Introduction
Yessotoxin (YTX) and its analogs are a group of
lipophilic marine toxins mainly produced by the dinoflagellates
Protoceratium reticulatum, Gonyaulax spinifera and
Lingulodinium polyedrum. These toxins tend to accumulate in
filter-feeding molluscs, have been found in numerous countries
worldwide (1–6) and were first detected in shellfish
samples collected from Chinese coastal areas in 2009 (7).
YTX and its analogs were first isolated from
Patinopecten yessoensis in Japan (8) and were long classified as one of the
categories of diarrheic shellfish poisoning (DSP) toxins (9). However, as these toxins cannot induce
diarrhea, nor inhibit protein phosphatase 2A, like other DSP
toxins, they were classified as an independent group by the
European Union Commission in 2002 (10).
Apoptosis is a programmed form of cell suicide. The
process of apoptosis is controlled by genes and is crucial in
disease outbreaks, including cancer. Once the signaling pathway of
apoptosis is activated, the process cannot be easily undergone,
even by tumor cells. Thus, there is an increasing number of studies
on tumor cell apoptosis, with the aim to design an effective cancer
treatment (11–13). Several studies indicated that YTX
may induce apoptosis in different types of cell lines (14–21).
However, the exact underlying mechanisms have not been
elucidated.
As the chemical structure of YTX is similar to that
of brevetoxins and ciguatoxins, which were shown to interfere with
voltage-gated sodium channels, the effects of YTX on transmembrane
ion channels were previously investigated (22,23).
The present study mainly investigated the YTX-induced alterations
in intracellular Ca2+ levels in Bel7402 human
hepatocellular carcinoma cells and the possible underlying
mechanisms.
Materials and methods
Reagents
Pure YTX was purchased from the National Research
Council (NRC; Ottawa, ON, Canada). Fluo-3 acetoxymethyl ester (AM)
solution (5 mM) was purchased from Beyotime Institute of
Biotechnology (Shanghai, China). Nifedipine was purchased from
Sigma (St. Louis, MO, USA) and was dissolved in dimethyl sulfoxide
(Merck KGaA, Darmstadt, Germany). All other chemicals were of
analytical reagent grade or higher purity.
The Hanks’ balanced salt solution (HBSS) used for
cell washing consisted of 137 mM NaCl, 5.6 mM KCl, 1.26 mM
CaCl2, 0.81 mM MgSO4, 0.38 mM
Na2HPO4, 0.44 mM KH2PO4
and 4.2 mM NaHCO3. The pH of the HBSS was adjusted to
7.4 with 0.1 M HCl and NaOH.
Cell culture
The Bel7402 human hepatocellular carcinoma cell line
was purchased from the Cell Bank of the Shanghai Institute of
Biochemistry and Cell Biology, Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in RPMI-1640 medium
(Gibco, Carlsbad, CA, USA) supplemented with 300 mg/l L-glutamine,
100 μg/ml streptomycin and 100 U/ml penicillin, plus 10% fetal calf
serum (TBD, Beijing, China) and incubated at 37°C in a humidified
5% CO2 atmosphere.
Analysis of intracellular Ca2+
levels
The Bel7402 cells were transferred to a 24-well
microplate and incubated at 37°C. When 90% confluence was reached,
the cells were washed twice with HBSS and loaded with Fluo-3 AM at
a final concentration of 5 μM for 1 h at 37°C in the dark. The
solution containing the dye was then removed and the cells were
washed twice with HBSS.
The average fluorescence intensity of intracellular
Ca2+ concentration in labeled cells was detected under a
laser scanning confocal microscope (FluoView FV1000; Olympus Co.,
Tokyo, Japan). The wavelength of excitation was set at 488 nm and
the emission wavelength at 525 nm for Fluo-3 fluorescence reading.
Newly-developed FV300/FV500 Application software was used for the
measurement and analysis of Ca2+ concentration.
Ethylene glycol tetraacetic acid (EGTA) and
nifedipine, at a final concentration of 2 mM and 1 μM,
respectively, were added to the reaction system to investigate the
exact mechanism of YTX-evoked Ca2+ increase.
Results
Effect of YTX on intracellular
Ca2+ levels
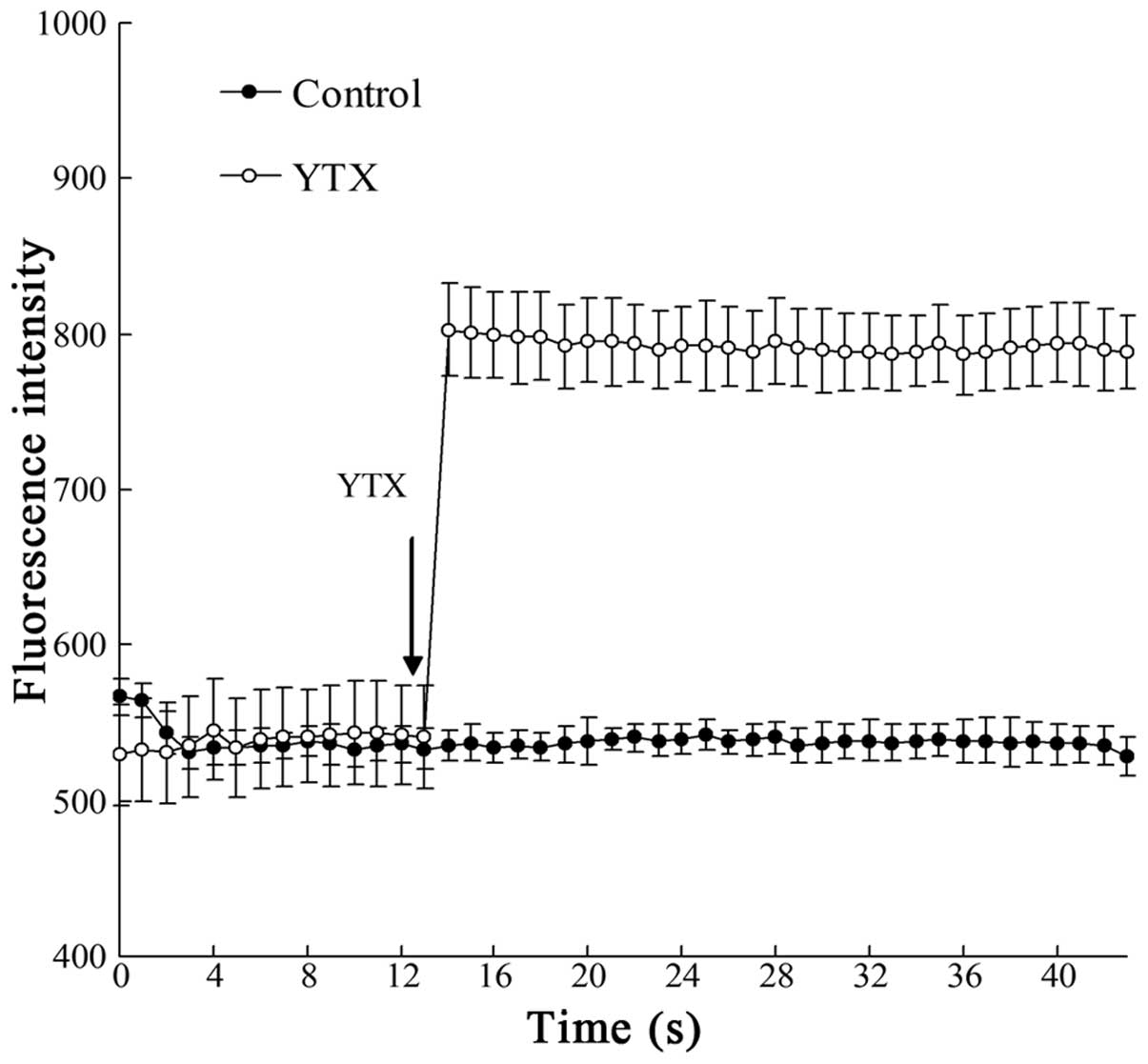
As shown in Fig. 1,
the intracellular Ca2+ levels in Bel7402 cells were
obviously increased following the addition of YTX to the medium,
with the increase of Ca2+ levels lasting for >40
sec.
Addition of EGTA
EGTA at a final concentration of 2 mM was used to
investigate whether the YTX-evoked Ca2+ increase was
caused by Ca2+ in the extracellular medium or released
from cytoplasmic organoids. As shown in Fig. 2, in a Ca2+-free solution,
YTX was not able to induce an elevation of the Ca2+
concentration. Although 2 mM CaCl2 was then added to the
medium, the fluctuation of Fluo-3 fluorescence was marginal.
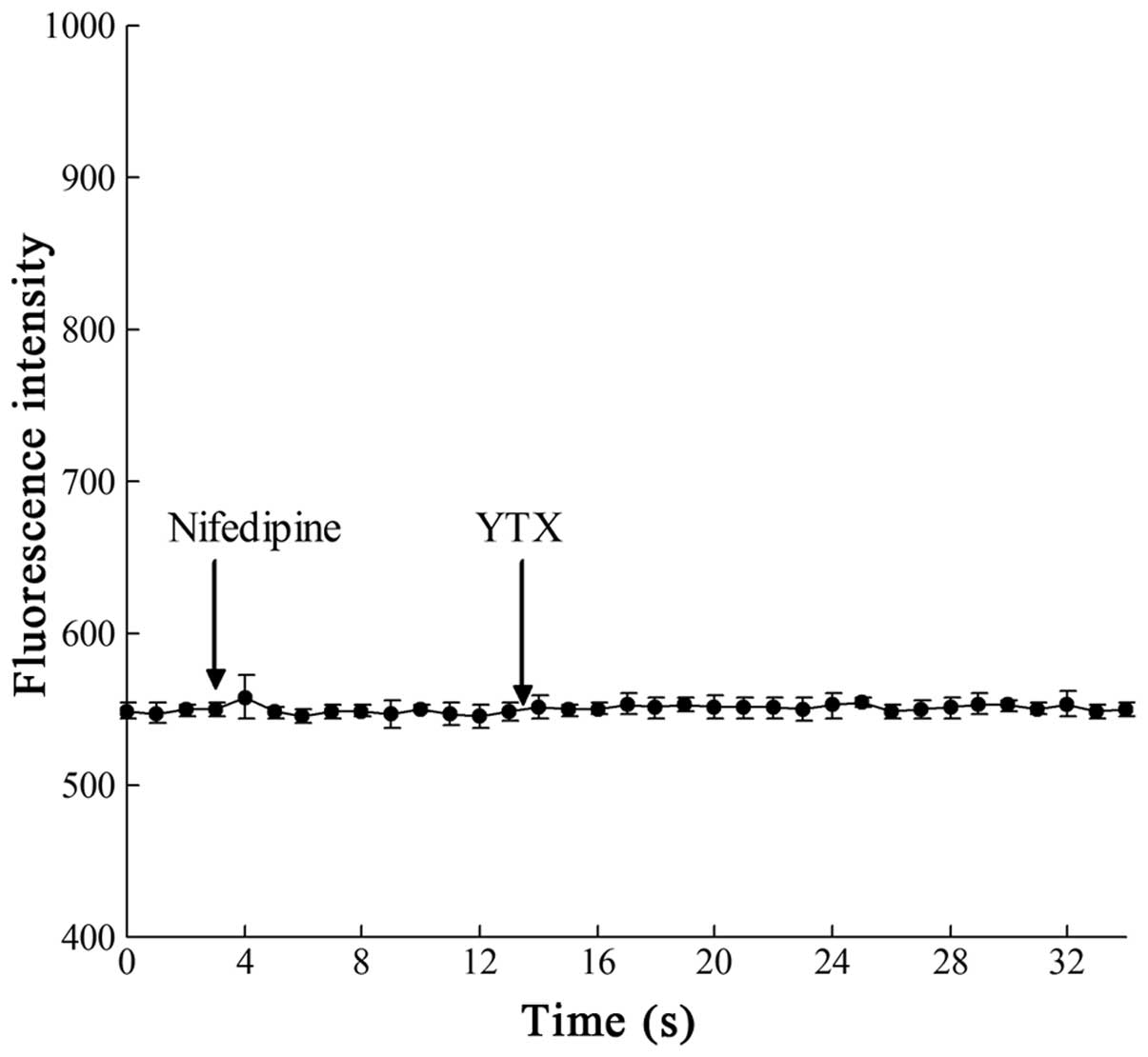
Addition of nifedipine
The possible involvement of Ca2+ channels
in the elevation of Ca2+ concentration evoked by YTX was
also investigated. Several drugs are known to block different types
of Ca2+ channels. Nifedipine, at a final concentration
of 1 μM, was used in this study to block the L-type Ca2+
channels; YTX was then added to observe the fluorescence change in
the Bel7402 cells. As shown in Fig.
3, the YTX-evoked Ca2+ increase was completely
blocked by nifedipine.
Discussion
YTX is a low-molecular weight compound that is toxic
to different mammalian and insect cell types, including cancer
cells (14,15,23–26).
Our previous studies demonstrated the apoptosis induced by YTX in
HeLa human cervical cancer cells, Bel7402 human hepatocellular
carcinoma cells and HL7702 human liver cells (19–21).
The identification of cell death signalling pathways and cellular
factors involved in apoptosis induced by YTX may provide useful
information for evaluating the potential use of YTX for therapeutic
purposes, including cancer research. Botana Lopez et
al(27) indicated that YTX may
be used as an antitumor agent, with this possibility already
considered in the European patent application EP1875906.
Apoptosis, the process of programmed cell death, is
often characterized by an orderly progression of morphological
changes, including cell shrinkage, condensation of chromatin and
externalization of membrane phosphatidylserine, and is controlled
by cell signals that may originate extracellularly or
intracellularly (28). These
signals may positively or negatively affect apoptosis.
Extracellular signals, including toxins, hormones, growth factors,
nitric oxide and cytokines (29),
may either cross the plasma membrane or transduce into
intracellular signals in order to affect other responses.
Ca2+ is an important intracellular second messenger
which is crucial in numerous processes, including cell
proliferation, differentiation, DNA damage and apoptosis (30,31).
Thus, the changes in the cytoplasmic Ca2+ concentration
may induce the process of apoptosis (32,33).
Previous studies indicated that YTX may induce the increase of
Ca2+ levels in several types of cells (22,23,26).
Our previous studies also demonstrated that YTX may induce
Ca2+ entry in different types of human cells (21,34).
Ca2+ ions are crucial in the process of apoptosis and
cytoplasmic Ca2+ concentration is involved in the
modulation of several cell functions. It was previously suggested
that the capacitative Ca2+ influx through
Ca2+ channels and the Ca2+ released in
cytoplasm from intracellular organelles are apoptogenic (35–37).
As described by Putney (38,39),
the release of Ca2+ from intercellular stores may induce
Ca2+ influx from the extracellular medium. However, de
la Rosa et al(22)
demonstrated that the Ca2+ influx induced by YTX was not
affected by internal stores. Our previous study also indicated that
YTX did not affect internal Ca2+ levels in a
Ca2+-free medium (21).
Although the changes in the Ca2+ levels
may be associated with the apoptosis induced by YTX, the exact
mechanism underlying this increase in Ca2+ has not been
elucidated. The chemical structure of YTX is similar to that of
brevetoxins, which are known to interfere with sodium channel
function. Therefore, it is possible that YTX is also able to
interact with cellular ion channels. We previously demonstrated
that nifedipine, a specific L-type Ca2+ channel blocker,
was able to inhibit the Ca2+ increase induced by YTX in
several human cell lines (21,34).
YTX may exert an effect on L-type Ca2+ channel in cells.
Although YTX may induce the Ca2+ increase in HL7702
normal human liver cells and Bel7402 human hepatocellular carcinoma
cells, the increasing trend of Ca2+ levels in Bel7402
cells was different from that observed in HL7702 cells (34). This difference may provide new
insight into cancer therapy. Further studies are required to
elucidate the exact mechanisms underlying apoptosis induced by YTX
in tumor cells.
In conclusion, YTX was shown to exert a cytotoxic
effect on normal human liver cells and human hepatocellular
carcinoma cells. Although YTX may induce Ca2+ influx in
these two cell types, the detailed increasing trend of
Ca2+ levels was different. The difference between normal
human liver cells and human hepatocellular carcinoma cells may
provide new insight into cancer therapy.
Acknowledgements
This study was supported by the Fundamental Science
Research Project of FIO (no. 2011T07), the youth fund of State
Oceanic Administration (no. 2012123) and project of the Key
Laboratory for Ecological Environment in Coastal Areas, SOA (no.
201106).
Abbreviations:
|
AM
|
acetoxymethyl ester
|
|
DSP
|
diarrheic shellfish poisoning
|
|
FACS
|
fluorescent-activated cell sorting
|
|
HBSS
|
Hanks’ balanced salt solution
|
|
LSCM
|
laser scanning confocal microscopy
|
|
NRC
|
National Research Council
|
|
YTX
|
yessotoxin
|
References
|
1
|
Satake M, MacKenzie L and Yasumoto T:
Identification of Protoceratium reticulatum as the
biogenetic origin of yessotoxin. Nat Toxins. 5:164–167. 1997.
|
|
2
|
Draisci R, Ferretti E, Palleschi L, et al:
High levels of yessotoxin in mussels and presence of yessotoxin and
homoyessotoxin in dinoflagellates of the Adriatic Sea. Toxicon.
37:1187–1193. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stobo LA, Lewis J, Quilliam MA, et al:
Detection of yessotoxin in UK and Canadian isolates of
phytoplankton and optimization and validation of LC-MS methods.
Proceedings of the Eighth Canadian Workshop on Harmful Marine
Algae; Bates S: Can Tech Rep Fish Aquat Sci. Gulf Fisheries Centre;
Moncton: pp. 8–14. 2003
|
|
4
|
Ciminiello P, Dell’Aversano C, Fattorusso
E, et al: Complex yessotoxins profile in Protoceratium
reticulatum from northwestern Adriatic sea revealed by LC-MS
analysis. Toxicon. 42:7–14. 2003.(In Chinese).
|
|
5
|
Arévalo F, Pazos Y, Correa J, Salgado C,
Moroño A, Paz B and Franco JM: First report of yessotoxins in
mussels of Galician Rías during a bloom of Lingulodinium
polyedra Stein (Dodge). Henshilwood K, Deegan B, McMahon T,
Cusack C, Keaveney S, Silke J, O’Cinneide M, Lyons D and Hess P:
Galway. pp. 184–189. 2006
|
|
6
|
Morton SL, Vershinin A, Leighfield TA,
Smith L and Quilliam M: Identification of yessotoxin in mussels
from the Caucasian Black Sea Coast of the Russian Federation.
Toxicon. 50:581–584. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao CL, Liu RY, Liang YB, et al: First
report of the presence of yessotoxins (YTXs) in shellfish from
China’s coastal areas. Acta Oceanol Sin. 3:129–137. 2010.PubMed/NCBI
|
|
8
|
Murata M, Masanori K, Lee JS and Yasumoto
T: Isolation and structure of yessotoxin, a novel polyether
compound implicated in diarrhetic shellfish poisoning. Tetrahedron
Lett. 28:5869–5872. 1987. View Article : Google Scholar
|
|
9
|
Yasumoto T and Takizawa A: Fluorometric
measurement of yessotoxins in shellfish by high-pressure liquid
chromatography. Biosci Biotechnol Biochem. 61:1775–1777. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
2002/225/EC. Commission Decision of 15
March 2002 laying down detailed rules for the implementation of
Council Directive 91/492/EEC as regards the maximum levels and the
methods of analysis of certain marine biotoxins in bivalve
molluscs, echinoderms, tunicates and marine gastropods. Official
Journal of the European Communities. 622002.
|
|
11
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar
|
|
12
|
Green DR and Evan GI: A matter of life and
death. Cancer Cell. 1:19–30. 2002. View Article : Google Scholar
|
|
13
|
Green DR and Kroemer G: The
pathophysiology of mitochondrial cell death. Science. 305:626–629.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malagutia C, Ciminiellob P, Fattorussob E
and Rossinia GP: Caspase activation and death induced by yessotoxin
in HeLa cells. Toxicol In Vitro. 16:357–363. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leira F, Alvarez C, Vieites JM, Vieytes MR
and Botana LM: Characterization of distinct apoptotic changes
induced by okadaic acid and yessotoxin in the BE(2)-M17
neuroblastoma cell line. Toxicol In Vitro. 16:23–31. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suárez Korsnes M, Hetland DL, Espenes A
and Aune T: Induction of apoptosis by YTX in myoblast cell lines
via mitochondrial signalling transduction pathway. Toxicol In
Vitro. 20:1419–1426. 2006.PubMed/NCBI
|
|
17
|
Suárez Korsnes M, Hetland DL, Espenes A,
Tranulis MA and Aune T: Apoptotic events induced by yessotoxin in
myoblast cell lines from rat and mouse. Toxicol In Vitro.
20:1077–1087. 2006.PubMed/NCBI
|
|
18
|
Suárez Korsnes M, Hetland DL, Espenes A
and Aune T: Cleavage of tensin during cytoskeleton disruption in
YTX-induced apoptosis. Toxicol In Vitro. 21:9–15. 2007.PubMed/NCBI
|
|
19
|
Pang M, Gao CL, Wu ZX, Lv N, Wang ZL, Tang
XX and Qu P: Apoptosis induced by yessotoxins in HeLa human
cervical cancer cells in vitro. Mol Med Rep. 3:629–634.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pang M, Wang ZL, Gao CL, Qu P and Li HD:
Characterization of apoptotic changes induced by yessotoxin in the
Bel7402 human hepatoma cell line. Mol Med Rep. 4:547–552.
2011.PubMed/NCBI
|
|
21
|
Pang M, Qu P, Gao CL and Wang ZL:
Yessotoxin induces apoptosis in HL7702 human liver cells. Mol Med
Rep. 5:211–216. 2012.PubMed/NCBI
|
|
22
|
de la Rosa LA, Alfonso A, Vilarino N,
Vieytes MR and Botana LM: Modulation of cytosolic calcium levels of
human lymphocytes by yessotoxin, a novel marine phycotoxin. Biochem
Pharmacol. 61:827–833. 2001.PubMed/NCBI
|
|
23
|
Perez-Gomez A, Fererro-Gutierrez A,
Novelli A, Franco JM, Paz B and Fernandez-Sanchez MT: Potent
neurotoxic action of the shellfish biotoxin yessotoxin on cultured
cerebellar neurons. Toxicol Sci. 90:168–177. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Korsnes MS, Hetland DL, Espenes A and Aune
T: Induction of apoptosis by YTX in myoblast cell lines via
mitochondrial signalling transduction pathway. Toxicol In Vitro.
20:1419–1426. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Korsnes MS, Hetland DL, Espenes A,
Tranulis MA and Aune T: Apoptotic events induced by yessotoxin in
myoblast cell lines from rat and mouse. Toxicol In Vitro.
20:1077–1087. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Malagoli D, Marchesini E and Ottaviani E:
Lysosomes as the target of yessotoxin in invertebrate and
vertebrate cell lines. Toxicol Lett. 167:75–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Botana Lopez LM, Alfonso Rancano A,
Rodriguez Vieytes M and Loza Garcia MI: Therapeutic use of
yessotoxins as human tumor cell growth inhibitors EP 1875906 B1.
Filed July 21, 2004; issued June 29, 2011.
|
|
28
|
Martin SJ, Reutelingsperger CP, McGahon
AJ, Rader JA, van Schie RC, LaFace DM and Green DR: Early
redistribution of plasma membrane phosphatidylserines is a general
feature of apoptosis regardless of the initiating stimulus:
inhibition by overexpression of Bcl-2 and Abl. J Exp Med.
182:1545–1556. 1995. View Article : Google Scholar
|
|
29
|
Popov SG, Villasmil R, Bernardi J, et al:
Lethal toxin of Bacillus anthracis causes apoptosis of
macrophages. Biochem Bioph Res Commun. 293:349–355. 2002.PubMed/NCBI
|
|
30
|
Berridge MJ, Lipp P and Bootman MD: The
versatility and universality of calcium signalling. Nat Rev Mol
Cell Biol. 1:11–21. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mattson MP and Chan SL: Calcium
orchestrates apoptosis. Nat Cell Biol. 5:1041–1043. 2003.
View Article : Google Scholar
|
|
32
|
Ferrari D, Pinton P, Szabadkai G, Chami M,
Campanella M, Pozzan T and Rizzuto R: Endoplasmic reticulum, Bcl-2
and Ca2+handling in apoptosis. Cell Calcium. 32:413–420.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Smaili SS, Hsu YT, Carvalho ACP,
Rosenstock TR, Sharpe JC and Youle RJ: Mitochondria, calcium and
pro-apoptotic proteins as mediators in cell death signaling. Braz J
Med Biol Res. 36:183–190. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pang M, Qu P, Gao CL, Wang ZL and Zhang Y:
Apoptosis and intracellular calcium level change induced by
yessotoxin in HeLa cells. J Fish China. 35:1729–1735. 2011.
|
|
35
|
Jiang S, Chow SC, Nicotera P and Orrenius
S: Intracellular Ca2+signals activate apoptosis in
thymocytes: studies using the Ca2+-ATPase inhibitor
thapsigargin. Exp Cell Res. 212:84–92. 1994.
|
|
36
|
Wertz IE and Dixit VM: Characterization of
calcium release-activated apoptosis of LNCaP prostate cancer cells.
J Biol Chem. 275:11470–11477. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pinton P, Ferrari D, Rapizzi E, Di
Virgilio F, Pozzan T and Rizzuto R: The
Ca2+concentration of the endoplasmic reticulum is a key
determinant of ceramide-induced apoptosis: significance for the
molecular mechanism of Bcl-2 action. EMBO J. 20:2690–2701.
2001.
|
|
38
|
Putney JW Jr: Receptor-regulated calcium
entry. Pharmacol Ther. 48:427–434. 1990. View Article : Google Scholar
|
|
39
|
Putney JW Jr: Capacitative calcium entry
revisited: Review article. Cell Calcium. 11:611–624. 1990.
View Article : Google Scholar : PubMed/NCBI
|