Introduction
Organophosphorus compounds (OPs) are commonly used
in agriculture as insecticides. Acute OP intoxication results
primarily from the irreversible inhibition of acetylcholinesterase
(AChE), which leads to acetylcholine accumulation and,
consequently, overstimulation of cholinergic receptors in the
peripheral and central nervous systems. Exposure to OPs is
responsible for a significant number of deaths by poisoning
annually (1).
The therapeutic strategies to decrease OP toxicity
include atropine, to reduce the muscarinic syndrome, and
pralidoxime, to reactivate OP-inhibited AChE (2). However, AChE inhibition by certain OPs
may be refractory to reactivation by the clinically available
oximes. Consequently, the fatality rate from OP poisoning remains
high, despite treatment. Therefore, there is a need for a novel
medical countermeasure for individuals who are prone to OP
poisoning.
It was previously demonstrated that antibodies
exhibited scavenger characteristics in the detoxication of OP
poisoning (3). However, the
limitations of immunotherapy are well recognized, as it takes a
long time to provoke a potent antibody response against OP
intoxication. It was demonstrated that pretreatment with
physostigmine may protect against the incapacitating and lethal
effects of OP poisoning (4). It was
also demonstrated that OP toxicity and lethality are counteracted
when galantamine is administered prior to acute exposure of guinea
pigs to OPs (5). However, the
ultimate usefulness of pyridostigmine and galantamine is
questionable, as the time period during which they are effective
against OPs intoxication is limited.
Huperzine A (HupA), an alkaloid isolated from the
Chinese club moss Huperzia serrata, has been established as
a slow, reversible inhibitor of AChE at the peripheral and central
level (6). HupA sustained-release
microspheres (HSMs), a new formulation of HupA, were prepared by an
oil/water solvent evaporation technique loading poly(D,
L-lactic-co-glycolic) acid (PLGA) microspheres with HupA. Following
intramuscular injection of HSMs in mice, HupA was steadily released
from the PLGA microspheres and the AChE activity was continually
inhibited for 14 days (7). The
present study aimed to investigate whether HSM pretreatment is
effective in counteracting the toxicity of OPs.
Materials and methods
Drug and chemicals
Methyl parathion (MP) (80%, w/w) was obtained from
Shandong Dacheng Pesticide Co., Ltd.(Zibo, China). The HSMs were
provided by Shandong Luye Pharmaceutical Co., Ltd. (Yantai,
China).
Animals
A total of 370 Swiss mice, weighing 20–22 g, were
provided by the Experimental Animal Center of the Shandong
Engineering Research Center for Natural Drugs (Yantai, China). The
animals were housed in a climate-controlled room, maintained on a
12 h/12 h light/dark cycle and had ad libitum access to food
and water. The experiments were performed according to the National
Institute of Health Guidelines for the Care and Use of Laboratory
Animals (publication no. 86-23, revised in 1986) and were approved
by the local Ethics Committee. All efforts were made to minimize
the number of animals used and their suffering.
Acute toxicity of MP in HSM-pretreated
mice
The mice were randomly divided into 5 groups (n=50
mice/group) as follows: control, 2-h HSMs, 5-day HSMs, 10-day HSMs
and 15-day HSMs groups. According to the doses of MP, the mice were
further subdivided into 5 subgroups (n=10 mice/subgroup) (Table I). After fasting for 12 h, the mice
in the control group were treated intramuscularly with 2.5% (w/v)
sodium carboxymethyl cellulose. The mice were then challenged by a
single intragastric exposure to MP at doses of 43.0, 47.8, 53.1,
59.0 and 65.6 mg/kg. The mice in the 2-h HSMs subgroups were
treated intramuscularly with HSMs at a dose of 1.5 mg/kg. Two hours
later, they were intragastrically challenged with MP at doses of
59.0, 65.6, 72.9, 81.0 and 90.0 mg/kg. The mice in the 5-day HSMs
subgroups were treated intramuscularly with HSMs at a dose of 1.5
mg/kg. Five days later, they were intragastrically challenged with
MP at doses of 53.1, 59.0, 65.6, 72.9 and 81.0 mg/kg. The mice in
the 10-day HSMs subgroups were treated intramuscularly with HSMs at
a dose of 1.5 mg/kg. Ten days later, they were intragastrically
challenged with MP at doses of 53.1, 59.0, 65.6, 72.9 and 81.0
mg/kg. The mice in the 15-day HSMs subgroups were treated
intramuscularly with HSMs at a dose of 1.5 mg/kg. Fifteen days
later, they were intragastrically challenged with MP at doses of
47.8, 53.1, 59.0, 65.6 and 72.9 mg/kg. The general behavior and
signs of toxicity in mice were observed continuously for 1 h
following MP administration and then intermittently for 4 h over a
period of 24 h. The mice were further observed once a day up to 14
days for behavioral changes and signs of toxicity and/or death. The
value of the median lethal dose (LD50) was determined as
previously described (8).
 | Table IGroups for the evaluation of acute
toxicity of methyl parathion. |
Table I
Groups for the evaluation of acute
toxicity of methyl parathion.
| Subgroups according
to dose of methyl parathion (mg/kg) |
|---|
|
|
|---|
| Groups | 1 (n=10) | 2 (n=10) | 3 (n=10) | 4 (n=10) | 5 (n=10) |
|---|
| Control | 43.0 | 47.8 | 53.1 | 59.0 | 65.6 |
| 2-h HSMs | 59.0 | 65.6 | 72.9 | 81.0 | 90.0 |
| 5-day HSMs | 53.1 | 59.0 | 65.6 | 72.9 | 81.0 |
| 10-day HSMs | 53.1 | 59.0 | 65.6 | 72.9 | 81.0 |
| 15-day HSMs | 47.8 | 53.1 | 59.0 | 65.6 | 72.9 |
Evaluation of HSM pretreatment on MP
poisoning
The mice were randomly divided into 3 groups (n=40
mice/group): Control, MP and HSMs groups. The mice in the control
group were further subdivided into 4 subgroups: 2-h, 5-day, 10-day
and 15-day control subgroups. The mice in the MP group were further
subdivided into 4 subgroups: 2-h, 5-day, 10-day and 15-day MP
subgroups. The mice in the HSMs group were further divided into 4
subgroups: 2-h, 5-day, 10-day and 15-day HSMs subgroups. The mice
in the control subgroups were treated intramuscularly with 2.5%
(w/v) sodium carboxymethyl cellulose. Two hours, 5, 10 and 15 days
later, they were intragastrically administered distilled water. The
mice in the MP subgroups were treated intramuscularly with 2.5%
(w/v) sodium carboxymethyl cellulose. Two hours, 5, 10 and 15 days
later, they were intragastrically administered MP at a dose of 60.0
mg/kg (1.2 × LD50). The mice in the HSMs subgroups were
treated intramuscularly with HSMs at a dose of 1.5 mg/kg. Two
hours, 5, 10 and 15 days later, they were intragastrically
administered MP at a dose of 60.0 mg/kg. The general behavior and
signs of toxicity in mice were observed continuously for 1 h after
the MP administration and then intermittently for 4 h over a period
of 24 h. The mice were further observed once a day up to 14 days
for behavioral changes and signs of toxicity and/or death. Acute
reactions to treatment were scored according to a modified Racine
scale (9). Whenever animals
developed life-threatening signs of intoxication, such as gasping
and unremitting motor convulsions, they were euthanized according
to the Institutional Animal Care and Use Committee-approved
protocol.
Histopathological analysis
Following euthanasia, the brain, heart, liver,
lungs, kidneys and intercostal muscles of the mice were harvested.
Subsequently, paraformaldehyde (4%)-fixed, paraffin-embedded
samples were cut into 4-μm sections, deparaffinized in xylene and
rehydrated through a series of decreasing concentrations of
ethanol. The sections were stained with hematoxylin and eosin and
pathological observation of the tissues was performed under a light
microscope.
Statistical analysis
In order to calculate the LD50, a linear
regression analysis corrected for the number of animals at each
dose level was used. The proportion of dead mice and the
logarithmic transformed dose levels were used as the dependent and
independent variables, respectively. The survival rates were
compared using the Kaplan-Meier log-rank test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of HSMs on the LD50 of
MP
The LD50 of MP in the mice of the control
group was 51.4 mg/kg. Compared to the control group, animals in the
2-h, 5-day and 10-day HSMs groups were more resistant to the toxic
effects of MP (LD50=70.0, 67.5 and 63.4 mg/kg,
respectively). However, the LD50 value of MP in the
15-day HSMs group (53.5 mg/kg) was the same as that of the control
group (Table II).
 | Table IIEffect of HSMs on the LD50
of methyl parathion. |
Table II
Effect of HSMs on the LD50
of methyl parathion.
| Groups | HSMs dose
(mg/kg) | LD50
(mg/kg) | 95% CI |
|---|
| Contro | - | 51.4 | 46.8–56.4 |
| 2-h HSMs | 1.5 | 70.0 | 64.6–75.8 |
| 5-day HSMs | 1.5 | 67.5 | 62.3–73.0 |
| 10-day HSMs | 1.5 | 63.4 | 57.8–69.7 |
| 15-day HSMs | 1.5 | 53.5 | 49.2–58.2 |
Effect of HSMs on the acute toxicity of
1.2 × LD50 MP
Within 25 min after a single intragastric
administration of MP at a dose of 60 mg/kg, ~40% of the mice
developed a cholinergic crisis characterized by chewing, miosis,
hypersecretion, diarrhea, bruxism, muscle fasciculations and
tremors. According to the Institutional Animal Care and Use
Committee-approved protocol, the animals were euthanized as soon as
signs of intoxication became life threatening. Approximately 20–40%
of the mice survived for 24 h after exposure to 1.2 ×
LD50 MP.
Effects of HSMs on the manifestation of
MP poisoning in mice
Following MP exposure, all the mice in the 2-h HSMs
group survived, with no apparent toxic signs. The mice in the 5-day
and 10-day HSMs groups developed mild adverse symptoms, such as
increased chewing, hypersalivation and tremors. The toxic signs in
the mice of the 2-h, 5-day and 10-day HSMs groups were
significantly attenuated. Furthermore, the 24-h survival of the
animals in the 2-h, 5-day and 10-day HSMs groups was 100, 90 and
80%, respectively (P<0.05 or P<0.01). The animals in the
15-day HSMs group developed severe signs of intoxication, with a
24-h survival of 30% (Table
III).
 | Table IIIEffects of HSMs on methyl parathion
poisoning in mice. |
Table III
Effects of HSMs on methyl parathion
poisoning in mice.
| No. of euthanized
animals | | |
|---|
|
| | |
|---|
| Groups | 5–15 min | 15–25 min | 35–45 min | 45–60 min | 12–24 h | Total deaths | Survival, % |
|---|
| A |
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 100 |
| MP | 1 | 3 | 0 | 0 | 2 | 6 | 40 |
| 2-h HSMs | 0 | 0 | 0 | 0 | 0 | 0 | 100b |
| B |
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 100 |
| MP | 1 | 3 | 0 | 0 | 2 | 6 | 40 |
| 5-day HSMs | 0 | 0 | 0 | 0 | 1 | 1 | 90a |
| C |
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 100 |
| MP | 2 | 3 | 0 | 1 | 2 | 8 | 20 |
| 10-day HSMs | 0 | 1 | 0 | 0 | 1 | 2 | 80b |
| D |
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 100 |
| MP | 2 | 2 | 1 | 0 | 2 | 7 | 30 |
| 15-day HSMs | 0 | 3 | 0 | 2 | 2 | 7 | 30 |
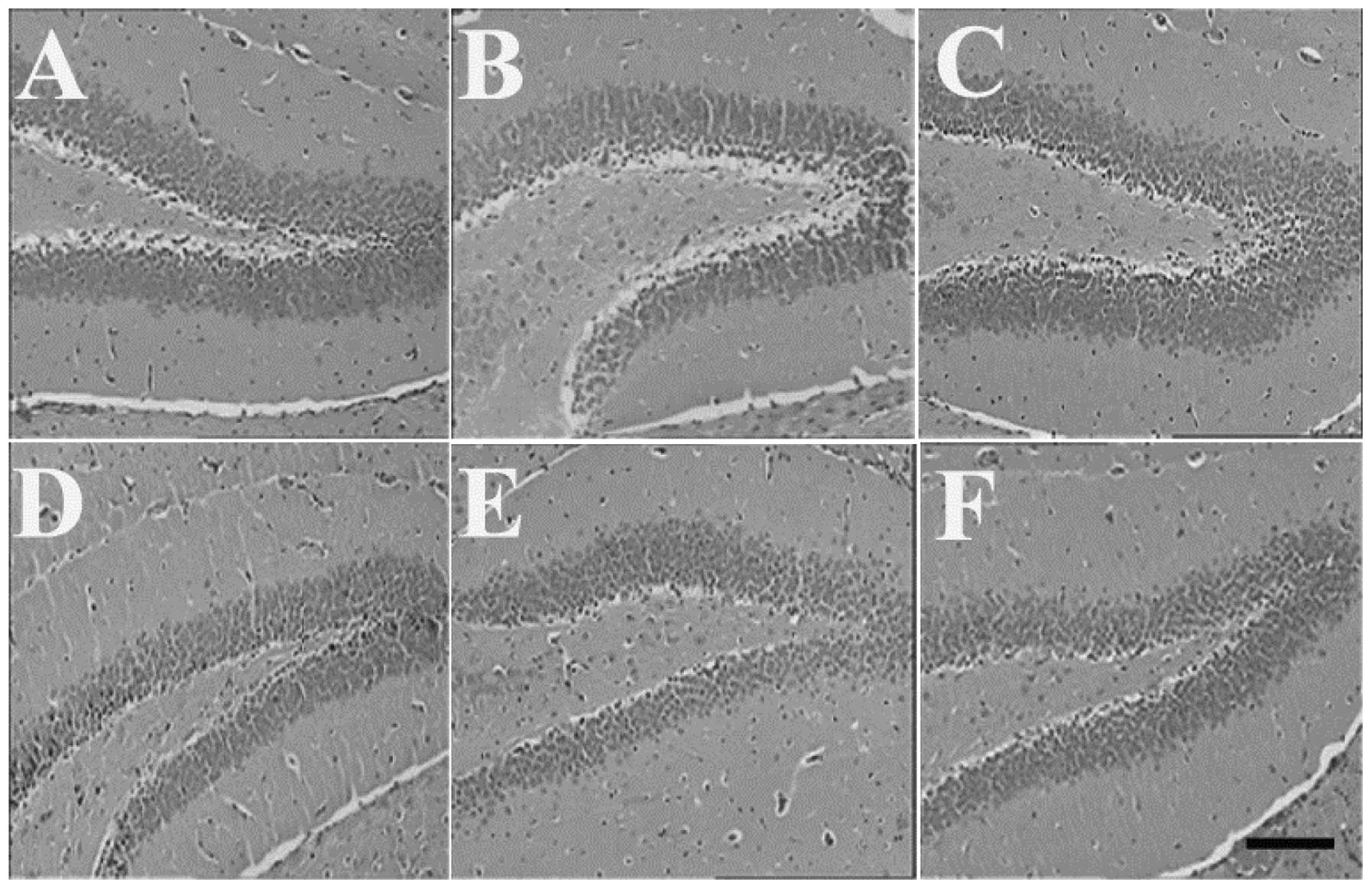
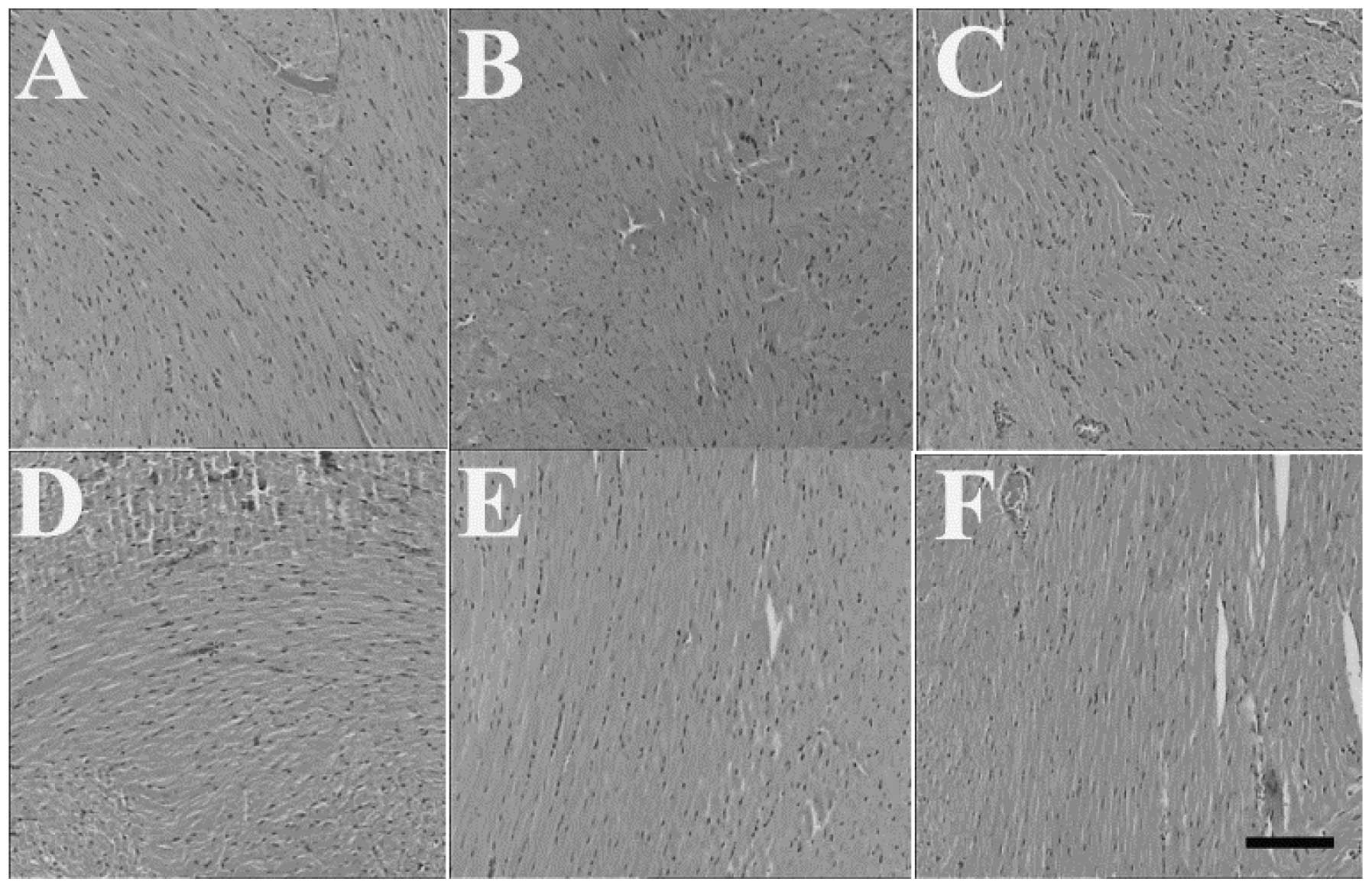
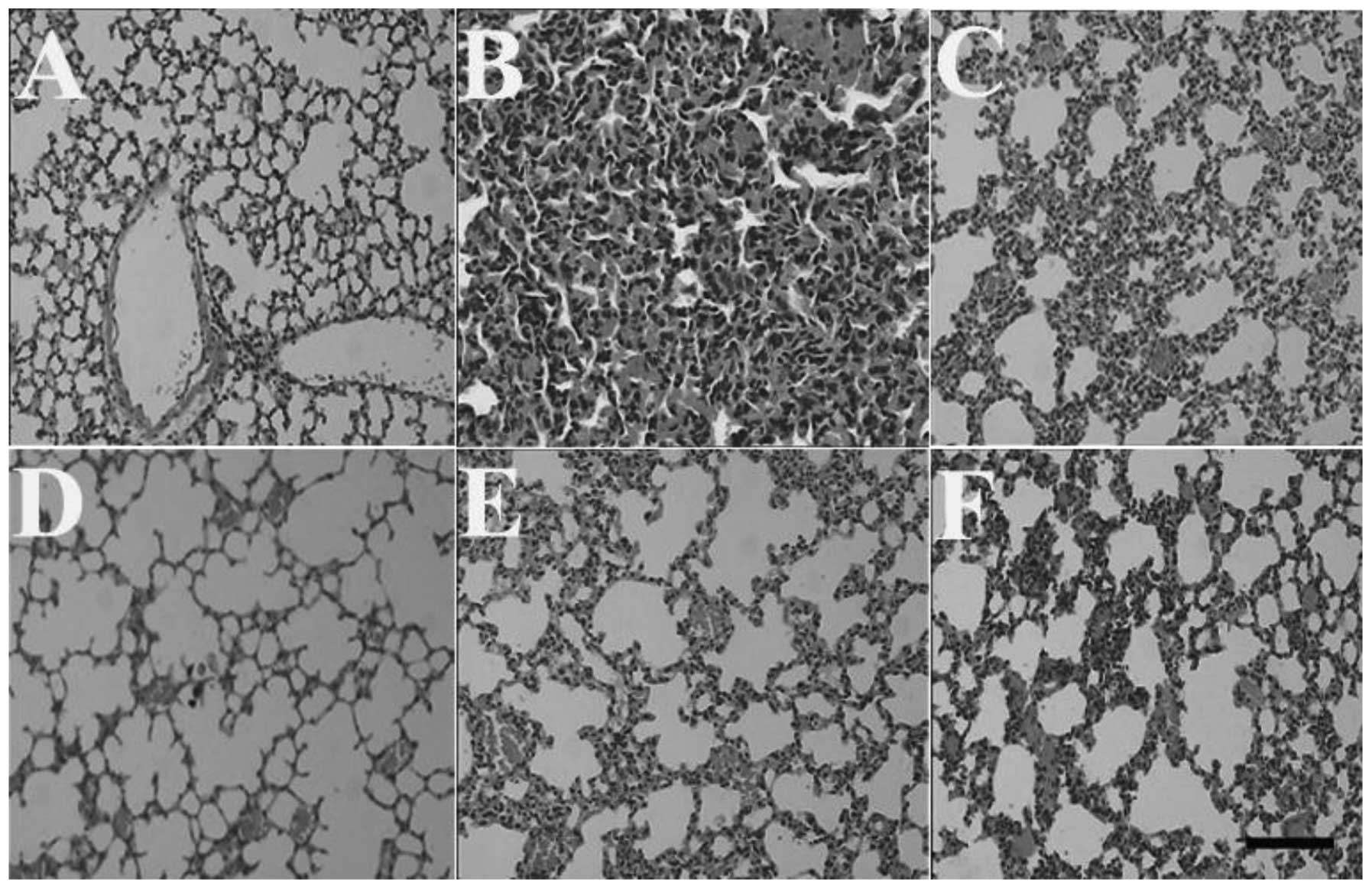
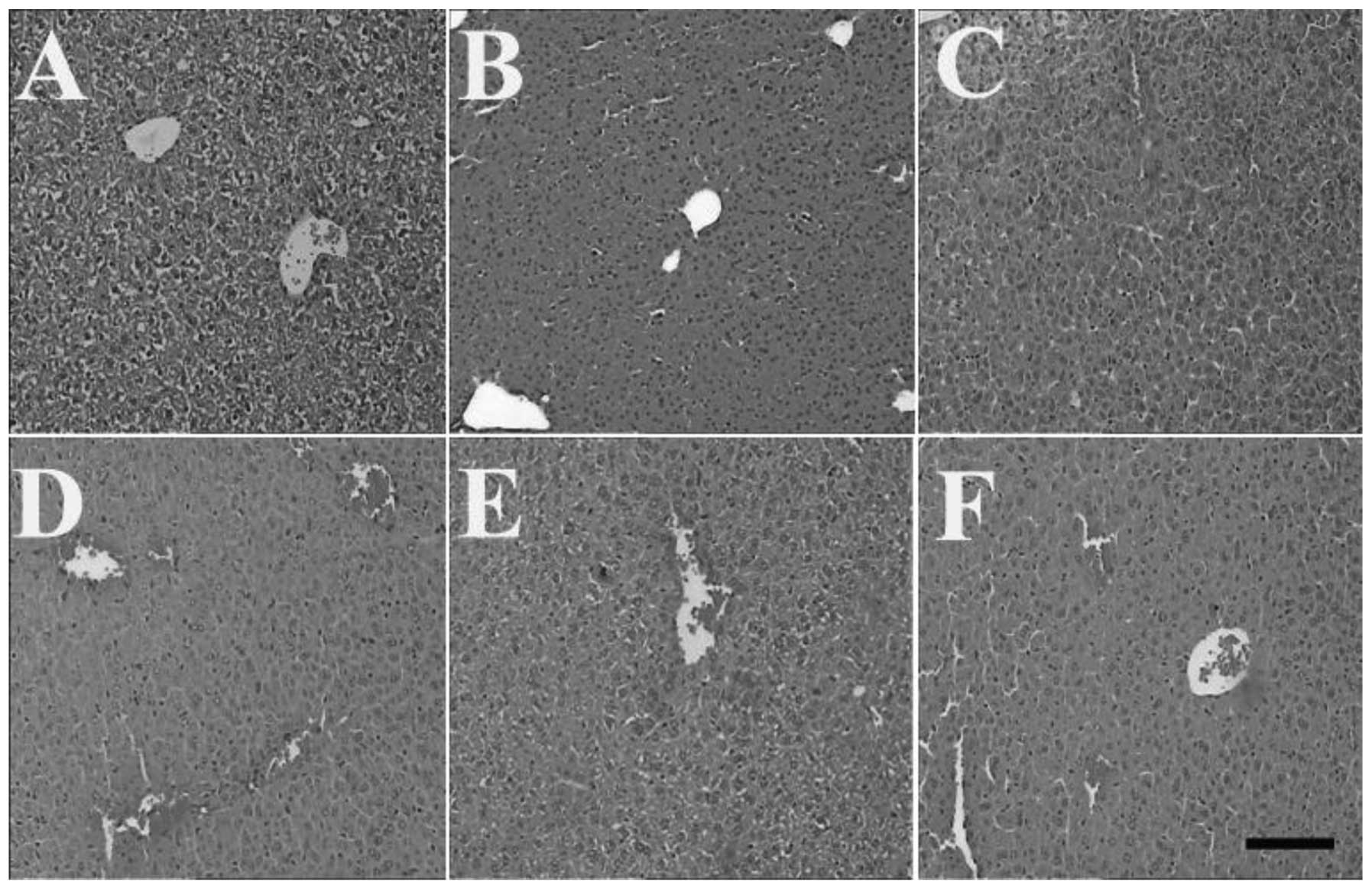
Effects of HSMs on histopathological
changes induced by MP poisoning
The examination under a light microscope revealed a
normal structure of the brain, heart and kidneys of the control
animals. The brain, heart and kidneys of the mice in the MP and
HSMs groups also revealed no histological changes (Figs. 1, 2
and 3, respectively). However, the
livers of the mice in the MP and HSMs groups displayed central vein
congestion as a result of the exposure to MP. The lungs of the
animals in the MP group exhibited a notable pulmonary edema that
was characterized by edema and congestion of the alveolar septae.
However, the histopathological changes of the lungs in the 2-h,
5-day and 10-day HSMs groups were significantly less prominent,
whereas the histopathological changes of the lungs in the 15-day
HSMs group were identical with those of the MP group (Fig. 4). Furthermore, pretreatment with
HSMs did not attenuate the MP-induced central vein congestion in
the liver (Fig. 5).
Discussion
In the present study, HSMs pretreatment was shown to
increase the value of the LD50 and the survival of
animals poisoned by MP. The results demonstrated that pretreatment
with HSMs, even 10-days prior to MP exposure, may effectively
counteract the toxicity of OPs.
MP poisoning leads to cholinergic overstimulation,
with signs of toxicity such as sweating, dizziness, vomiting,
diarrhea, convulsions, cardiac arrest, respiratory arrest and, in
extreme cases, death. In toxicology, the median LD50 is
frequently used as a general indicator of the acute toxicity of a
substance. The LD50 of MP in the control group was 51.4
mg/kg. However, the LD50 values of the 2-h, 5-day and
10-day HSMs groups were 70.0, 67.5 and 63.4 mg/kg, respectively,
which were equivalent to 1.36, 1.31 and 1.23 times the dose in mice
without administering HSMs. There was no significant difference in
the LD50 between the control group and the 15-day HSMs
group. The acute toxicity of MP (1.2 × LD50) was also
effectively counteracted by pretreatment with HSMs. Approximately
60–80% of the mice in the MP groups developed life threatening
symptoms and were euthanized. By contrast, the mice pretreated with
HSMs 2 h, 5 days and 10 days prior to MP exposure survived (100, 90
and 80%, respectively), with mild signs of toxicity. These results
demonstrated the high, long-term effectiveness of HSMs against the
toxicity of 1.2 × LD50 MP.
The organ injury caused by OP exposure in insects
and humans is associated with the ability of the OPs to bind to
AChE and prevent the hydrolysis of acetylcholine (10). The present study demonstrated that
the histopathological changes of the lungs in the 2-h, 5-day and
10-day HSMs groups were significantly less prominent compared to
those in the control group. It was previously demonstrated that
respiratory failure is a prominent characteristic of acute OP
poisoning (11) and that the
morbidity and mortality of acute OP poisoning is associated with
respiratory failure (12).
Therefore, it is hypothesized that the effectiveness of HSMs is at
least partly associated with ameliorating MP-induced pulmonary
edema.
The development of an effective and safe approach
against OP toxicity may help to reduce the mortality associated
with OP poisoning worldwide. Our results indicated that
pretreatment with HSMs effectively and safely counteracts OP
poisoning and may therefore be of benefit to individuals who are
prone to exposure to OPs or nerve agents. In conclusion, the
present study demonstrated a novel approach to effectively
protecting against OPs toxicity and nerve agent-induced organ
injury or death.
Acknowledgements
This study was supported by the Project of
Fundamental Study Funds of the Shandong Provincial Education
Department (grant no. J11LF56), the Doctoral Foundation of Yantai
University (grant no. YX12B30) and the Shandong Provincial Natural
Science Foundation (grant no. ZR2010HQ049). The authors would like
to thank Professor Lon Clark for the English language revision.
References
|
1
|
Soltaninejad K and Abdollahi M: Current
opinion on the science of organophosphate pesticides and toxic
stress: a systematic review. Med Sci Monit. 15:RA75–RA90.
2009.PubMed/NCBI
|
|
2
|
Bajgar J: Organophosphates/nerve agent
poisoning: mechanism of action, diagnosis, prophylaxis, and
treatment. Adv Clin Chem. 38:151–216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jia P, Wang Y, Yu M, et al: An
organophosphorus hapten used in the preparation of monoclonal
antibody and as an active immunization vaccine in the detoxication
of soman poisoning. Toxicol Lett. 187:45–51. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Muggleton NG, Bowditch AP, Crofts HS,
Scott EA and Pearce PC: Assessment of a combination of
physostigmine and scopolamine as pretreatment against the
behavioural effects of organophosphates in the common marmoset
(Callithrix jacchus). Psychopharmacology (Berl).
166:212–220. 2003.PubMed/NCBI
|
|
5
|
Albuquerque EX, Pereira EF, Aracava Y, et
al: Effective countermeasure against poisoning by organophosphorus
insecticides and nerve agents. Proc Natl Acad Sci USA.
103:13220–13225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ashani Y, Peggins JO III and Doctor BP:
Mechanism of inhibition of cholinesterases by huperzine A. Biochem
Biophys Res Commun. 184:719–726. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang C, Zhang T, Ma H, Liu J, Fu F and Liu
K: Prolonged effects of poly(lactic-co-glycolic acid)
microsphere-containing huperzine A on mouse memory dysfunction
induced by scopolamine. Basic Clin Pharmacol Toxicol. 100:190–195.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi SC: Interval estimation of the
LD50based on an up-and-down experiment. Biometrics.
46:485–492. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aracava Y, Pereira EF, Akkerman M, Adler M
and Albuquerque EX: Effectiveness of donepezil, rivastigmine, and
(+/−)huperzine A in counteracting the acute toxicity of
organophosphorus nerve agents: comparison with galantamine. J
Pharmacol Exp Ther. 331:1014–1024. 2009.
|
|
10
|
Lu L, Wang X, Lang L and Fu F: Protective
effect of reduced glutathione on the liver injury induced by acute
omethoate poisoning. Environ Toxicol Pharmacol. 30:279–283. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Munidasa UA, Gawarammana IB, Kularatne SA,
Kumarasiri PV and Goonasekera CD: Survival pattern in patients with
acute organophosphate poisoning receiving intensive care. J Toxicol
Clin Toxicol. 42:343–347. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tafuri J and Roberts J: Organophosphate
poisoning. Ann Emerg Med. 16:193–202. 1987. View Article : Google Scholar
|