Introduction
Type 1 diabetes (T1D) is a chronic autoimmune
disease characterized by the T-cell-mediated destruction of
pancreatic β cells and subsequent dependence on exogenous insulin
(1). T1D affects millions of
individuals worldwide and its incidence is on the increase,
particularly in young children (2,3). The
etiology of T1D is multifactorial and complex, with genetic and
environmental factors playing a role in its pathogenesis (4–6). Human
leukocyte antigen (HLA) genes are considered to greatly affect the
development of T1D. Genetic determinants such as polymorphisms in
the HLA region are crucial, accounting for 40–50% of the
inheritable diabetes risk (7). In
addition, there is evidence that proinflammatory cytokines
contribute to the genetic susceptibility to T1D, such as
interleukin (IL)-1, IL-2, IL-12, IL-18 and interferon (IFN)-γ
(8).
IL-18, a member of the IL-1 superfamily, is a
proinflammatory cytokine produced by activated
monocytes/macrophages. Through a concerted action with IL-12, IL-18
promotes the development of Th1 lymphocyte response by induction of
IFN-γ production. IL-18 also modulates the activity of NK cells,
increases tumor necrosis factor (TNF)-α and IL-1. Evidence suggests
that IL-18 is involved in the regulation of the innate and adaptive
immune response and may have a pathogenic role in T1D. Nicoletti
et al(9) reported that serum
IL-18 levels were elevated in the subclinical stage of T1D in
first-degree relatives of T1D patients. Rothe et al(10,11)
found that increased IL-18 mRNA production by macrophages followed
by increased IFN-γ levels was associated with an active stage of
autoimmune diabetes in non-obese diabetic (NOD) mice. Moreover, it
has been found that in the animal model of autoimmune diabetes a
short prophylactic treatment with IL-18 inhibitors resulted in
significant protection against development of this disease
(12).
The IL-18 gene is located on chromosome
11q22.2-q22.3, and comprises six exons and five introns (13). Of the IL-18 gene polymorphisms
identified, the genetic association between single nucleotide
polymorphisms (SNPs) at positions −137, −607 in IL-18 gene promoter
and T1D were widely evaluated. However, the results were
inconsistent. Meta-analysis is a statistical procedure for
combining the results of several studies to produce a single
estimate of the major effect with enhanced precision. Thus, in
order to examine the relationship between IL-18 gene polymorphisms
(−137 C/G, −607 A/C) and susceptibility to T1D, a meta-analysis was
performed.
Materials and methods
Identification of eligible studies and
data extraction
Studies examining the association of IL-18 −137 C/G
(rs187238), −607 A/C (rs1946518) polymorphisms with T1D were fully
considered and carefully selected. The literature was searched
using the following electronic databases: Medline, PubMed, China
National Knowledge Infrastructure (CNKI) and CBM. The search was
based on the combinations of ‘type 1 diabetes’, ‘polymorphisms’ or
‘variant’, ‘interleukin-18’ or ‘IL-18’. No language restrictions
were applied. We only recruited complete published studies, and not
any data from conference abstracts or meetings. A study was
included when the following criteria were met: i) it was published
up to April 2013; ii) it was a case-control study; iii) it provided
sufficient data for estimating the odds ratio (OR). When more than
one study of the same patient population was included in several
publications, only the most recent or complete study was used in
this meta-analysis. Furthermore, two investigators independently
searched the electronic database and extracted available
information from each study, including first author’s name, year of
publication, country where the trial was conducted, ethnicity,
number of cases and controls, as well as data available on allele
and genotype frequency of IL-18 −137 C/G, −607 A/C
polymorphisms.
Evaluation of statistical
associations
Allele frequencies of IL-18 −137 C/G, −607 A/C
polymorphisms in each of the studies were determined using the
allele counting method. The strength of association between these
variants and T1D was achieved by calculating OR with 95% confidence
interval (CI). We assessed the within- and between- study variation
or heterogeneity by means of the Cochran’s Q-statistic (14). P>0.10 for the Q-test indicated a
lack of heterogeneity among studies, and then the fixed-effect
model was used. Otherwise, results of the random-effect model were
selected. The effect of heterogeneity was quantified by using the
measurement method, I2=100% × (Q−df)/Q (15). The I2 measures the degree
of inconsistency in the studies by calculating the percentage of
total variation across studies as being due to heterogeneity rather
than chance. I2 values of 25, 50, and 75% were nominally
considered low, moderate and high estimates.
Evaluation of publication bias
Publication bias was investigated by using a funnel
plot, in which the standard error of log (OR) of each study was
plotted against its OR. The degree of asymmetry was achieved using
the method of Egger’s linear regression test (16). The significance of the intercept was
determined by the t-test suggested by Egger. P<0.05 was
considered representative of statistically significant publication
bias.
Statistical analysis
All the statistical analyses were carried out using
the software package Stata Version 10.0 (Stata Corporation, College
Station, TX, USA), using two-sided P-values. P<0.05 was
considered to indicate a statistically significant difference.
Results
Characteristics of the articles in our
meta-analysis
The search words yielded 26 relevant studies.
Following careful review, 15 of the 26 articles were excluded (8
were conducted to examine other IL-18 polymorphisms or other
diseases; 4 were reviews; 2 were duplicated data publications; and
1 was letter). Therefore, a total of 11 case-control studies were
considered in this meta-analysis (17–27).
Table I presents the studies
identified and their main characteristics. Of the 11 studies, 7
studies were of Europeans, 3 of Asians and 1 study of South
Americans. The results of the Hardy-Weinberg equilibrium (HWE) test
for the distribution of the genotype in the control population are
also shown in Table I. One study
was not in HWE in the eligible studies (23).
 | Table IThe characteristics of studies
included in the meta-analysis. |
Table I
The characteristics of studies
included in the meta-analysis.
| | | Nos. | Allele frequency
(%)a | HWE for controls | |
|---|
| | |
|
|
| |
|---|
| Polymorphisms | Author (year) | Ethnic group | T1D | Control | T1D | Control | χ2 | P-value | Refs. |
|---|
| −137C/G | Kretowski et
al(2002) | Poland (E) | 201 | 194 | 37.6 | 27.3 | 9.400 | <0.01 | (23) |
| Ide et al
(2004) | Japan (A) | 116 | 114 | 12.1 | 11.4 | 0.230 | 0.63 | (22) |
| Novota et
al(2005) | Czech Republic
(E) | 49 | 139 | 34.7 | 28.8 | 1.080 | 0.30 | (26) |
| Martin et
al(2005) | UK (E) | 433 | 426 | 25.8 | 28.1 | 0.013 | 0.91 | (24) |
| Boraska et
al(2006) | Croatia (E) | 134 | 132 | 28.7 | 28.8 | 0.001 | 0.98 | (20) |
| Szeszko et
al(2006) | UK (E) | 4,323 | 4,610 | 26.4 | 26.6 | 1.418 | 0.23 | (27) |
| Mojtahedi et
al(2006) | Iran (A) | 112 | 194 | 30.8 | 24.0 | 0.003 | 0.95 | (25) |
| Dong et
al(2007) | China (A) | 118 | 150 | 14.0 | 10.7 | 0.004 | 0.95 | (21) |
| Altinova et
al(2010) | Turkey (E) | 91 | 87 | 26.4 | 25.9 | 0.010 | 0.92 | (19) |
| Hadžija et
al(2013) | Croatia (E) | 187 | 236 | 29.7 | 33.1 | 0.274 | 0.60 | (18) |
| Tavares et
al(2013) | Brazil (S) | 181 | 122 | 28.5 | 43.9 | 0.320 | 0.57 | (17) |
| −607A/C | Kretowski et
al(2002) | Poland (E) | 201 | 194 | 32.1 | 34.0 | 13.421 | <0.01 | (23) |
| Ide et
al(2004) | Japan (A) | 116 | 114 | 46.6 | 55.3 | 3.328 | 0.07 | (22) |
| Novota et
al(2005) | Czech Republic
(E) | 49 | 139 | 56.1 | 42.4 | 1.116 | 0.29 | (26) |
| Martin et
al(2005) | UK (E) | 433 | 426 | 36.5 | 40.8 | 0.172 | 0.68 | (24) |
| Szeszko et
al(2006) | UK (E) | 1,560 | 1,715 | 39.2 | 38.1 | 0.462 | 0.50 | (27) |
| Mojtahedi et
al(2006) | Iran (A) | 112 | 194 | 44.6 | 41.2 | 0.795 | 0.37 | (25) |
| Dong et
al(2007) | China (A) | 118 | 150 | 41.2 | 53.0 | 0.138 | 0.71 | (21) |
| Altinova et
al(2010) | Turkey (E) | 91 | 87 | 35.7 | 35.6 | 2.027 | 0.15 | (19) |
| Hadžija et
al(2013) | Croatia (E) | 187 | 236 | 42.5 | 44.9 | 1.471 | 0.23 | (18) |
| Tavares et
al(2013) | Brazil | 181 | 122 | 42.5 | 46.3 | 0.180 | 0.67 | (17) |
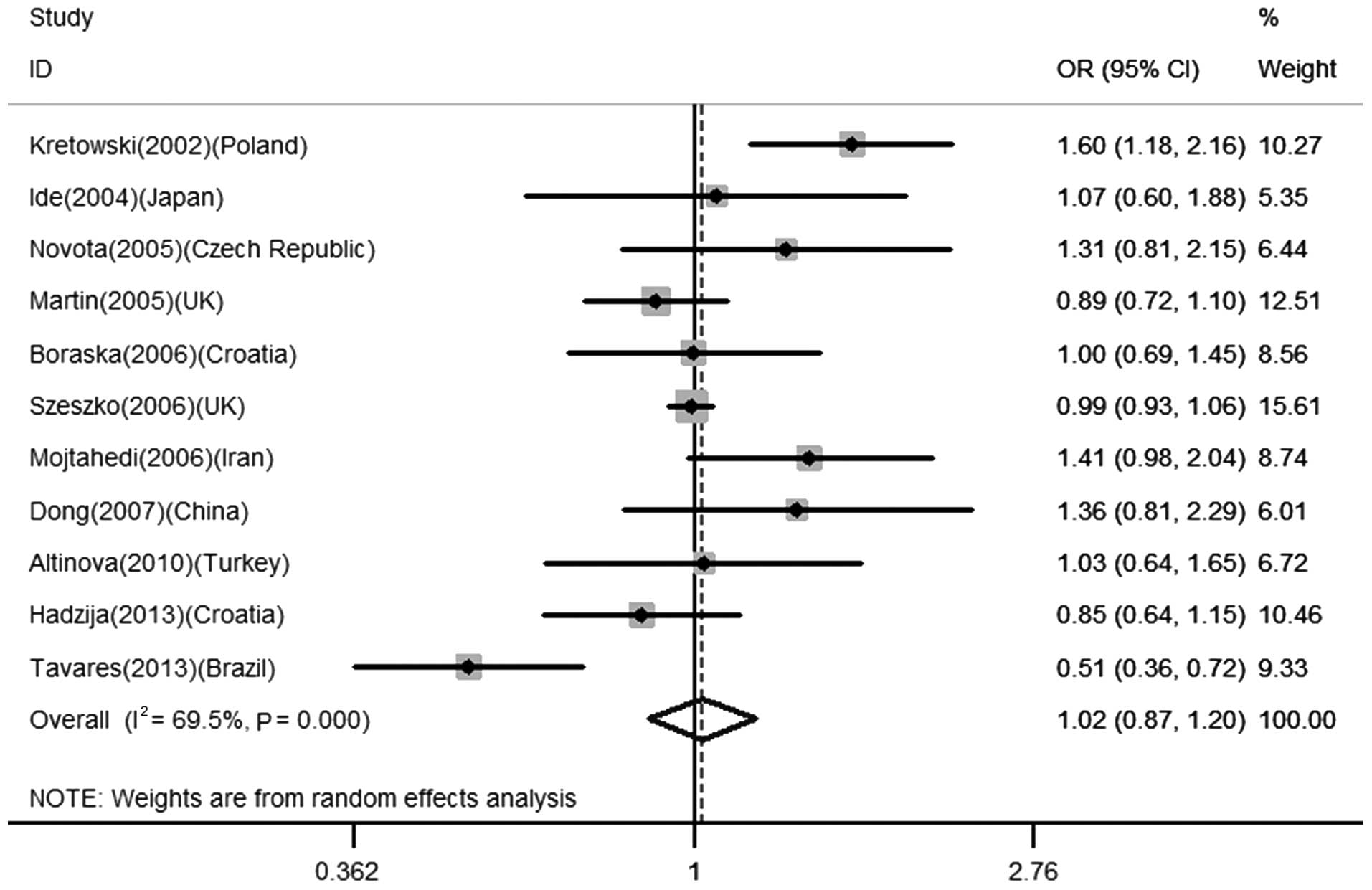
Association between IL-18 −137 C/G
polymorphism and the risk for T1D
A total of 11 studies comprising 5,945 cases and
6,404 controls were included in order to analyze the association
between −137 C/G and T1D risk. Overall, no significant relationship
was observed in the total population (for C vs. G: OR=1.02, 95%
CI=0.87–1.20, P=0.770; CC + CG vs. GG: OR=1.05, 95% CI=0.87–1.25,
P=0.630; CC vs. CG + GG: OR=0.96, 95% CI=0.68–1.36, P=0.814), as
shown in Table II and Fig. 1. Similarly, in the subsequent
stratified analysis, no significant association was identified
between the IL-18 −137 C/G polymorphism and T1D in the European
population (for C vs. G: OR=1.04, 95% CI=0.90–1.20, P=0.620; CC +
CG vs. GG: OR=1.07, 95% CI=0.87–1.30, P=0.534; CC vs. CG + GG:
OR=1.00, 95% CI=0.87–1.16, P=0.988).
 | Table IIMain results of the
meta-analysis. |
Table II
Main results of the
meta-analysis.
| Polymorphism | Comparisons | Study | No. of studies | Test of
association | Test of
heterogeneity | Publication
biasa |
|---|
|
|
|
|---|
| OR | 95% CI | Z | P-value | Model | Q | P-value | I2
(%) | t | P-value |
|---|
| −137 C/G | C vs G | Overall | 11 | 1.02 | 0.87–1.20 | 0.29 | 0.770 | R | 32.75 | <0.001 | 69.5 | 0.41 | 0.689 |
| | European | 7 | 1.04 | 0.90–1.20 | 0.50 | 0.620 | R | 12.93 | 0.044 | 53.6 | 0.70 | 0.517 |
| CC + CG vs GG | Overall | 11 | 1.05 | 0.87–1.25 | 0.48 | 0.630 | R | 25.29 | 0.005 | 60.5 | 0.61 | 0.559 |
| | European | 7 | 1.07 | 0.87–1.30 | 0.62 | 0.534 | R | 14.48 | 0.025 | 58.6 | 0.79 | 0.464 |
| CC vs CG + GG | Overall | 11 | 0.96 | 0.68–1.36 | 0.24 | 0.814 | R | 22.91 | 0.011 | 56.4 | −0.04 | 0.967 |
| | European | 7 | 1.00 | 0.87–1.16 | 0.02 | 0.988 | F | 6.69 | 0.350 | 10.4 | 0.39 | 0.709 |
| −607 A/C | A vs C | Overall | 10 | 0.93 | 0.81–1.06 | 1.08 | 0.281 | R | 22.12 | 0.009 | 59.3 | −0.80 | 0.449 |
| | European | 6 | 0.99 | 0.85–1.15 | 0.15 | 0.884 | R | 10.52 | 0.062 | 52.5 | 0.04 | 0.970 |
| AA + AC vs CC | Overall | 10 | 0.99 | 0.89–1.10 | 0.24 | 0.808 | F | 13.34 | 0.148 | 32.5 | −0.29 | 0.782 |
| | European | 6 | 1.01 | 0.90–1.13 | 0.18 | 0.856 | F | 7.02 | 0.219 | 28.8 | 0.40 | 0.710 |
| AA vs AC + CC | Overall | 10 | 0.81 | 0.60–1.09 | 1.40 | 0.162 | R | 25.25 | 0.003 | 64.4 | −1.85 | 0.102 |
| | European | 6 | 0.96 | 0.66–1.39 | 0.23 | 0.817 | R | 13.40 | 0.020 | 62.7 | −0.95 | 0.398 |
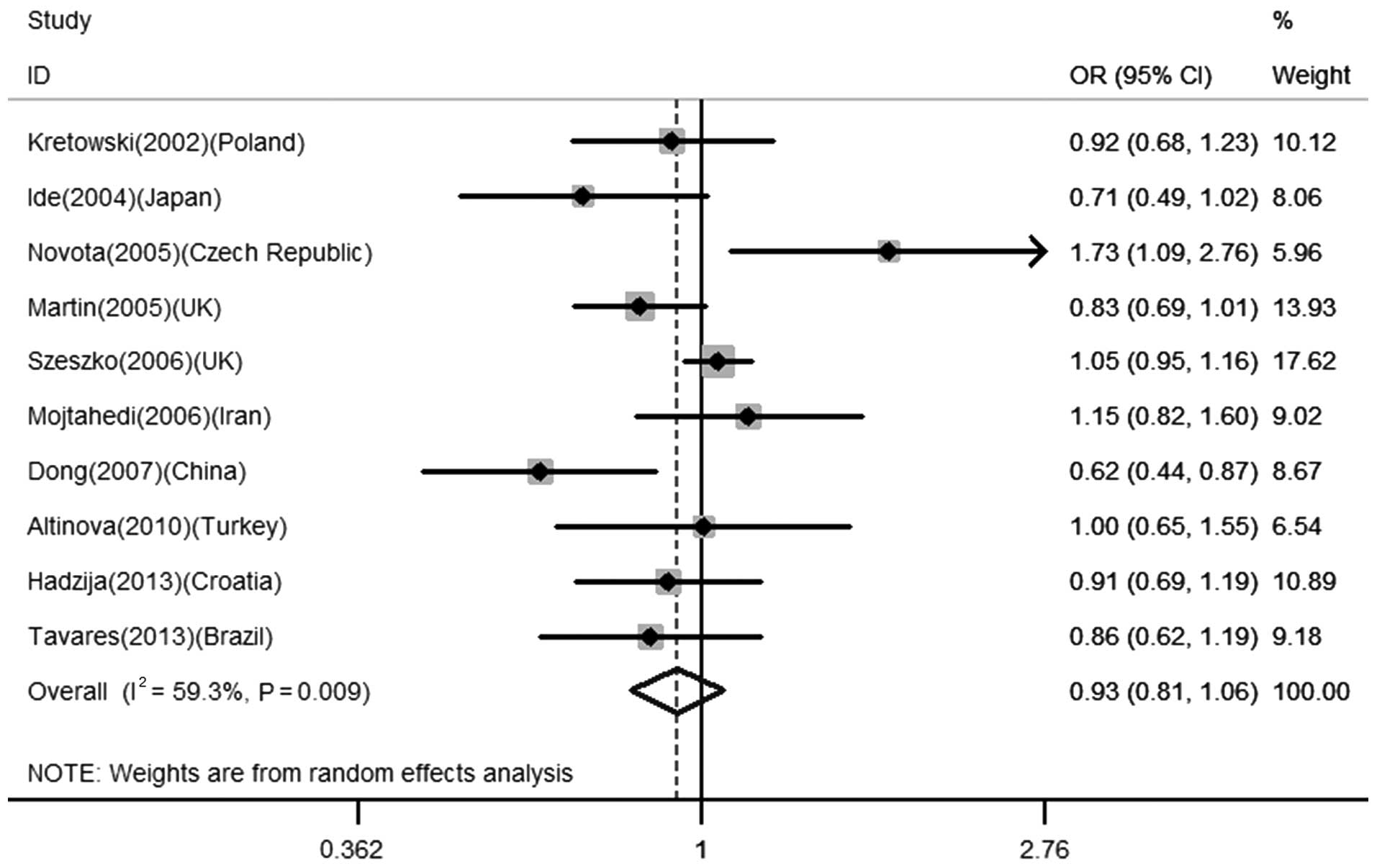
Association between −607 A/C polymorphism
and the risk for T1D
The association between −607 A/C polymorphism and
T1D was investigated in 10 studies with a total of 3,048 cases and
3,377 controls. No significant association was found between this
polymorphism and T1D in any of the genetic models in the total
population (for A vs. C: OR=0.93, 95% CI=0.81–1.06, P=0.281; AA +
AC vs. CC: OR=0.99, 95% CI=0.89–1.10, P=0.808; AA vs. AC + CC:
OR=0.81, 95% CI=0.60–1.09, P=0.162), as shown in Table II and Fig. 2. While stratified by ethnicity with
European populations, similar results were obtained (for A vs. C:
OR=0.99, 95% CI=0.85–1.15, P=0.884; AA + AC vs. CC: OR=1.01, 95%
CI=0.90–1.13, P=0.856; AA vs. AC + CC: OR=0.96, 95% CI=0.66–1.39,
P=0.817).
Evaluation of publication bias
Egger’s test was performed to estimate the
publication bias of literature. The results of Egger’s linear
regression test are listed in Table
II. No publication bias was identified for any of the
comparisons (P>0.05).
Discussion
Significant familial aggregation, convincing
demonstrations of multiple genetic linkages and its associations
indicate a genetic component as a risk factor in the pathogenesis
of T1D (28). Candidate gene
studies have identified numerous susceptibility genes for T1D, such
as MHC, PTPN22 and CTLA4 (29).
IL-18 is a strong candidate for T1D as it has been
shown to have a central role in autoimmunity in experimental models
(30,31). Several variants in the IL-18 gene
have been identified, with the −137 C/G and −607 A/C variants in
the promoter region having been the most extensively examined in
epidemiologic studies. A change at position −137 from G to C alters
the H4TF-1 nuclear factor binding site and a change from C to A at
position −607 disrupts a potential cAMP-responsive element-binding
protein binding site. Cloning and gene expression analysis showed
that IL-18 −137C/G and −607A/C polymorphisms were suggested to
cause a difference in transcription factor binding and have an
impact on IL-18 gene activity (32).
Since the first study by Kretowski et
al(23) revealed a significant
correlation between IL-18 (−137 C/G, −607 A/C) gene polymorphisms
and T1D risk in a Polish polulation, numerous independent
investigators have undertaken to replicate this association.
However, the results are conflicting. The present meta-analysis of
11 eligible studies suggested that IL-18 −137 C/G and −607 A/C
polymorphisms did not contribute to the development of T1D in the
total population. In the subgroup analysis concerning ethnicity,
similar results were identified in all the genetic models in the
European population.
The reasons that the same polymorphism plays a
different role in different ethnic populations or across different
studies may arise from many factors. Firstly, this contradiction
might be due to exposure to different environmental factors. It is
generally accepted that environmental factors may influence the
penetrance of diabetes susceptibility genes (33). Exposure to a wide range of
environmental microbes in early life may play a role in the
modulation of the immune system, thus preventing the development of
T1D (34). Secondly, deviations
from HWE might contribute to the inconsistency. Testing for
deviations from HWE is an important quality control step in
population genetic studies. According to the Polish population,
genotype frequencies for IL-18 −137 C/G and −607 A/C polymorphisms
in controls slightly deviated from HWE (P<0.05), which may
increase the chances of obtaining false-positive results. Therefore
conclusions of those authors must be interpreted with caution.
Thirdly, linkage disequilibrium (LD) is relatively strong across
IL-18, which may distort the results observed (35). Ide et al(22) found that a higher promoter activity
of haplotype −137G/−607C of the IL-18 gene might increase the
expression of IL-18, resulting in upregulation of the
IFN-γ-producing T-cells. It is reported that the two loci (−137
C/G, −607 A/C) were in LD (19,24).
Lastly, clinical heterogeneity might also explain the discrepancy.
The potential contribution of differences in patient populations
(e.g., age and years from onset, as well as disease severity) might
lead to different results. In the study by Martin et
al(24), the age at diagnosis
of cases was under 15 years, whereas in the Polish and Japanese
studies the age at diagnosis was under 30 years.
The present study has some limitations that should
be considered. First, publication bias, heterogeneity, and
confounding factors may have distorted the meta-analysis. Second,
all of the studies were performed in European-, Asian- and South
American-descent populations. Future studies are needed in other
ethnic populations due to possible ethnic difference of the IL-18
−137 C/G and −607 A/C polymorphisms.
In conclusion, the combined results of independent
association studies by meta-analysis showed that IL-18 −137 C/G and
−607 A/C polymorphisms may not be risk factors for T1D. Prospective
studies with larger patient cohorts and relevant confounding risk
factors, such as age, ethnicity and life style, are required to
examine the possible effects of IL-18 polymorphisms on T1D to
confirm our conclusion.
Acknowledgements
This study was financed by the Doctoral Scientific
Research Foundation of Anhui University of Chinese Medicine (grant
no. 2013RC002).
Abbreviations:
|
IL-18
|
interleukin-18
|
|
T1D
|
type 1 diabetes
|
|
OR
|
odds ratio
|
|
CI
|
confidence interval
|
|
HWE
|
Hardy-Weinberg equilibrium
|
References
|
1
|
Nokoff NJ, Rewers M and Cree Green M: The
interplay of autoimmunity and insulin resistance in type 1
diabetes. Discov Med. 13:115–122. 2012.PubMed/NCBI
|
|
2
|
Tooley JE, Waldron-Lynch F and Herold KC:
New and future immunomodulatory therapy in type 1 diabetes. Trends
Mol Med. 18:173–181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Daneman D: Type 1 diabetes. Lancet.
367:847–858. 2006. View Article : Google Scholar
|
|
4
|
Redondo MJ and Eisenbarth GS: Genetic
control of autoimmunity in Type I diabetes and associated
disorders. Diabetologia. 45:605–622. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gan MJ, Albanese-O’Neill A and Haller MJ:
Type 1 diabetes: current concepts in epidemiology, pathophysiology,
clinical care, and research. Curr Probl Pediatr Adolesc Health
Care. 42:269–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Knip M: Pathogenesis of type 1 diabetes:
implications for incidence trends. Horm Res Paediatr. 76:57–64.
2011. View Article : Google Scholar
|
|
7
|
Risch N: Assessing the role of HLA-linked
and unlinked determinants of disease. Am J Hum Genet. 40:1–14.
1987.PubMed/NCBI
|
|
8
|
Rabinovitch A: An update on cytokines in
the pathogenesis of insulin-dependent diabetes mellitus. Diabetes
Metab Rev. 14:129–151. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nicoletti F, Conget I, Di Marco R, et al:
Serum levels of the interferon-gamma-inducing cytokine
interleukin-18 are increased in individuals at high risk of
developing type I diabetes. Diabetologia. 44:309–311. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rothe H, Ito Y and Kolb H: Disease
resistant, NOD-related strains reveal checkpoints of
immunoregulation in the pancreas. J Mol Med (Berl). 79:190–197.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rothe H, Jenkins NA, Copeland NG and Kolb
H: Active stage of autoimmune diabetes is associated with the
expression of a novel cytokine, IGIF, which is located near Idd2. J
Clin Invest. 99:469–474. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zaccone P, Phillips J, Conget I, Cooke A
and Nicoletti F: IL-18 binding protein fusion construct delays the
development of diabetes in adoptive transfer and
cyclophosphamide-induced diabetes in NOD mouse. Clin Immunol.
115:74–79. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kruse S, Kuehr J, Moseler M, et al:
Polymorphisms in the IL 18 gene are associated with specific
sensitization to common allergens and allergic rhinitis. J Allergy
Clin Immunol. 111:117–122. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cochran WG: The combination of estimates
from different experiments. Biometrics. 10:101–129. 1954.
View Article : Google Scholar
|
|
15
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Egger M, Smith GD, Schneider M and Minder
C: Bias in meta-analysis detected by a simple, graphical test. BMJ.
315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tavares NA, Santos MM, Moura R, Araújo J,
Guimarães R, Crovella S and Brandão L: Interleukin 18 (IL18) gene
promoter polymorphisms are associated with type 1 diabetes mellitus
in Brazilian patients. Cytokine. 62:286–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hadžija MP, Korolija M, Jemin N, et al:
Polymorphisms in the IL-18 and IL-12B genes and their association
with the clinical outcome in Croatian patients with Type 1
diabetes. Gene. 512:477–481. 2013.PubMed/NCBI
|
|
19
|
Altinova AE, Engin D, Akbay E, et al:
Association of polymorphisms in the IL-18 and IL-12 genes with
susceptibility to Type 1 diabetes in Turkish patients. J Endocrinol
Invest. 33:451–454. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boraska V, Terzić J, Skrabić V, et al:
NeuroD1 gene and interleukin-18 gene polymorphisms in type 1
diabetes in Dalmatian population of Southern Croatia. Croat Med J.
47:571–578. 2006.PubMed/NCBI
|
|
21
|
Dong GP, Yu ZS, Liang L, Zou CC, Fu JF and
Wang CL: IL-18 gene promoter −137C/G and −607C/A polymorphisms in
Chinese Han children with type 1 diabetes mellitus. Int J
Immunogenet. 34:75–79. 2007.
|
|
22
|
Ide A, Kawasaki E, Abiru N, et al:
Association between IL-18 gene promoter polymorphisms and CTLA-4
gene 49A/G polymorphism in Japanese patients with type 1 diabetes.
J Autoimmun. 22:73–78. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kretowski A, Mironczuk K, Karpinska A, et
al: Interleukin-18 promoter polymorphisms in type 1 diabetes.
Diabetes. 51:3347–3349. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martin RJ, Savage DA, Carson DJ, Maxwell
AP and Patterson CC: Interleukin 18 promoter polymorphisms are not
strongly associated with type I diabetes in a UK population. Genes
Immun. 6:171–174. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mojtahedi Z, Naeimi S, Farjadian S, Omrani
GR and Ghaderi A: Association of IL-18 promoter polymorphisms with
predisposition to Type 1 diabetes. Diabet Med. 23:235–239. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Novota P, Kolostova K, Pinterova D, et al:
Interleukin IL-18 gene promoter polymorphisms in adult patients
with type 1 diabetes mellitus and latent autoimmune diabetes in
adults. Immunol Lett. 96:247–251. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Szeszko JS, Howson JM, Cooper JD, et al:
Analysis of polymorphisms of the interleukin-18 gene in type 1
diabetes and Hardy-Weinberg equilibrium testing. Diabetes.
55:559–562. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Noble JA and Erlich HA: Genetics of type 1
diabetes. Cold Spring Harb Perspect Med. 2:a0077322012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bradfield JP, Qu HQ, Wang K, et al: A
genome-wide meta-analysis of six type 1 diabetes cohorts identifies
multiple associated loci. PloS Genet. 7:e10022932011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Frigerio S, Hollander GA and Zumsteg U:
Functional IL-18 is produced by primary pancreatic mouse islets and
NIT-1 beta cells and participates in the progression towards
destructive insulitis. Horm Res. 57:94–104. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lukic ML, Mensah-Brown E, Wei X, Shahin A
and Liew FY: Lack of the mediators of innate immunity attenuate the
development of autoimmune diabetes in mice. J Autoimmun.
21:239–246. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Giedraitis V, He B, Huang WX and Hillert
J: Cloning and mutation analysis of the human IL-18 promoter: a
possible role of polymorphisms in expression regulation. J
Neuroimmunol. 112:146–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yoon JW and Jun HS: Role of viruses in the
pathogenesis of type 1 diabetes mellitus. Diabetes Mellitus.
LeRoith D, Taylor SI and Olefsky JM: 2nd edition. Lippincott
Williams and Wilkins; New York: pp. 575–589. 2004
|
|
34
|
McKinney PA, Okasha M, Parslow RC, et al:
Early social mixing and childhood Type 1 diabetes mellitus: a
case-control study in Yorkshire, UK. Diabet Med. 17:236–242. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thompson SR and Humphries SE:
Interleukin-18 genetics and inflammatory disease susceptibility.
Genes Immun. 8:91–99. 2007. View Article : Google Scholar : PubMed/NCBI
|