenol-induced cardiac hypertrophy. Circ Res.
107:1094–1101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stansfield WE, Tang RH, Moss NC, Baldwin
AS, Willis MS and Selzman CH: Proteasome inhibition promotes
regression of left ventricular hypertrophy. Am J Physiol Heart Circ
Physiol. 294:H645–H650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hedhli N, Lizano P, Hong C, Fritzky LF,
Dhar SK, Liu H, Tian Y, Gao S, Madura K, Vatner SF and Depre C:
Proteasome inhibition decreases cardiac remodeling after initiation
of pressure overload. Am J Physiol Heart Circ Physiol.
295:H1385–H1393. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Probstfield JL and O’Brien KD: Progression
of cardiovascular damage: the role of renin-angiotensin system
blockade. Am J Cardiol. 105:10A–20A. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamamoto E, Kataoka K, Shintaku H,
Yamashita T, Tokutomi Y, Dong YF, Matsuba S, Ichijo H, Ogawa H and
Kim-Mitsuyama S: Novel mechanism and role of angiotensin II induced
vascular endothelial injury in hypertensive diastolic heart
failure. Arterioscler Thromb Vasc Biol. 27:2569–2575. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamamoto E, Kataoka K, Yamashita T,
Tokutomi Y, Dong YF, Matsuba S, Ogawa H and Kim-Mitsuyama S: Role
of xanthine oxidoreductase in the reversal of diastolic heart
failure by candesartan in the salt-sensitive hypertensive rat.
Hypertension. 50:657–662. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gervais M, Fornes P, Richer C, Nisato D
and Giudicelli JF: Effects of angiotensin II AT1-receptor blockade
on coronary dynamics, function, and structure in postischemic heart
failure in rats. J Cardiovasc Pharmacol. 36:329–337. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Richer C, Fornes P, Cazaubon C, Domergue
V, Nisato D and Giudicelli JF: Effects of long-term angiotensin II
AT1 receptor blockade on survival, hemodynamics and cardiac
remodeling in chronic heart failure in rats. Cardiovasc Res.
41:100–108. 1999. View Article : Google Scholar
|
|
12
|
Whaley-Connell A, Habibi J, Cooper SA,
Demarco VG, Hayden MR, Stump CS, Link D, Ferrario CM and Sowers JR:
Effect of renin inhibition and AT1R blockade on myocardial
remodeling in the transgenic Ren2 rat. Am J Physiol Endocrinol
Metab. 295:E103–E109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Forni V, Wuerzner G, Pruijm M and Burnier
M: Long-term use and tolerability of irbesartan for control of
hypertension. Integr Blood Press Control. 4:17–26. 2011.PubMed/NCBI
|
|
14
|
Richer C, Gervais M, Fornes P and
Giudicelli JF: Combined selective angiotensin II AT1-receptor
blockade and angiotensin I-converting enzyme inhibition on coronary
flow reserve in postischemic heart failure in rats. J Cardiovasc
Pharmacol. 34:772–781. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsutsui H, Matsushima S, Kinugawa S, Ide
T, Inoue N, Ohta Y, Yokota T, Hamaguchi S and Sunagawa K:
Angiotensin II type 1 receptor blocker attenuates myocardial
remodeling and preserves diastolic function in diabetic heart.
Hypertens Res. 30:439–449. 2007. View Article : Google Scholar
|
|
16
|
Zhang RY, Wang LF, Zhang L, Meng XN, Li SJ
and Wang WR: Effects of angiotensin converting enzyme inhibitor,
angiotensin II type I receptor blocker and their combination on
postinfarcted ventricular remodeling in rats. Chin Med J (Engl).
119:649–655. 2006.
|
|
17
|
Kim BH, Cho KI, Kim SM, Kim JY, Choi BG,
Kang JH, Jeon YK, Kim SS, Kim SJ, Kim YK and Kim IJ: Irbesartan
prevents myocardial remodeling in experimental thyrotoxic
cardiomyopathy. Endocr J. 59:919–929. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Biswas S, Ghoshal PK, Mandal SC and Mandal
N: Relation of anti- to pro-inflammatory cytokine ratios with acute
myocardial infarction. Korean J Intern Med. 25:44–50. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bradham WS, Bozkurt B, Gunasinghe H, Mann
D and Spinale FG: Tumor necrosis factor-alpha and myocardial
remodeling in progression of heart failure: a current perspective.
Cardiovasc Res. 53:822–830. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kelly ML, Wang M, Crisostomo PR,
Abarbanell AM, Herrmann JL, Weil BR and Meldrum DR: TNF receptor 2,
not TNF receptor 1, enhances mesenchymal stem cell-mediated cardiac
protection following acute ischemia. Shock. 33:602–607. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Daniels MC, Keller RS and de Tombe PP:
Losartan prevents contractile dysfunction in rat myocardium after
left ventricular myocardial infarction. Am J Physiol Heart Circ
Physiol. 281:H2150–H2158. 2001.PubMed/NCBI
|
|
22
|
Gurlek A, Kilickap M, Dincer I, Dandachi
R, Tutkak H and Oral D: Effect of losartan on circulating TNFα
levels and left ventricular systolic performance in patients with
heart failure. J Cardiovasc Risk. 8:279–282. 2001.
|
|
23
|
Tsutamoto T, Wada A, Maeda K, Mabuchi N,
Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, Matsumoto T and
Kinoshita M: Angiotensin II type 1 receptor antagonist decreases
plasma levels of tumor necrosis factor alpha, interleukin-6 and
soluble adhesion molecules in patients with chronic heart failure.
J Am Coll Cardiol. 35:714–721. 2000. View Article : Google Scholar
|
|
24
|
Sixt SU and Dahlmann B: Extracellular,
circulating proteasomes and ubiquitin - incidence and relevance.
Biochim Biophys Acta. 1782:817–823. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marfella R, Di Filippo C, Portoghese M,
Siniscalchi M, Martis S, Ferraraccio F, Guastafierro S, Nicoletti
G, Barbieri M, Coppola A, Rossi F, Paolisso G and D’Amico M: The
ubiquitin-proteasome system contributes to the inflammatory injury
in ischemic diabetic myocardium: the role of glycemic control.
Cardiovasc Pathol. 18:332–345. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Hees HW, Li YP, Ottenheijm CA, Jin B,
Pigmans CJ, Linkels M, Dekhuijzen PN and Heunks LM: Proteasome
inhibition improves diaphragm function in congestive heart failure
rats. Am J Physiol Lung Cell Mol Physiol. 294:L1260–L1268.
2008.PubMed/NCBI
|
|
27
|
Barbieri M, Marfella R, Rizzo MR, Boccardi
V, Siniscalchi M, Schiattarella C, Siciliano S, Lemme P and
Paolisso G: The -8 UTR C/G polymorphism of PSMA6 gene is associated
with susceptibility to myocardial infarction in type 2 diabetic
patients. Atherosclerosis. 201:117–123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Friehs I: Proteasome inhibition in
hypertrophied myocardium. Am J Physiol Heart Circ Physiol.
295:H1373–H1374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Su H and Wang X: The ubiquitin-proteasome
system in cardiac proteinopathy: a quality control perspective.
Cardiovasc Res. 85:253–262. 2010. View Article : Google Scholar : PubMed/NCBI
|