Introduction
The hypothalamus integrates signals originating in
the brain, peripheral circulatory system and gastrointestinal
tract, in order to regulate food intake and the expenditure of
energy (1). The hypothalamic
arcuate nucleus (ARC) plays a major role in the integration of
signals regulating appetite. A neuronal circuit inhibits food
intake via the expression of pro-opiomelanocortin (POMC) and
cocaine- and amphetamine-regulated transcript (CART), whereas a
different circuit stimulates food intake via the expression of
neuropeptide Y (NPY) and Agouti-related peptide (AgRP), which are
two potent orexigenic peptides (2,3). This
network responds to several hormonal and metabolic signals,
including leptin, insulin, ghrelin, corticosterone and glucose
(4).
Adiponectin, which is secreted by adipose tissue,
may be a direct signal involved in appetite and the control of body
weight (BW) (5). Adiponectin plays
a central role in the stimulation of food intake by activating
AMP-activated protein kinase (AMPK) in the ARC. Previous studies on
the effects of adiponectin on energy expenditure demonstrated that
oxygen consumption was significantly decreased by adiponectin. By
contrast, adiponectin-knockout mice exhibited reduced food intake
and increased oxygen consumption. Furthermore, the phosphorylation
of AMPK was significantly suppressed, the expression of NPY was
significantly reduced and the expression of POMC was increased in
the ARC of adiponectin-knockout mice following fasting (6).
Nesfatin-1 was recently identified as an
anorexigenic factor ssociated with melanocortin signaling in the
hypothalamus. Nesfatin-1 is an 82-amino acid residue derived from
the nucleobindin-2 (NUCB2) peptide, possibly through proteolysis by
prohormone convertases (7).
Nesfatin-1/NUCB2 immunoreactive cells were found to be expressed in
the pancreas, stomach, duodenum, adipose tissue, central amygdaloid
nucleus, hypothalamus, nucleus accumbens, cerebellum and
microdissected lumbar spinal cord in rodents, suggesting that
nesfatin-1/NUCB2 may be crucial in the physiological regulation of
carbohydrate metabolism, gastrointestinal function and nutrient
absorption (8–14). Furthermore, the nesfatin-1-induced
inhibition of food intake may be mediated through the inhibition of
orexigenic NPY neurons (15) and
the activation of POMC and CART (16).
Over the last few years, numerous studies
demonstrated that the NPY, CART and POMC mRNA expression levels and
the plasma levels of the respective proteins are altered with
advancing age (4,17–19).
However, although it was demonstrated that nesfatin-1 suppresses
appetite via the regulation of NPY, CART and POMC, and that
adiponectin regulates energy metabolism in association with NPY and
POMC (6,7,20), the
mechanisms underlying the changes in nesfatin-1 and adiponectin
levels with advancing age have not been fully elucidated. The
present study aimed to investigate the effects of aging on the
plasma levels of nesfatin-1 and adiponectin.
Materials and methods
Laboratory animals and breeding
environment
A total of 23 female BALB/c mice, aged 2, 6 and 24
months, were obtained from CLEA Japan Inc., (Tokyo, Japan). The
mice were housed in breeding rooms at a temperature of 22±2°C and a
humidity of 55±10%, under a 12-h light cycle, with the the light
period initiated at 7 a.m. daily. A normal diet (CE-2 containing
11% kcal fat, 59% kcal carbohydrate and 30% kcal protein; CLEA
Japan Inc.) and water were freely available. All the experiments
were approved by the Animal Experimental Ethics Committee of the
Kagoshima University Graduate School of Medical and Dental Sciences
(Kagoshima, Japan).
Blood and tissue sampling
The mice were deprived of food for 6 h prior to
tissue sampling. The rectal temperature was measured using a
digital thermometer (Technol Seven Co. Ltd., Yokohama, Japan) in a
room maintained at 22±0.5°C. A lubricated thermocouple was inserted
1.5 cm into the rectum of conscious mice. Blood samples were
obtained from the orbital sinus under diethyl ether anesthesia.
Immediately after collection, the blood samples were transferred to
chilled tubes containing EDTA-2Na (1 mg/ml) and aprotinin (500
U/ml), centrifuged and stored at −80°C until analysis. For peptide
measurements, the samples were not further aliquoted, nor were they
repeatedly frozen and thawed. The mice were sacrificed by cervical
dislocation. The liver and visceral fat (VF) were removed and
weighed.
Measurement of nesfatin-1 and
adiponectin
The plasma nesfatin-1 levels were measured using a
commercial ELISA kit (Phoenix Pharmaceuticals, Belmont, CA, USA)
following the manufacturer’s instructions. The plasma adiponectin
levels were determined with an adiponectin ELISA kit (Otsuka
Pharmaceutical Co., Tokyo, Japan).
Statistical analysis
Statistical analysis was performed using SPSS/PASW
Statistics for Windows, version 18.0 (SPSS, Inc., Chicago, IL,
USA). The nesfatin-1 and adiponectin data were analyzed using
analysis of variance followed by the post hoc least significant
difference test. The correlation between plasma nesfatin-1 and
adiponectin levels, VF and other related variables were assessed by
two-way invariant correlations (Pearson’s correlation coefficient).
P<0.05 was considered to indicate a statistically significant
difference.
Results
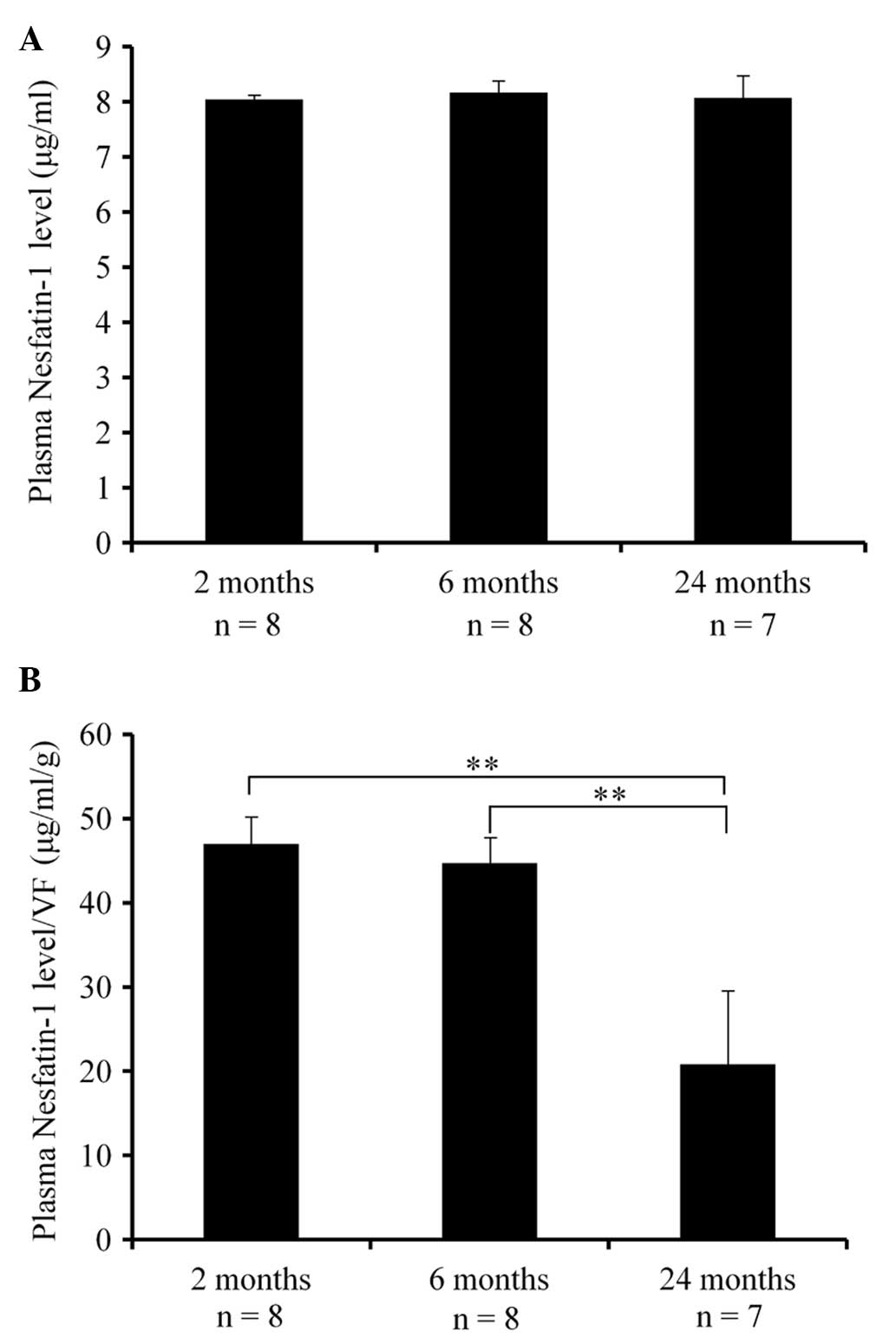
Changes in plasma nesfatin-1 levels
(μg/ml) and nesfatin-1 level/VF ratio with age
The plasma nesfatin-1 levels and the plasma
nesfatin-1 level/VF ratio of the mice are presented in Table I. There were no significant
differences between the groups regarding the plasma nesfatin-1
levels [F (2,20)=0.068] (Fig. 1A).
However, there were significant differences in the plasma
nesfatin-1 level/VF ratio among the three age groups [F=
(2,20)=7.19, P<0.01], with the ratio in the 24-month-old group
being significantly lower compared to that in the 2- and
6-month-old groups (P<0.01) (Fig.
1B).
 | Table IComparison of plasma nesfatin-1 and
adipotectin levels among the three age groups (2, 6 and 24 months)
of BALB/c mice. |
Table I
Comparison of plasma nesfatin-1 and
adipotectin levels among the three age groups (2, 6 and 24 months)
of BALB/c mice.
| Variables | 2 months | 6 months | 24 months | Difference by
group |
|---|
| Plasma nesfatin-1
level (μg/ml) | 8.04±0.07 | 8.17±0.23 | 8.07±0.41 | N.S. |
| Plasma nesfatin-1
level/VF | 46.98±3.29 | 44.7±3.01 | 20.83±8.66 | 24 vs. 2, 6 months,
P<0.01 |
| Plasma adiponectin
level (μg/ml) | 32.07±1.49 | 38.61±1.70 | 33.01±3.47 | N.S. |
| Plasma adiponectin
level/VF | 185.79±13.62 | 211.14±15.32 | 68.65±15.29 | 24 vs. 2, 6 months,
P<0.001 |
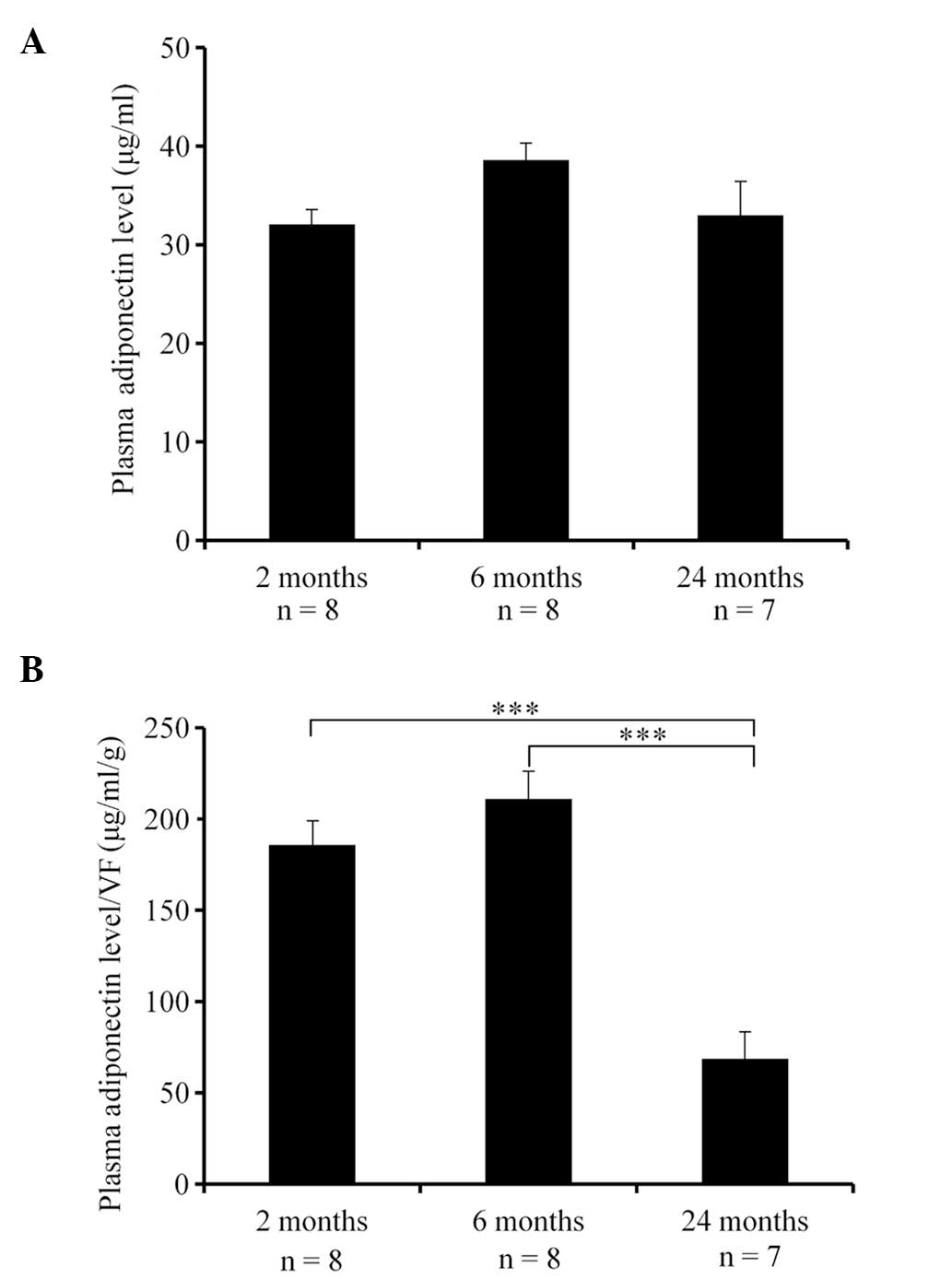
Changes in plasma adiponectin levels
(μg/ml) and in plasma adiponectin/VF ratio with age
The plasma adiponectin levels and plasma adiponectin
level/VF ratio of the mice are presented in Table I. There were no significant
differences in the plasma adiponectin levels between the different
age groups [F (2,20)=2.488] (Fig.
2A). However, there were significant differences in the plasma
adiponectin level/VF ratio [F (2,20)=25.39, P<0.001], with the
plasma adiponectin level/VF ratio being significantly lower in the
24-month-old group compared to that in the 2- and 6-month-old
groups (P<0.001) (Fig. 2B).
Changes in body and liver weight with
age
There were significant differences in BW and liver
weight among the three age groups [F (2,20)=143.96, P<0.01 and F
(2,20)=25.44, P<0.01, respectively]. The BW and liver weight
were significantly lower in the 2-month-old group compared to the
6- and 24-month-old groups (P<0.001) (Table II).
 | Table IIComparison of body weight, visceral
fat, liver weight and body temperature among the three age groups
(2, 6 and 24 months) of BALB/c mice. |
Table II
Comparison of body weight, visceral
fat, liver weight and body temperature among the three age groups
(2, 6 and 24 months) of BALB/c mice.
| Variables | 2 months | 6 months | 24 months | Difference by
group |
|---|
| Body weight
(g) | 18.95±0.17 | 25.56±0.49 | 26.69±0.30 | 2 vs. 6, 24 months,
P <0.01 |
| Visceral fat
(g) | 0.18±0.01 | 0.19±0.02 | 0.61±0.11 | 24 vs. 2, 6 months,
P <0.01 |
| Liver weight
(g) | 1.02±0.03 | 1.37±0.05 | 1.32±0.04 | 2 vs. 6, 24 months,
P <0.01 |
| Temperature
(°C) | 38.19±0.12 | 37.40±0.17 | 35.72±0.34 | 2 vs. 6 months, P
<0.05
2 vs. 24 and 6 vs.24 months, P <0.01 |
Changes in the VF level with age
There were significant differences in the VF levels
among the three age groups [F (2,20)=16.64]. The VF level was
higher in 24-month-old group compared to that in the 2- and
6-month-old groups (P<0.01) (Table
II).
Changes in body temperature with age
There were significant differences in the body
temperature among the three age groups [F (2,20)=32.31]. The body
temperature was higher in the 2-month-old group compared to that of
the 6- and 24-month-old groups (P<0.05 and P<0.01,
respectively), and that of the 6 months group was significantly
higher compared to that of 24 months group (P<0.01) (Table II).
Correlation analysis
There was no significant correlation of the plasma
nesfatin-1 and adiponectin levels with BW, liver weight, VF and
body temperature.
Discussion
Nesfatin-1 was identified as a novel
appetite-suppressing peptide in 2006 and studies on its
physiological effects, distribution and effects on energy
metabolism are currently underway (7,9–12,16).
It was previously demonstrated that fasting for 24 h decreased
NUCB2 mRNA expression in a pool of enriched small gastric endocrine
cells (21) and significantly
reduced nesfatin-1 plasma levels in rats (11). Li et al(22) reported that older healthy human
subjects (average age, 47.3 years) exhibited a higher mean fasting
plasma nesfatin-1 concentration compared to that of young healthy
subjects (average age, 19.4 years); no differences in plasma
nesfatin-1 levels were observed between healthy male and female
subjects.
Previous studies demonstrated that the NPY content
in the hypothalamus was significantly increased in rats with
streptozotocin-induced diabetes mellitus and in spontaneously
diabetic Brattleboro rats, and that the increased NPY content of
the hypothalamus may result in diabetic hyperphagia (23,24).
Moreover, the vasoconstrictive properties of NPY may contribute to
the mechanism underlying hypertension in obesity (25,26).
In aging studies, it was demonstrated that plasma NPY levels and
NPY gene expression in the hypothalamus increased with age
(17–19). However, the POMC mRNA levels in the
ARC were not found to be affected by age in the basal state,
whereas an age-associated increase of CART mRNA in the ARC and an
age-associated decrease in CART mRNA in the PVN were previously
reported (4). The prevalence of
diabetes and hypertension were shown to increase with age (27,28).
Those findings suggested that changes in NPY, CART and POMC with
age may be associated with the risk of onset of diabetes or
hypertension. The nesfatin-1-induced inhibition of food intake may
be mediated through the inhibition of orexigenic NPY neurons
(15) and the activation of POMC
and CART (16). If nesfatin-1
levels increase with age, the risk of onset of diabetes or
hypertension may decrease via the activation of NPY, CART and POMC.
In our study, there was a marginal change in the plasma nesfatin-1
levels in mice aged 2–24 months, although the plasma nesfatin-1
level/VF ratio was markedly decreased with advancing age. These
results indicated that nesfatin-1 may be relatively insufficient
when taking into consideration the changes in the amount of VF with
age. To the best of our knowledge, this study was the first to
report the effects of age on plasma nesfatin-1 concentrations in
mice.
Previous studies demonstrated that the plasma
adiponectin levels increased with age (18,29,30).
By contrast, Takenouchi et al(31) reported that there was no change in
the plasma adiponectin levels associated with age. In our study,
the plasma adiponectin levels in mice exhibited no differences with
advancing age, which is in accordance with the findings of
Takenouchi et al(31).
However, the plasma adiponectin level/VF ratio was decreased from 6
to 24 months of age. This result indicated that, as with
nesfatin-1, adiponectin may be relatively insufficient when taking
into consideration the changes in the VF amount with age.
Adiponectin, an adipose tissue-derived protein, possesses
antidiabetic, anti-atherogenic and insulin-sensitizing properties.
Adiponectin induces a decrease in circulating free fatty acids
(FFA) through increasing fatty acid oxidation by skeletal muscles,
decreases liver FFA influx and stimulates glucose uptake by
adipocytes and myocytes through the activation of AMPK (32–35). A
decrease in the triglyceride content of the muscles and the liver
results in increased insulin sensitivity. In addition to its
metabolic effects, adiponectin possesses anti-inflammatory and
atheroprotective properties that affect endothelial vascular
function (36). Thus, we
hypothesized that the relative insufficiency of nesfatin-1 and
adiponectin per VF with age may be associated with an increased
risk of the progression of aging or cardiovascular and
cerebrovascular diseases.
In conclusion, our results demonstrated that the
concentrations of plasma nesfatin-1 and adiponectin, which are
crucial in regulating food intake and energy metabolism, per VF,
are significantly decreased with age and these changes may affect
the onset and progression of various diseases.
Acknowledgements
This study was supported by the Institute of
Laboratory Animal Sciences, Kagoshima University (Frontier Science
Research Center).
References
|
1
|
Neary NM, Goldstone AP and Bloom SR:
Appetite regulation: from the gut to the hypothalamus. Clin
Endocrinol (Oxf). 60:153–160. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sahu A and Kalra SP: Neuropeptidergic
regulation of feeding behavior Neuropeptide Y. Trends Endocrinol
Metab. 4:217–224. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wynne K, Stanley S and Bloom S: The gut
and regulation of body weight. J Clin Endocrinol Metab.
89:2576–2582. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wolden-Hanson T, Marck BT and Matsumoto
AM: Blunted hypothalamic neuropeptide gene expression in response
to fasting, but preservation of feeding responses to AgRP in aging
male Brown Norway rats. Am J Physiol Regul Integr Comp Physiol.
287:R138–R146. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shklyaev S, Aslanidi G, Tennant M, et al:
Sustained peripheral expression of transgene adiponectin offsets
the development of diet-induced obesity in rats. Proc Natl Acad Sci
USA. 100:14217–14222. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kadowaki T, Yamauchi T and Kubota N: The
physiological and pathophysiological role of adiponectin and
adiponectin receptors in the peripheral tissues and CNS. FEBS Lett.
582:74–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oh-I S, Shimizu H, Satoh T, et al:
Identification of nesfatin-1 as a satiety molecule in the
hypothalamus. Nature. 443:709–712. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ramanjaneya M, Chen J, Brown JE, Tripathi
G, Hallschmid M, et al: Identification of nesfatin-1 in human and
murine adipose tissue: a novel depot-specific adipokine with
increased levels in obesity. Endocrinology. 151:3169–3180. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang AQ, Li XL, Jiang CY, et al:
Expression of nesfatin-1/NUCB2 in rodent digestive system. World J
Gastroenterol. 16:1735–1741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Su Y, Zhang J, Tang Y, et al: The novel
function of nesfatin-1: anti-hyperglycemia. Biochem Biophys Res
Commun. 391:1039–1042. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stengel A, Goebel M, Wang L, et al:
Central nesfatin-1 reduces dark-phase food intake and gastric
emptying in rats: differential role of corticotropin-releasing
factor2 receptor. Endocrinology. 150:4911–4919. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goebel M, Stengel A, Wang L, et al:
Nesfatin-1 immunoreactivity in rat brain and spinal cord autonomic
nuclei. Neurosci Lett. 452:241–246. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ogiso K, Asakawa A, Amitani H, et al:
Plasma nesfatin-1 concentrations in restricting-type anorexia
nervosa. Peptides. 32:150–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan BK, Hallschmid M, Kern W, et al:
Decreased cerebrospinal fluid/plasma ratio of the novel satiety
molecule, nesfatin-1/NUCB-2, in obese humans: evidence of
nesfatin-1/NUCB-2 resistance and implications for obesity
treatment. J Clin Endocrinol Metab. 96:669–673. 2011. View Article : Google Scholar
|
|
15
|
Price CJ, Samson WK and Ferguson AV:
Nesfatin-1 inhibits NPY neurons in the arcuate nucleus. Brain Res.
1230:99–106. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shimizu H, Oh-I S, Hashimoto K, et al:
Peripheral administration of nesfatin-1 reduces food intake in
mice: the leptin-independent mechanism. Endocrinology. 150:662–671.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Escobar CM, Krajewski SJ, Sandoval-Guzmán
T, et al: Neuropeptide Y gene expression is increased in the
hypothalamus of older women. J Clin Endocrinol Metab. 89:2338–2343.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baranowska B, Bik W, Baranowska-Bik A, et
al: Neuroendocrine control of metabolic homeostasis in Polish
centenarians. J Physiol Pharmacol. 57(Suppl 6): 55–61.
2006.PubMed/NCBI
|
|
19
|
Baranowska B, Wolinska-Witort E, Bik W,
Baranowska-Bik A, et al: Evaluation of neuroendocrine status in
longevity. Neurobiol Aging. 28:774–783. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stengel A and Taché Y: Nesfatin-1 - Role
as possible new potent regulator of food intake. Regul Pept.
163:18–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stengel A, Goebel M, Yakubov I, et al:
Identification and characterization of nesfatin-1 immunoreactivity
in endocrine cell types of the rat gastric oxyntic mucosa.
Endocrinology. 150:232–238. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li QC, Wang HY, Chen X, et al: Fasting
plasma levels of nesfatin-1 in patients with type 1 and type 2
diabetes mellitus and the nutrient-related fluctuation of
nesfatin-1 level in normal humans. Regul Pept. 159:72–77. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sahu A, Sninsky CA, Kalra PS, et al:
Neuropeptide Y concentration in microdissected hypothalamic regions
and in vitro release from the medial basal hypothalamus-preoptic
area of streptozotocin-diabetic rats with and without insulin
substitution therapy. Endocrinology. 126:192–198. 1990. View Article : Google Scholar
|
|
24
|
Wiliams G, Steel JH, Cardoso H, et al:
Increased hypothalamic neuropeptide Y concentrations in diabetic
rats. Diabetes. 37:763–772. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ekblad E, Edvinsson L, Wahlestedt C, et
al: Neuropeptide Y co-exists and co-operates with noradrenaline in
perivascular nerve fibers. Regul Pept. 8:225–235. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lundberg JM, Hua X and Franco-Cereceda A:
Effects of NPY on mechanical activity and neurotransmission in the
heart, vas deferens and urinary bladder of the guinea-pig. Acta
Physiol Scand. 121:325–332. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang H, Qiu Q, Tan LL, et al: Prevalence
and determinants of diabetes and impaired fasting glucose among
urban community-dwelling adults in Guangzhou, China. Diabetes
Metab. 35:378–384. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim KI, Chang HJ, Cho YS, et al: Current
status and characteristics of hypertension control in community
resident elderly Korean people: data from a Korean longitudinal
study on health and aging (KLoSHa study). Hypertens Res. 31:97–105.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koh SJ, Hyun YJ, Choi SY, et al: Influence
of age and visceral fat area on plasma adiponectin concentrations
in women with normal glucose tolerance. Clin Chim Acta. 389:45–50.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Atzmon G, Pollin TI, Crandall J, et al:
Adiponectin levels and genotype: a potential regulator of life span
in humans. J Gerontol A Biol Sci Med Sci. 63:447–453. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takenouchi Y, Kobayashi T, Matsumoto T, et
al: Gender differences in age-related endothelial function in the
murine aorta. Atherosclerosis. 206:397–404. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamauchi T, Kamon J, Waki H, et al: The
fat-derived hormone adiponectin reverses insulin resistance
associated with both lipoatrophy and obesity. Nat Med. 7:941–946.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sheng T and Yang K: Adiponectin and its
association with insulin resistance and type 2 diabetes. J Genet
Genomics. 35:321–326. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yamauchi T, Kamon J, Minokoshi Y, et al:
Adiponectin stimulates glucose utilization and fatty acid oxidation
by activating AMP-activated protein kinase. Nat Med. 8:1288–1295.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kadowaki T and Yamauchi T: Adiponectin and
adiponectin receptors. Endocrine Rev. 26:439–451. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Goldstein BJ and Scalia R: Adiponectin: a
novel adipokine linking adipocytes and vascular function. J Clin
Endocrinol Metab. 89:2563–2568. 2004. View Article : Google Scholar : PubMed/NCBI
|