Introduction
Ovarian cancer is the most frequent cause of death
from gyne cological cancer and the fourth most frequent cause of
cancer-related death in women worldwide (1). Vascular endothelial growth factor
(VEGF) plays a crucial role through its involvement in ovarian
biology. Since it is closely associated with the normal function of
ovaries, VEGF is also involved in ovarian pathologies, including
malignant neoplasms (2). The
observation that angiogenesis occurs around a neoplastic tumor was
made over a century ago (3). Tumor
growth and metastasis were subsequently suggested to depend on
angiogenesis and blockage of angiogenesis may thus provide one
strategy for inhibiting tumor growth. The participation of
angiogenesis and VEGF in the pathogenesis of neoplastic diseases
has been previously described (4).
VEGF, previously known as vascular permeability
factor, has a mass of 45 kDa and belongs to a family of
platelet-derived growth factors. Thus far, several forms of VEGF
have been distinguished, including isoforms A, B, C, D and E
(5,6). The biological significance of the
different forms of VEGF has yet to be definitively determined.
Elevated VEGF levels are reportedly associated with advanced-stage
melanoma, together with negative immune reactions, including type 2
helper T-cell (Th2) dominance and impaired dendritic cell function
(7). Recent studies have reported
that immune-suppressing myeloid cells that in increase in number in
cancer are expandable by VEGF (8,9).
Cachexia due to cancer is a complex metabolic
disorder that includes loss of adipose tissue due to lipolysis,
loss of skeletal muscle, elevation of resting energy consumption,
anorexia and reduction of oral food intake (10). Acute-phase response proteins
including VEGF are reportedly associated with the development of
cachexia in patients with cancer (11). The present study investigated the
status of VEGF and examined relationships in patients with ovarian
cancer between serum levels of VEGF and markers of nutrition and
inflammation.
Materials and methods
Sample collection
Blood samples were collected from 27 patients with
ovarian cancer. The patient group included 4 patients with stage I
disease, 2 with stage II, 13 with stage III and 8 with stage IV
disease. The enrolled patients had received surgery or chemotherapy
in the Department of Obstetrics and Gynecology at Fukushima Medical
University Hospital (Fukushima, Japan) between May, 2011 and
August, 2012 and were 38–83 years old (median, 58.5 years) with
histological confirmation of the diagnosis. The patients were newly
diagnosed and blood samples were collected prior to initiation of
any treatment.
This study was approved by the ethics committee at
Fukushima Medical University (2010–2014) and written informed
consent was obtained from the subjects enrolled in this study.
Serum levels of VEGF
Peripheral venous blood sera from the subjects were
stored at −80°C until use. Serum concentrations of VEGF were
measured by enzyme-linked immunosorbent assay (ELISA) (R&D
Systems, Minneapolis, MN, USA) according to the manufacturer's
instructions.
Markers for nutritional status
Nutritional status was determined by measuring serum
concentrations of albumin (nephelometry), prealbumin (turbidimetric
immunoassay), retinol-binding protein (latex agglutination
immunoassay) and transferrin (turbidimetric immunoassay).
Statistical analysis
Differences between groups were determined using the
Student's t-test. Relationships between two variables were
quantified by the Spearman's rank correlation coefficient.
P<0.05 was considered statistically significant. Notably, not
all the blood samples were of sufficient volume for the
measurements.
Results
We tested sera from 27 patients with ovarian cancer
and from 18 healthy volunteers. The serum levels of VEGF in whole
patients, stage I, II, III and IV patients were 576.7±110.6,
460.1±176.9, 248.3±61.6, 440.6±160.9 and 1006.3±218.3 pg/ml. Serum
levels were significantly increased for whole patients (P<0.01)
and stage IV patients (P<0.0001) compared to healthy volunteers
(238.5±27.5 pg/ml) (Fig. 1). Serum
levels of stage IV patients were significantly higher compared to
those of stage II (P<0.00005) and stage III (P<0.05)
patients. The serum levels of CRP in stage I, II, III and IV
patients were 0.64±0.04, 0.34±0.27, 6.76±1.76 and 4.15±1.92 mg/dl,
with significant increases being detected in the serum levels for
stage III compared to stage I (P<0.01) and stage II (P<0.01)
patients, and for stage IV patients compared to stage I (P<0.05)
and stage II (P<0.05) patients (Fig.
2).
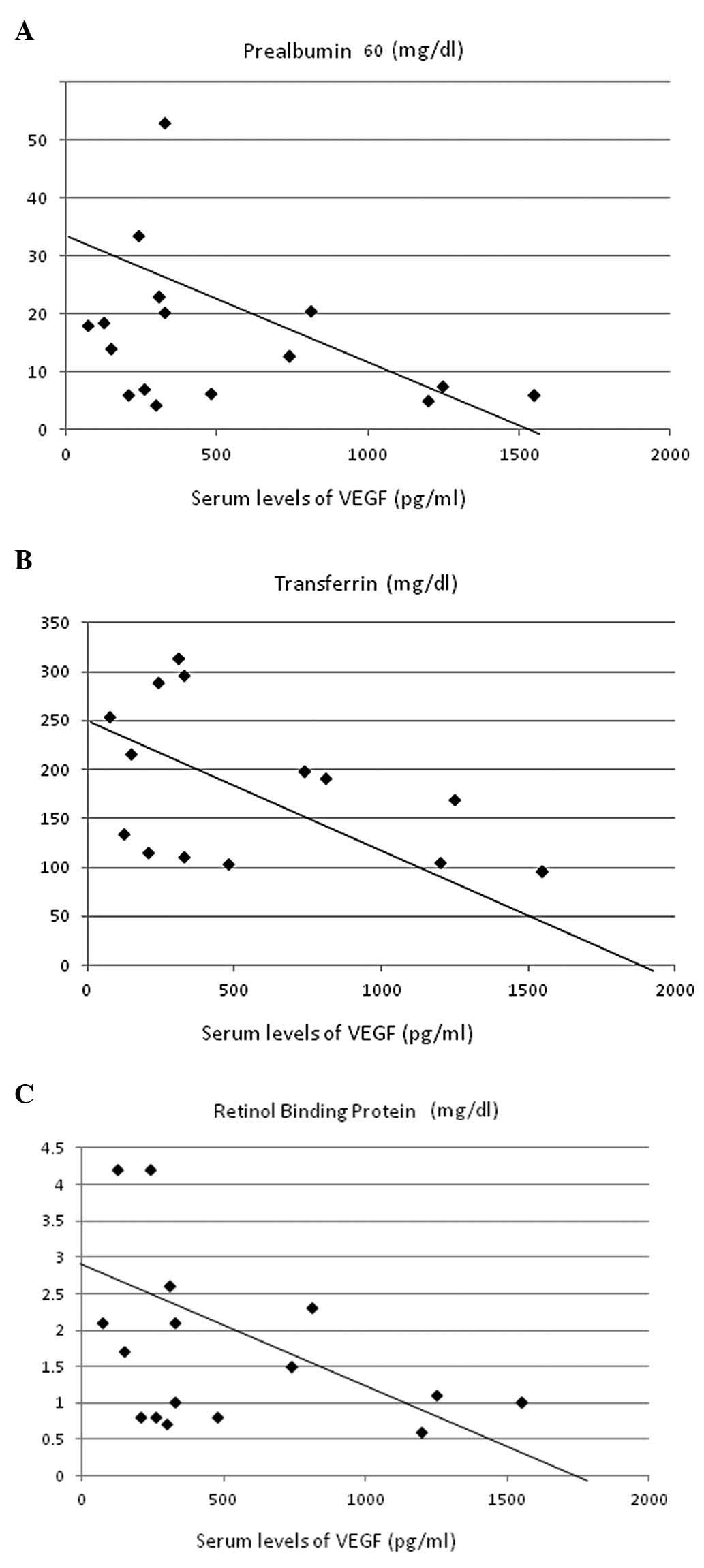
These data were analyzed for correlations with
parameters of nutritional status and inflammation. VEGF levels
showed significant inverse correlations with serum concentrations
of prealbumin (r=–0.467, P<0.005; Fig. 3A), transferrin (r=–0.640,
P<0.00005; Fig. 3B) and
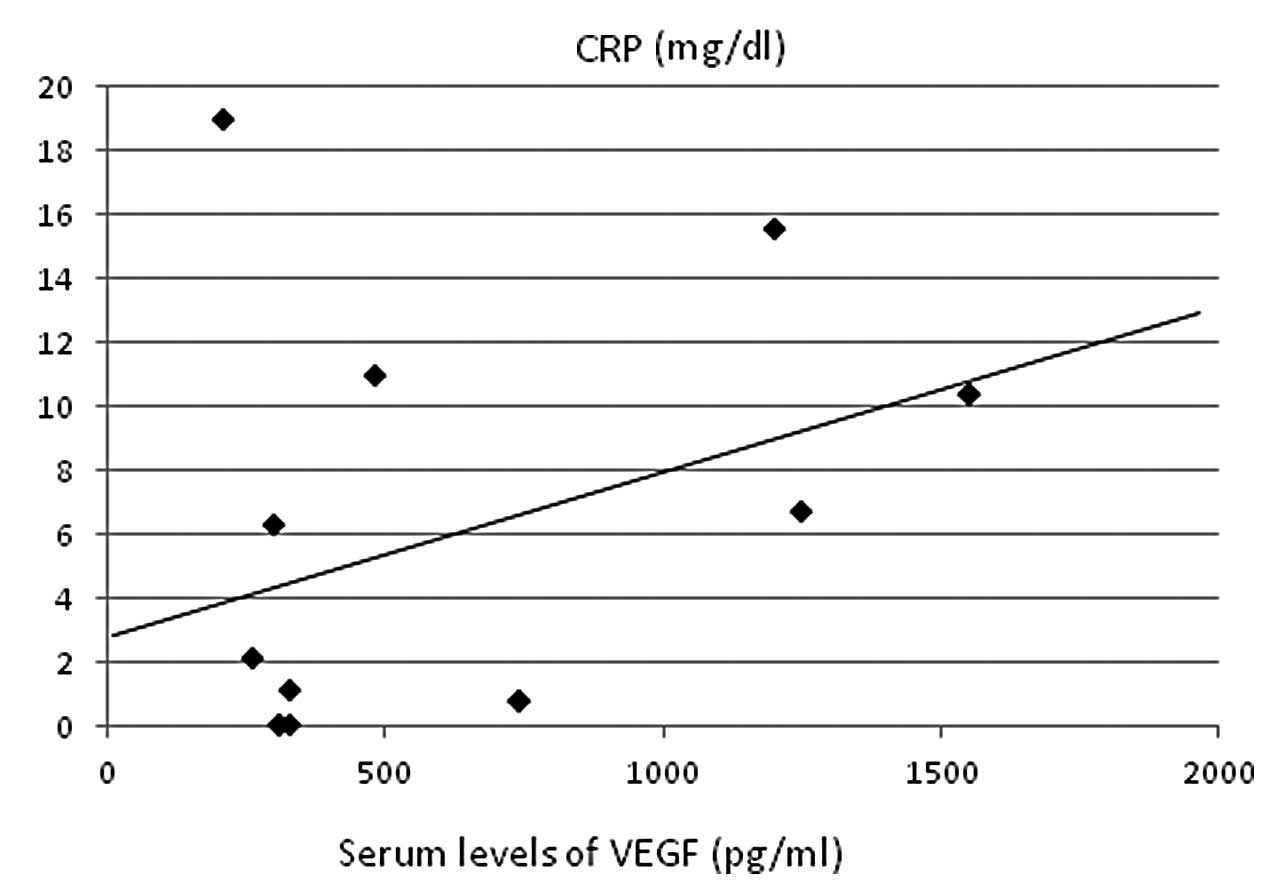
retinol-binding protein (r=–0.457, P<0.005; Fig. 3C). A strong positive correlation was
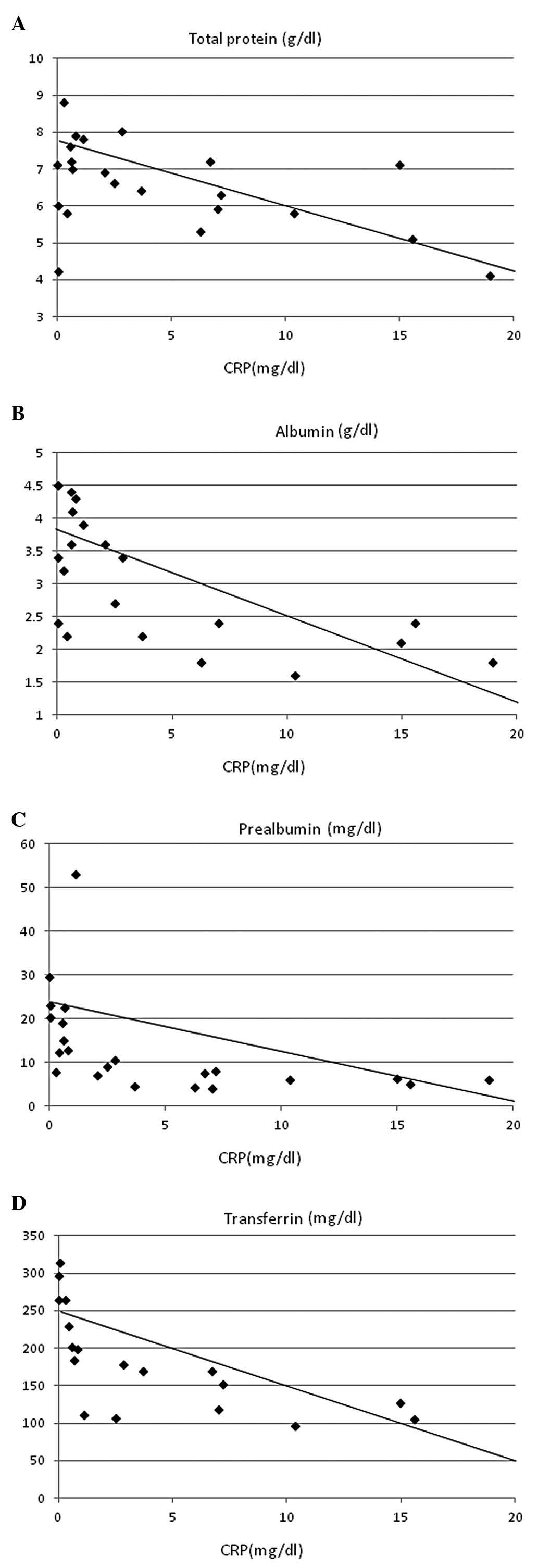
identified with CRP (r=0.422, P<0.01; Fig. 4) and CRP levels showed significant
inverse correlations with serum concentrations of total protein
(r=–0.402, P<0.05; Fig. 5A),
albumin (r=–0.668, P<0.0005; Fig.
5B), prealbumin (r=–0.511, P=0.005; Fig 5C), transferrin (r=–0.623,
P<0.0005; Fig. 5D) and
retinol-binding protein (r=–0.410, P<0.05; Fig. 5E).
Discussion
VEGF is important in the progression of malignant
neoplasms (12). In the present
study, we investigated the association of VEGF with nutritional
damage and systemic inflammation. VEGF levels were significantly
higher in patients with ovarian cancer than in healthy volunteers
as well as in patients with stage IV patients. VEGF levels showed
significant inverse correlations with nutritional status as
reflected by prealbumin, transferrin and retinol-binding protein
and the marker of inflammation, CRP, which has a strong association
with nutritional damage. CRP levels were also significantly
elevated in advanced diseases. These results strongly support the
hypothesis that VEGF plays a significant role in malnutrition and
inflammation, which are essential factors for the progression of
ovarian cancer, supporting the possible involvement of VEGF in the
pathogenesis of cachexia.
Decreased albumin concentrations are involved with
cachexia and are common laboratory features in malignant diseases.
Hypoalbuminemia has been demonstrated to be a predictive factor for
poor responsiveness (13,14). The ongoing systemic inflammatory
response in terms of CRP has recently gained some interest, as an
easily measured and standardized predictor of outcomes in patients
after treatment (15,16).
The immunosuppressive properties of malignant tumors
have been previously reported (17). Central to this hypothesis is the
polarization of the immune system towards a state of inflammation
driven by immunological mediators produced by tumor and immune
cells (17,18). CRP has been reported as a marker of
systemic inflammatory response and an independent predictor of
clinical benefit, good prognosis and survival in patients receiving
cancer chemotherapy. We recently reported that myeloid-derived
suppressor cells (MDSCs) are increased in various types of cancer
(19,20) and strong correlations of MDSC with
malnutrition were identified in patients with digestive system
cancer. Although the exact mechanisms involved in the increased
production of immature myeloid cells in cancer patients remain
unclear, it has been reported that VEGF is one of the key molecules
involved in the induction and expansion of MDSC (21–23).
In conclusion, results of this study have demonstrated that an
increased production of VEGF correlated with nutritional impairment
and systemic inflammation. Future studies should be conducted to
investigate possibilities for clinical control of chronic
inflammation through the modulation of VEGF.
Acknowledgements
We would like to thank Dr Mineyuki
Haruta, The Nihon University College of Engineering for processing
ELISA data.
References
|
1
|
Jacobs IJ and Menon U: Progress and
challenges in screening for early detection of ovarian cancer. Mol
Cell Proteomics. 3:355–366. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Geva E and Jaffe RB: Role of vascular
endothelial growth factor in ovarian physiology and pathology.
Fertil Steril. 74:429–438. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goldman E: The growth of malignant disease
in man and the lower animals with special reference to the vascular
system. Proc R Soc Med. 1:1–13. 1908.PubMed/NCBI
|
|
4
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Senger DR, Galli SJ, Dvorak AM, et al:
Tumor cell secrete a vascular permeability factor that promotes
accumulation of ascites fluid. Science. 219:983–985. 1983.
View Article : Google Scholar
|
|
6
|
Ferrara N: Vascular endothelial growth
factor and the regulation of angiogenesis. Recent Prog Horm Res.
55:15–36. 2000.PubMed/NCBI
|
|
7
|
Terheyden P, Schrama D, Pedersen LO, et
al: Longitudinal analysis of MART-1/HLA-A2-reactive T cells over
the course of melanoma progression. Scand J Immunol. 58:566–571.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ostrand-Rosenberg S and Sinha P:
Myeloid-derived suppressor cells: linking inflammation and cancer.
J Immunol. 182:4499–4506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rubin H: Cancer cachexia: its correlations
and causes. Proc Natl Acad Sci USA. 100:5384–5389. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deans C and Wigmore SJ: Systemic
inflammation, cachexia and prognosis in patients with cancer. Curr
Opin Clin Nutr Metab Care. 8:265–269. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kajdaniuk D, Marek B, Foltyn W and
Kos-Kudla B: Vascular endothelial growth factor (VEGF)-part 2: in
endocrinology and oncology. Endocrinol Pol. 62:456–464.
2011.PubMed/NCBI
|
|
13
|
Sanz L, Ovejero VJ, Gonzalez JJ, et al:
Mortality risk scales in esophagotomy for cancer: their usefulness
in preoperative patients selection. Hepatogastroenterology.
53:869–873. 2006.PubMed/NCBI
|
|
14
|
Onate-Ocana LF, Aiello-Crocifoglio V,
Gallardo-Rincon D, et al: Serum albumin as a significant prognostic
factor for patients with gastric carcinoma. Ann Surg Oncol.
14:381–389. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chua W, Charles KA, Baracos VE and Clarke
SJ: Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in
patients with advanced colorectal cancer. Brit J Cancer.
104:1288–1295. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cho H, Hur HW, Kim SW, et al:
Pre-treatment neutrophil to lymphocyte ratio is elevated in
epithelial ovarian cancer and predicts survival after treatment.
Cancer Immunol Immunother. 58:15–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nevala WK, Vachon CM, Leontovich AA, Scott
CG, Thompson MA and Markovic SN: Evidence of systemic Th2-driven
chronic inflammation in patients with metastatic melanoma. Clin
Cancer Res. 15:1931–1939. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shibata M, Shimura T, Gonda K, Suzuki S,
Nakamura I, Ohki S and Takenoshita S: Relationship of
myeloid-derived suppressor cells (MDSC) and immune suppression,
nutritional impairment, and inflammatory markers in patients with
gastrointestinal cancer. 2012 ASCO Gastrointestinal Cancer
Symposium: ASCO abs. 675,. 2012.
|
|
20
|
Ohki S, Shibata M, Gonda K, et al:
Circulating myeloid-derived suppressor cells are increased and
correlate to immune suppression, inflammation and hypoalbuminemia
in patients with cancer. Oncol Rep. 28:453–458. 2012.PubMed/NCBI
|
|
21
|
Gabrilovich DI, Chen HL, Girgis KR, et al:
Production of vascular endothelial growth factor by human tumors
inhibits the functional maturation of dendritic cells. Nat Med.
2:1096–1103. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Menetrier-Caux C, Montmain G, Dieu MC, et
al: Inhibition of the differentiation of dendritic cells from CD34+
progenitors by tumor cells: role of interleukin-6 and
macrophage-colony-stimulating factor. Blood. 92:4778–4791.
1998.
|
|
23
|
Gabrilovich D, Ishida T, Oyama T, et al:
Vascular endothelial growth factor inhibits the development of
dendritic cells and dramatically affects the differentiation of
multiple hematopoietic lineages in vivo. Blood. 92:4150–4166.
1998.
|